Abstract
Melanocortins play an important role in regulating blood pressure (BP) and sympathetic nervous system (SNS) activity as well as energy balance, glucose and other metabolic functions in humans and experimental animals. In experimental models of hypertension with high SNS activity, blockade of the melanocortin-4 receptor (MC4R) reduces BP despite causing marked hyperphagia and obesity. Activation of the central nervous system (CNS) pro-opiomelanocortin (POMC)–MC4R pathway appears to be an important link between obesity, SNS activation and hypertension. Despite having severe obesity, subjects with MC4R deficiency exhibit reductions in BP, heart rate, urinary catecholamine excretion and SNS responses to cold stimuli compared to obese subjects with normal MC4R function. In this review we discuss the importance of the brain POMC-MC4R system in regulating SNS activity and BP in obesity and other forms of hypertension. We also highlight potential mechanisms and brain circuitry by which the melanocortin system regulates cardiovascular function.
Keywords: hypertension, sympathetic activity, obesity, pro-opiomelanocortin, melanocortin-4 receptor
INTRODUCTION
The melanocortin system (Figure 1) consists of several pro-opiomelanocortin (POMC) derived melanocortin peptides including α, β and γ-melanocyte stimulating hormone (α, β and γ-MSH); adrenocorticotropic hormone (ACTH); N-terminal peptide of POMC (NPP or pro-γ-MSH); corticotropin-like intermediate peptide (CLIP); β–lipotropin (β–LPH) lipotropin gamma (γ-LPH); β–endorphin and [Met] enkephalin. Cleavage of POMC into biologically active peptides is driven by two prohomone convertases, proconvertase 1 (PC1) and proconvertase 2 (PC2). There are five melanocortin receptor subtypes, namely melanocortin receptors 1–5 (MC1R-MC5R), two endogenous antagonists (agouti and agouti-related protein, AgRP), and two ancillary proteins (mahogany and syndecan-3) [1–4].
Figure 1.
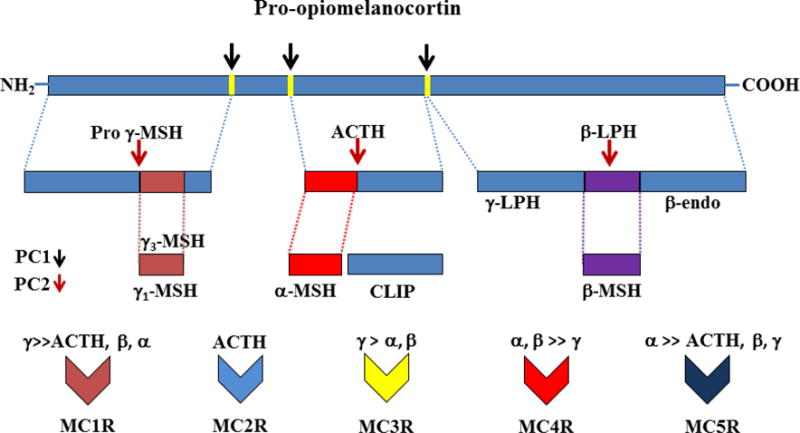
Schematic representation of sequential processing of pro-opiomelanocortin peptide and affinities of its byproducts for melanocortin receptors (MC1R-MC5R). PC1, proconvertase 1; PC2, proconvertase 2; LPH, lipotropin; ACTH, adrenocorticotrophic hormone; CLIP, corticotrophin-like peptide; β-endo, β-endorphin; MSH, melanocyte-stimulating hormone;
POMC is synthesized mainly by cells in the anterior and intermediate lobes of the pituitary, the arcuate nucleus (ARC) of hypothalamus, the nucleus tractus solitarius (NTS) of the brainstem, and several extracranial tissues such as thyroid, testis, placenta, pancreas, kidney, gastrointestinal tract, liver, and skin. Circulating POMC-derived peptides are thought to be secreted mainly from the pituitary gland whereas peptides in extrapituitary tissues function as autocrines, paracrines, and neurotransmitters [1–3]. The specific POMC-derived peptides produced in different tissues and their degradation depend on tissue-specific expression of several enzymes including prohormone convertases, carboxypeptidase E (CPE), peptidyl α-amidating monooxygenase (PAM), N-acetyltransferase (N-AT), and prolylcarboxypeptidase (PRCP).
The peptide fragments cleaved from POMC play a crucial role in controlling multiple physiological functions including skin pigmentation, adrenal steroid synthesis, inflammation, food intake, energy expenditure, glucose homeostasis, sympathetic nervous system (SNS) activity, and cardiovascular functions, including blood pressure (BP) and heart rate (HR) regulation [2, 5–9]. This review focuses on BP regulation by the brain melanocortin system, with emphasis on POMC neurons, α-MSH, and the melancortin-4 receptor (MC4R), and a brief discussion of γ-MSH and ACTH. Although some of the other POMC-derived peptides also influence BP regulation, discussion of these peptides is beyond the scope of this brief review.
POMC, MELANOCORTIN PEPTIDES AND BLOOD PRESSURE REGULATION
Besides its effects on food intake and body weight regulation, the melanocortin system also plays an important role in regulating SNS activity and BP. Infusions of several of the melanocortin peptides as well as pharmacological blockade or genetic deficiency of POMC-derived peptides and melanocortin receptors have been reported to have significant effects on BP and HR regulation. Some of the melanocortins such as ACTH and γ-MSH function as circulating hormones to influence BP regulation via multiple neurohumoral and renal mechanisms. Others such as α-MSH control BP mainly through their effects on the central nervous system (CNS).
Effects of Adrenocorticotropic Hormone (ACTH) on BP Regulation
ACTH has multiple homeostatic functions, including a key role in regulating secretion of adrenocortical hormones which, in turn, regulate metabolism, sodium balance, and BP. Chronic high circulating levels of ACTH, caused by excess anterior pituitary secretion (e.g. Cushing’s disease) or by infusion of ACTH, are associated with hypertension in humans and experimental animals [10–12].
ACTH-induced hypertension depends on intact adrenal gland function since adrenal insufficiency or adrenalectomy prevents increased BP in subjects with high levels of ACTH [13]. Although the mechanisms by which ACTH raises BP regulation are still unclear, sodium and water retention secondary to adrenal secretion of glucocorticoids/mineralocorticoid hormones appears to play an important role [12, 14]. The importance of the renal actions of the ACTH-adrenocortical axis on BP is evident by the fact that the hypertensive effects of ACTH are potentiated when renal excretory capacity is reduced by surgical removal of kidney mass [14]. ACTH also greatly enhances the chronic BP effects of norepinephrine or angiotensin II through mechanisms that are independent of increased glucocorticoids [12]. Although the kidneys are importantly involved in mediating the chronic cardiovascular actions of the ACTH-adrenocortical axis, the complex mechanisms involved are still the subject of investigation and have been extensively reviewed by others [13].
Effects of γ-MSH on BP Regulation
Previous studies have suggested that γ-MSH has pro-hypertensive as well as antihypertensive actions [15]. Injections of γ-MSH (i.v. or ICV) have been reported to raise BP and HR, although injections into the NTS lower BP and HR [15]. The Arg-Phe sequence in γ-MSH is critical for its acute hypertensive actions since synthetic analog peptides lacking this sequence do not raise BP [16, 17]. The elevation in BP by γ-MSH appears to be independent of MC3/4R activation since agouti protein or inhibitors of MC3/4R did not alter the acute BP effect of γ-MSH. In additon, BP increased similarly following γ-MSH injections in wild-type (WT) as well as in MC3R and MC4R deficent mice [18]. These observations suggest that these receptors may not play an important role in mediating the SNS activity and BP responses of γ-MSH. However, the effects of γ-MSH on BP in WT mice were completety abolished by benzamil, an amiloride analog, injected into the lateral ventricle [19]. These findings suggest that γ-MSH interacts with Phe-Met-Arg-Phe-NH2 (FMRFamide) gated sodium channels to induce SNS activation and increased BP. The physiological significance of the acute pressor actions of pharmacological injections of γ-MSH is unclear as there have been no studies, to our our knowledge, indicating that blocking the effects of endogenous γ-MSH lowers BP in physiological or pathophysiological conditions.
Several studies have demonstrated that γ-MSH also has natriuretic effects when infused directly into the renal artery [15, 20]. Although the physiological significance of γ-MSH-induced natriuresis has not been fully elucidated, the effect appears to be mediated primarily by activation of MC3R and subsequent stimulation of cAMP production in the kidney [15]. High salt intake increases MC3R mRNA and protein in inner medullary collecting duct cells suggesting that γ-MSH–MC3R activation may contribute to natriuresis during increased sodium intake [21]. Support for this hypothesis comes from studies showing that mice with genetic deficiency of MC3R or PC2, which reduces formation of γ-MSH, increase salt sensitivity of BP in mice [22]. These observations suggest that physiological activation of the γ-MSH–MC3R pathway by high sodium intake may protect against the development of salt-sensitive hypertension [20].
Effects of α-MSH on BP Regulation
Several previous studies have shown that acute ICV injections of α-MSH increase SNS activity and BP [19, 23, 24]. The effects of α-MSH on BP are mediated mainly by direct actions on the CNS since i.v. injections of α-MSH have no measureable effect on BP [23, 24]. The BP effects of α-MSH also require activation of CNS MC4R since BP responses to α-MSH administered directly into the CNS were completety abolished in MC4R deficent mice [19].
The acute effects of CNS injections of α-MSH on BP depend on the brain area where it activates its receptors since microinjections of α-MSH into the dorsal motor nucleus of the vagus (DMV) complex or the NTS lowered BP [25, 26] and this response was attenuated by MC4R blockade. Thus, acute pharmacological injections of α-MSH can increase or reduce BP depending on the site of injection. However, as discussed below, chronic physiological/pathophysiological stimulation of MC4R, which is activated mainly by α-MSH, generally increases BP and is thought to play a key role in several forms of hypertension associated with increased SNS activity, including obesity-induced hypertension. Also, MC4R appears to be the dominant efferent arm of the actions of the brain melanocortin system on regulation of food intake and body weight [27, 28].
CENTRAL ACTIONS OF POMC-MC4R ON BLOOD PRESSURE REGULATION
POMC-containing neurons are mainly located in the arcuate nucleus (ARC) of the hypothalamus and a few nuclei in the hindbrain NTS where they release α-MSH, an agonist for MC3/4R. These neurons project to several other brain regions involved in cardiovascular and metabolic regulation. There are five melanocortin receptors but only MC3R and MC4R are abundantly located in the CNS. Although MC3Rs have been implicated in body weight regulation (mainly via effects on enery expenditure) and in preventing salt sensitivty of BP as previously discussed [22], MC4R, activated mainly by α-MSH, is believed to be the key component of the brain melanocortin system’s effects on SNS activation and BP regulation [9, 29, 30].
Previous studies showed that by embryonic day 19 MC4R is expressed in many brain regions including components of the autonomic nervous system [31]. In addition, MC4R mRNA is widely expressed in the adult brain, including cortex, thalamus, hypothalamus, brainstem and spinal cord [32, 33]. In the hypothalamus, MC4R is highly expressed in paraventricular nucleus (PVN) of the hypothalamus, including parvocellular and magnocellular neurons, lateral hypothalamus (LH), the amygdala, the dorsal motor complex which includes the NTS and the DMV [34, 35]. McMullan and Pilowsky showed MC4R-green fluorescent protein (MC4R-GFP) immunoreactive neurons in the rostral ventral lateral medulla (RVLM) and intermediolateral nucleus (IML) of spinal cord in MC4R-GFP transgenic mice [36]. Besides these areas, MC4R are expressed in preganglionic sympathetic neurons of the IML [35] which is an important site for regulation of autonomic activity and BP. However, the specific role of MC4R in different brain regions in regulating SNS activity and BP is still unclear. Only a few studies have examined chronic cardiovascular actions of activating or blocking MC4R in specific neuronal populations. There is evidence, however, that MC4R activation in cholinergic preganglionic parasympathetic and sympathetic neurons [35] may contribute to autonomic regulation and increased BP in obese mice fed a high fat diet, as discussed later.
Stimulation of CNS POMC-MC4R pathway increases SNS activity and BP
Previous acute experiments in anesthetized animals showed that microinjection of an MC4R agonist into the PVN increased RSNA and BP [18], and the effect of hyperinsulinemia to acutely raise lumbar SNS activity was prevented by blockade of MC4R in the PVN [37]. Iwasa et al. found increased HR after α-MSH was injected into the IML [38]. In addition, ICV injections of MC4R agonist increased brown adipose tissue (BAT) and lumbar SNS activity whereas MC4R blockade completely abolished these effects [39].
Chronic pharmacological activation of CNS MC4R in rats increased BP while reducing appetite and body weight [40]. The increase in BP after chronic activation of MC4R is completely abolished after α/β-adrenergic blockade suggesting that it is due to increased adrenergic activity [41]. Blockade of CNS MC4R in rodents reduces BP despite increasing appetite and causing rapid weight gain which would normally increase BP [42].
Role of POMC Neurons and MC4R Activation in Mediating Cardiovascular and Metabolic Effects of Leptin and Obesity-Induced Hypertension
Increased SNS activity mediates a major component of the increased BP associated with obesity [9, 43, 44]. Although the mechanisms of obesity-induced hypertension are not fully understood, leptin, a peptide hormone produced by adipose tissue, may play an important role in linking obesity, SNS activity and increased BP. Acute leptin administration in rodents and humans increases renal and muscle SNS activity [45, 46] while chronic increases in plasma leptin levels, comparable to those found in severe obesity, caused sustained increases in BP and HR in rodents despite causing weight loss which would normally reduce BP [47].
Administration of a leptin receptor antagonist reduced BP and renal SNS activity in obese rabbits supporting a role for endogenous leptin in mediating obesity-induced increases in BP [48]. Although leptin’s effects on SNS activity and BP in humans have not been extensively studied, humans with leptin deficiency are generally not hypertensive and do not have increased sympathetic activity despite severe obesity, hyperinsulinemia, insulin resistance and most other characteristics of the metabolic syndrome [49].
Leptin requires activation of POMC neurons and MC4R to mediate its effects on renal SNS activity and BP [28, 50, 51]. Leptin receptor (LR) deletion specifically in POMC neurons completely abolished the chronic effects of leptin to raise BP and to reduce plasma glucose and insulin levels but did not markedly attenuate the effects of leptin on food intake [50]. These findings suggest that LR activation in POMC neurons is critical for leptin’s ability to increase BP and improve glucose homeostasis but not for its effect to reduce food intake.
Leptin requires functional MC4R for most of its chronic cardiovascular and metabolic effects. Mice with whole-body MC4R deficiency are hyperphagic and obese, and have many characteristics of the metabolic syndrome including hyperglycemia, hyperinsulinemia, visceral adiposity and dyslipidemia despite markedly elevated blood leptin levels [27, 28, 52]; moreover, these mice are completely unresponsive to the effects of leptin to reduce appetite and to raise BP [27].
MC4R activation also plays an important role in regulating BP in obesity independent of leptin. In obese Zucker fatty rats with defective LR signaling, MC4R blockade caused greater BP reductions than in lean control rats [53], indicating a key role for MC4R in controlling SNS activity and BP in the absence of functional LR.
The importance of MC4R to obesity hypertension is further supported by observations in humans with MC4R deficiency. Mutations of the POMC gene or the MC4R are estimated to account for as much as 5–6% of early onset obesity in humans (48, 64). Patients with MC4R deficiency exhibit lower BP, reduced 24-hr norepinephrine excretion, and reduced prevalence of hypertension despite severe metabolic abnormalities, compared to obese subjects with normal MC4R function [54]. In additon, individuals with MC4R mutations exhibit reduced muscle SNS activity and impaired SNS responses to a hypoxia stress test [55]. These observations suggest that in obese humans and obese rodents functional MC4Rs may be required for normal sympathetic responses to acute stress as well as increases in SNS activity and BP.
Role of MC4R Activation in Non-Obese Forms of Hypertension
Tonic MC4R activation may also contribute to regulation of SNS activity and BP in non-obese normotensive and hypertensive subjects. In lean normotensive animals, chronic MC4R antagonism caused sustained bradycardia and reduced BP in spite of hyperphagia and rapid weight gain which normally would evoke tachycardia and elevated BP [56]. Blockade of endogenous MC4R activity also reduced BP in several non-obese experimental models of hypertension, especially those associated with increased SNS activity [42, 57] (Figure 2). For instance, the BP-lowering effects of MC4R antagonism are especially pronounced in spontaneously hypertensive rats (SHR), a genetic model of hypertension that has increased SNS activity [42]. Blockade of CNS MC4R for 12 consecutive days caused a much greater reduction in BP in SHR than in normotensive Sprague-Dawley or Wistar-Kyoto rats despite causing marked hyperphagia, weight gain, and insulin resistance [42, 56]. In addition, the fall in BP in SHR after MC4R blockade was similar to that observed after α/β-adrenergic blockade [42].
Figure 2.
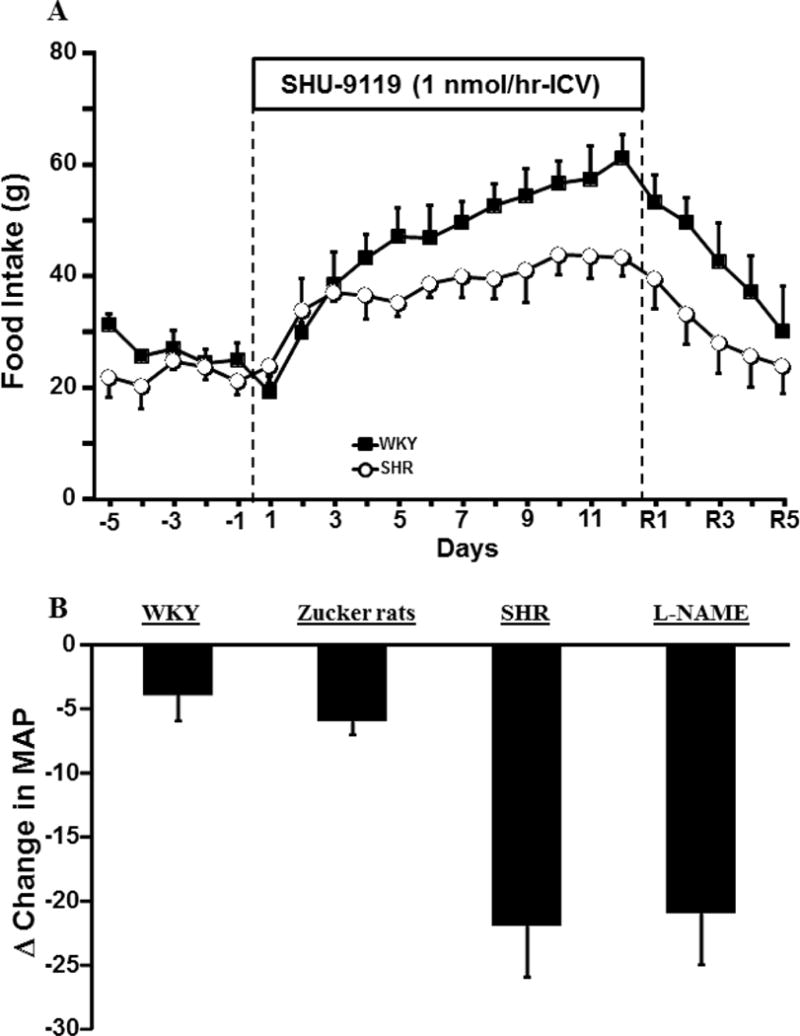
(A) Impact of chronic MC4R antagonism with SHU-9119 on food intake in Wistar Kyoto (WKY) and SHR rats. (B) The average changes (Δ) in mean arterial pressure in normotensive WKY, Zucker fatty rats, SHR and L-name-induced hypertension on the last day of SHU-9119 infusion. Figure 2A modified from data in reference 15. Data shown in Figure 2B are from references 15,17, and 20.
We also found that MC4R antagonism significantly attenuated hypertension induced by the nitric oxide synthase inhibitor L-NAME [58] (Figure 2). However, MC4R blockade failed to lower BP in angiotensin-II-induced hypertension, an experimental model with baroreflex mediated reductions in SNS activity [57]. These findings highlight the key role of the brain melanocortin system in maintenance of SNS activity and BP in normotensive subjects as well as in non-obese forms of hypertension that have increased sympathetic activity [41, 42, 57, 58].
CNS centers for BP and SNS regulation by POMC-MC4R
The CNS regions with the greatest abundance of MC4R are the hypothalamus and hindbrain preganglionic sympathetic neurons of the NTS, DMV and IML [35] (Figure 3) which are important sites for autonomic regulation. However, the specific brain regions where MC4R are most important in regulating SNS activity and BP have not been fully elucidated. The few studies that have examined chronic cardiovascular actions of MC4R in specific neuronal populations suggest a role for MC4R on cholinergic preganglionic parasympathetic and sympathetic neurons in contributing to obesity hypertension [59].
Figure 3.
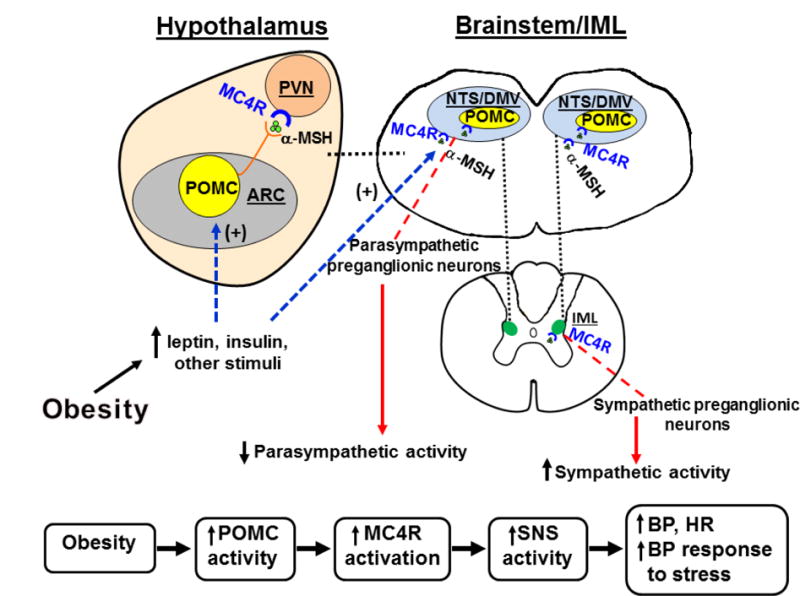
(A) Schematic representation of proopiomelanocortin (POMC) neuronal and melanocortin-4 receptor activation in forebrain and hindbrain as well as in the spinal cord IML leading to decreased parasympathetic nervous system (PSNS) activity, increased sympathetic nervous system (SNS) activity, increased blood pressure (BP), and enhanced BP response to stress. (α-MSH, alpha-melanocyte stimulating hormone; ARC, arcuate nucleus of the hypothalamus; DMV, dorsal motor nucleus of the vagus; IML, intermediolateal medulla; NTS, nucleus of the tractus solitaries; PVN, paraventricular nucleus of the hypothalamus.
MC4R activation may also play a role in autocrine control of POMC activity and autonomic function. Rescuing MC4R function specifically in POMC neurons of mice with whole-body MC4R deficiency partially restored BP responses to acute stress, suggesting that MC4R may serve to autopotentiate POMC neuronal activity [60]. In addition, MC4R located in PVN, RVLM and cholinergic preganglionic neurons of hindbrain and IML are important in mediating cardiovascular responses to acute stress [61, 62] (Figure 3). However, the specific neurons that mediate the effects of MC4R on SNS activity and BP are still largely unknown.
Downstream Mediators for MC4R Actions
The MC4R is a G protein-coupled 7 transmembrane receptor that increases cAMP phosphorylation and activates protein kinase A (PKA) [3, 26, 63]; therefore, blockade of these intracellular pathways attenuates MC4R actions [3, 63]. Although other cAMP-independent mediators of MC4R have been proposed [3, 64] their physiological importance is still unclear.
Several potential candidates, including brain-derived neurotrophic factor (BNDF), corticotrophin-releasing hormone (CRH), thyrotropin-releasing hormone (TRH), melanin-concentrating hormone (MCH), and orexins have been suggested to mediate or amplify the effects of MC4R activation. Studies by Bariohay et al showed that pharmacological activation or inhibition of MC4R, respectively, increased and reduced BDNF protein content in the DMV of adult rats [8]. In addition, the orexigenic effect of a selective MC4R antagonist injected into the 4th ventricle was completely blocked by co-administration of BDNF [8]. Nicholson and colleagues also found that the reduction in 24-hour food intake and increase in BP caused by the MC4R agonist, MK1, was reduced by prior central injection of an anti-BDNF antibody [64].
Others potential mediators of MC4R action including oxytocin, CRH, TRH and MCH have been proposed to contribute to MC4R effects on appetite. However, it is still unclear which of these signaling pathways may contribute to the cardiovascular effects of MC4R activation. Another potential mediator of MC4R cardiovascular actions is SIM1, a transcription factor required for development of the PVN. Although heterozygous mutation of SIM1 is one cause of monogenic obesity in humans, its role in mediating the BP effects of MC4R activation is unknown [3]. Further studies are needed to determine the downstream pathways that mediate most of MC4R actions on BP regulation and metabolic functions.
POSSIBLE ROLE OF MELANOCORTINS IN CARDIOVASCULAR PROTECTION
Activation of MC3R in the CNS has been suggested to protect the myocardium against acute ischemia/reperfusion injury following myocardial infarction [65]. For instance, α-MSH and γ1-MSH have protective effects in a model of transient myocardial infarction (MI) followed by reperfusion as well as in a model of permanent coronary artery occlusion in rats [66]. Administration of melanocortin peptides prevented oxygen release of free radicals, inflammatory responses, development of severe ventricular arrhythmia, and increased survival in a model of myocardial ischemia/reperfusion [2, 67].
Previous studies by Bazzani et al. suggest that the protective actions of melanocortins in MI/reperfusion-induced arrhytmias may be mediated by brain MC3R receptors [67]. However, ACTH, α-MSH and other fragments lacking the C-terminal Arg-Phe sequence may also have a life-saving effect in humans and experimental animals in conditions associated with severe tissue hypoxia [2, 67–69]. Previous studies showed that melanocortins can regulate myocyte contractility, hypertrophy, apoptosis and cardiac metabolism [70, 71]. However, the long-term protective actions of activating the brain melanocortin pathway in protecting against tissue damage following MI are still poorly understood.
Melanocortins have been also reported to reverse hemorrhagic shock in humans as well as in experimental models [65, 72]. A study by Guarini et al. suggested that MC4R activation mediates the beneficial effects of melanocortin peptides in hemorrhagic shock [73]. In addition, previous studies also showed that hemorrhagic shock reversal is mediated via CNS POMC-MC4Rs and may involve activation of efferent vagal cholinergic pathways [74]. For instance, microinjections of α-MSH in the nucleus ambiguus (nAMB) exerted excitatory effects on parasympathetic preganglionic neurons via activation of MC4R, resulting in increased vagal input to the heart and bradycardia responses [74–76]. These findings suggest that MC4R may play a role in mediating the parasympathetic component of baroreflex-induced bradycardia. Whether targeting MC4R is an effective therapeutic strategy for hemorrhagic or others forms of shock is still unclear.
MC4R AS A POTENTIAL THERAPEUTIC TARGET FOR OBESITY AND DIABETES
The actions of MC4R agonists to reduce food intake, increase energy expenditure, and improve glucose regulation [34] make this class of drugs attractive as therapeutic targets for obesity and diabetes. However, as discussed earlier, chronic activation of the MC4R also increases SNA and BP. Our early studies demonstrated, for example, that chronic ICV administration of MT-II, a non-selective MC3/4R agonist, raised BP in rodents due to adrenergic activation [40, 41]. These effects appear to be due almost entirely to activation of MC4R. Studies by Greenfield et al. [77] demonstrated that LY2112688, a selective MC4R agonist, caused dose-dependent increases in BP and HR during peripheral infusion for 24 hours in overweight or obese adults. Moreover, the cardiovascular effects were sustained for the entire 7 days of LY2112688 infusion. These findings in rodents and in humans have raised concerns that treating obese individuals with MC4R agonists may increase the risk of hypertension and associated adverse cardiovascular events, such as stroke and myocardial infarction, despite causing weight loss and improving glucose regulation.
Considerable effort has therefore been devoted to developing MC4R agonists that reduce body weight and blood glucose without having adverse cardiovascular effects. Kievit et al [78] reported that administration of another small peptide MC4R agonist, RM-493 (also known as BIM-22493 or setmelanotide), caused transient decreases in food intake (35%) with persistent weight loss and no significant increases in BP or HR over 8 weeks of treatment in a diet-induced obese nonhuman primate model. In a recent open-label study, two patients with POMC deficiency were treated with setmelanotide and experienced sustained reduction in hunger and substantial weight loss (51.0 kg after 42 weeks in one patient and 20.5 kg after 12 weeks in the second patient) without significant increases in BP or HR [79]. However, the large weight loss caused by setmelanotide did not appear to promote substantial reductions in BP that would normally be expected with such large decreases in body weight and fat mass. In another study [80], setmelanotide administration for 3 days to 12 in obese adults caused significant increases in resting energy expenditure and preferential increases in fat oxidation without raising BP.
The reasons for the differential effects of MC4R agonists on BP are still unclear but possible explanations include: 1) differences in brain penetration of the various agonists which may differentially activate cardiovascular control centers; 2) differences in receptor pharmacology, including the mechanisms by which the various compounds activate the MC4R and elicit second messenger signaling pathways; 3) different affinities of the various compounds for the MC3R since activation of the MC3R has been suggested to reduce BP. Further studies are needed to develop and test pharmacologically selective MC4R agonists that are able to reduce hyperglycemia, food intake, and body weight while increasing energy expenditure without causing adverse cardiovascular effects.
CONCLUSIONS
The CNS POMC-melanocortin system is a powerful regulator of cardiovascular function and appears to be a key link between obesity, SNS activation, and elevated BP. Understanding how cardiovascular and metabolic functions are differentially regulated by this complex system may lead to novel therapies that reduce the burden of obesity and associated metabolic disorders without causing adverse cardiovascular effects. As obesity is rapidly becoming one of the most important challenges to worldwide health care systems, development of more effective strategies for preventing and treating obesity are critical.
HIGHLIGHTS.
Activation of MC4R in the CNS increases sympathetic activity and blood pressure
Activation of POMC neurons mediates leptin’s effects on BP and SNS activity
Hypothalamic and brainstem MC4R contribute to cardiovascular and metabolic regulation
CNS MC4R activation is an important link between obesity and hypertension
Acknowledgments
This research was supported by the National Heart, Lung and Blood Institute (PO1HL51971) and the National Institute of General Medical Sciences (P20GM104357).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
References
- 1.Smith AI, Funder JW. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev. 1988;9(1):159–79. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 2.Corander MP, Fenech M, Coll AP. Science of self-preservation: how melanocortin action in the brain modulates body weight, blood pressure, and ischemic damage. Circulation. 2009;120(22):2260–8. doi: 10.1161/CIRCULATIONAHA.109.854612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31(4):506–43. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou A, Bloomquist BT, Mains RE. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J Biol Chem. 1993;268(3):1763–9. [PubMed] [Google Scholar]
- 5.Tsatmali M, et al. Skin POMC peptides: their actions at the human MC-1 receptor and roles in the tanning response. Pigment Cell Res. 2000;13(Suppl 8):125–9. doi: 10.1034/j.1600-0749.13.s8.22.x. [DOI] [PubMed] [Google Scholar]
- 6.Torda C, Wolff HG. Effects of adrenocorticotrophic hormone, cortisone acetate, and 17-hydroxycorticosterone-21-acetate on acetylcholine metabolism. Am J Physiol. 1952;169(1):150–8. doi: 10.1152/ajplegacy.1952.169.1.150. [DOI] [PubMed] [Google Scholar]
- 7.Cheung WW, Mak RH. Melanocortin antagonism ameliorates muscle wasting and inflammation in chronic kidney disease. Am J Physiol Renal Physiol. 2012;303(9):F1315–24. doi: 10.1152/ajprenal.00341.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bariohay B, et al. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology. 2009;150(6):2646–53. doi: 10.1210/en.2008-1184. [DOI] [PubMed] [Google Scholar]
- 9.Hall JE, et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285(23):17271–6. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connell JM, et al. Effects of ACTH and cortisol administration on blood pressure, electrolyte metabolism, atrial natriuretic peptide and renal function in normal man. J Hypertens. 1987;5(4):425–33. [PubMed] [Google Scholar]
- 11.Zhang Y, et al. Adrenocorticotropic hormone, blood pressure, and serum erythropoietin concentrations in the rat. Am J Hypertens. 2004;17(5 Pt 1):457–61. doi: 10.1016/j.amjhyper.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Woods LL, Mizelle HL, Hall JE. Control of sodium excretion in NE-ACTH hypertension: role of pressure natriuresis. Am J Physiol. 1988;255(6 Pt 2):R894–900. doi: 10.1152/ajpregu.1988.255.6.R894. [DOI] [PubMed] [Google Scholar]
- 13.Whitworth JA, et al. Species variability in cardiovascular research: the example of adrenocorticotrophin-induced hypertension. Clin Exp Pharmacol Physiol. 2006;33(9):887–91. doi: 10.1111/j.1440-1681.2006.04460.x. [DOI] [PubMed] [Google Scholar]
- 14.Whitworth JA, et al. Glucocorticoid-induced hypertension: from mouse to man. Clin Exp Pharmacol Physiol. 2001;28(12):993–6. doi: 10.1046/j.1440-1681.2001.03584.x. [DOI] [PubMed] [Google Scholar]
- 15.Humphreys MH, Ni XP, Pearce D. Cardiovascular effects of melanocortins. Eur J Pharmacol. 2011;660(1):43–52. doi: 10.1016/j.ejphar.2010.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nijsen MJ, et al. Relevance of the C-terminal Arg-Phe sequence in gamma(2)-melanocyte-stimulating hormone (gamma(2)-MSH) for inducing cardiovascular effects in conscious rats. Br J Pharmacol. 2000;131(7):1468–74. doi: 10.1038/sj.bjp.0703709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Bergen P, et al. Cardiovascular effects of gamma-MSH/ACTH-like peptides: structure-activity relationship. Eur J Pharmacol. 1995;294(2–3):795–803. doi: 10.1016/0014-2999(95)00657-5. [DOI] [PubMed] [Google Scholar]
- 18.Li SJ, et al. Melanocortin antagonists define two distinct pathways of cardiovascular control by alpha- and gamma-melanocyte-stimulating hormones. J Neurosci. 1996;16(16):5182–8. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni XP, et al. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens. 2006;24(11):2239–46. doi: 10.1097/01.hjh.0000249702.49854.fa. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys MH. Gamma-MSH, sodium metabolism, and salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R417–30. doi: 10.1152/ajpregu.00365.2003. [DOI] [PubMed] [Google Scholar]
- 21.Ni XP, et al. Modulation by dietary sodium intake of melanocortin 3 receptor mRNA and protein abundance in the rat kidney. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R560–7. doi: 10.1152/ajpregu.00279.2005. [DOI] [PubMed] [Google Scholar]
- 22.Ni XP, et al. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest. 2003;111(8):1251–8. doi: 10.1172/JCI16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumura K, et al. Central alpha-melanocyte-stimulating hormone acts at melanocortin-4 receptor to activate sympathetic nervous system in conscious rabbits. Brain Res. 2002;948(1–2):145–8. doi: 10.1016/s0006-8993(02)03045-7. [DOI] [PubMed] [Google Scholar]
- 24.Hill C, Dunbar JC. The effects of acute and chronic alpha melanocyte stimulating hormone (alphaMSH) on cardiovascular dynamics in conscious rats. Peptides. 2002;23(9):1625–30. doi: 10.1016/s0196-9781(02)00103-1. [DOI] [PubMed] [Google Scholar]
- 25.Pavia JM, Schioth HB, Morris MJ. Role of MC4 receptors in the depressor and bradycardic effects of alpha-MSH in the nucleus tractus solitarii of the rat. Neuroreport. 2003;14(5):703–7. doi: 10.1097/00001756-200304150-00009. [DOI] [PubMed] [Google Scholar]
- 26.Tai MH, et al. Role of nitric oxide in alpha-melanocyte-stimulating hormone-induced hypotension in the nucleus tractus solitarii of the spontaneously hypertensive rats. J Pharmacol Exp Ther. 2007;321(2):455–61. doi: 10.1124/jpet.106.118299. [DOI] [PubMed] [Google Scholar]
- 27.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48(1):58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 28.do Carmo JM, et al. Impact of obesity on renal structure and function in the presence and absence of hypertension: evidence from melanocortin-4 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R803–12. doi: 10.1152/ajpregu.00187.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall JE, et al. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.do Carmo JM, et al. Control of metabolic and cardiovascular function by the leptin-brain melanocortin pathway. IUBMB Life. 2013;65(8):692–8. doi: 10.1002/iub.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mountjoy KG, Wild JM. Melanocortin-4 receptor mRNA expression in the developing autonomic and central nervous systems. Brain Res Dev Brain Res. 1998;107(2):309–14. doi: 10.1016/s0165-3806(98)00015-7. [DOI] [PubMed] [Google Scholar]
- 32.Glavas MM, et al. Melanocortinergic activation by melanotan II inhibits feeding and increases uncoupling protein 1 messenger ribonucleic acid in the developing rat. Endocrinology. 2007;148(7):3279–87. doi: 10.1210/en.2007-0184. [DOI] [PubMed] [Google Scholar]
- 33.Coupe B, Bouret SG. Development of the hypothalamic melanocortin system. Front Endocrinol (Lausanne) 2013;4:38. doi: 10.3389/fendo.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27(7):736–49. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 35.Rossi J, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13(2):195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMullan S, Pilowsky PM. Sympathetic premotor neurones project to and are influenced by neurones in the contralateral rostral ventrolateral medulla of the rat in vivo. Brain Res. 2012;1439:34–43. doi: 10.1016/j.brainres.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 37.Ward KR, et al. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57(3):435–41. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwasa M, Kawabe K, Sapru HN. Activation of melanocortin receptors in the intermediolateral cell column of the upper thoracic cord elicits tachycardia in the rat. Am J Physiol Heart Circ Physiol. 2013;305(6):H885–93. doi: 10.1152/ajpheart.00443.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haynes WG, et al. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33(1 Pt 2):542–7. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 40.Kuo JJ, et al. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension. 2004;43(2):370–5. doi: 10.1161/01.HYP.0000111836.54204.93. [DOI] [PubMed] [Google Scholar]
- 41.Kuo JJ, Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension. 2003;41(3 Pt 2):768–74. doi: 10.1161/01.HYP.0000048194.97428.1A. [DOI] [PubMed] [Google Scholar]
- 42.da Silva AA, et al. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension. 2008;51(4):884–90. doi: 10.1161/HYPERTENSIONAHA.107.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall JE, et al. Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension. 1993;22(3):292–9. doi: 10.1161/01.hyp.22.3.292. [DOI] [PubMed] [Google Scholar]
- 44.Hall JE, et al. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002;324(3):127–37. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Haynes WG, et al. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100(2):270–8. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machleidt F, et al. Experimental hyperleptinemia acutely increases vasoconstrictory sympathetic nerve activity in healthy humans. J Clin Endocrinol Metab. 2013;98(3):E491–6. doi: 10.1210/jc.2012-3009. [DOI] [PubMed] [Google Scholar]
- 47.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31(1 Pt 2):409–14. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 48.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension. 2013;61(3):628–34. doi: 10.1161/HYPERTENSIONAHA.111.00705. [DOI] [PubMed] [Google Scholar]
- 49.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84(10):3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 50.do Carmo JM, et al. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57(5):918–26. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carlyle M, et al. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension. 2002;39(2 Pt 2):496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 52.da Silva AA, do Carmo JM, Hall JE. Role of leptin and central nervous system melanocortins in obesity hypertension. Curr Opin Nephrol Hypertens. 2013;22(2):135–40. doi: 10.1097/MNH.0b013e32835d0c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.do Carmo JM, et al. Activation of the central melanocortin system contributes to the increased arterial pressure in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2012;302(5):R561–7. doi: 10.1152/ajpregu.00392.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenfield JR. Melanocortin signalling and the regulation of blood pressure in human obesity. J Neuroendocrinol. 2011;23(2):186–93. doi: 10.1111/j.1365-2826.2010.02088.x. [DOI] [PubMed] [Google Scholar]
- 55.Sayk F, et al. Sympathetic function in human carriers of melanocortin-4 receptor gene mutations. J Clin Endocrinol Metab. 2010;95(4):1998–2002. doi: 10.1210/jc.2009-2297. [DOI] [PubMed] [Google Scholar]
- 56.da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension. 2004;43(6):1312–7. doi: 10.1161/01.HYP.0000128421.23499.b9. [DOI] [PubMed] [Google Scholar]
- 57.da Silva AA, et al. Chronic central nervous system MC3/4R blockade attenuates hypertension induced by nitric oxide synthase inhibition but not by angiotensin II infusion. Hypertension. 2015;65(1):171–7. doi: 10.1161/HYPERTENSIONAHA.114.03999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.do Carmo JM, et al. Systemic but not central nervous system nitric oxide synthase inhibition exacerbates the hypertensive effects of chronic melanocortin-3/4 receptor activation. Hypertension. 2011;57(3):428–34. doi: 10.1161/HYPERTENSIONAHA.110.163931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sohn JW, et al. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152(3):612–9. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.do Carmo JM, et al. Differential control of metabolic and cardiovascular functions by melanocortin-4 receptors in proopiomelanocortin neurons. Am J Physiol Regul Integr Comp Physiol. 2013;305(4):R359–68. doi: 10.1152/ajpregu.00518.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.do Carmo JMSP, Ebaady S, Freeman JN, Hall JE. da Silva AA Melanocortin-4 receptors in the PVN and RVLM are important in mediating cardiovascular responses to acute stress. FASEB J. 2014;28:686.7. [Google Scholar]
- 62.do Carmo JMTF, Moak Sydney P, Browning Jackson R, Hall JE. Melanocortin-4 receptors in cholinergic preganglionic neurons of the hindbrain and spinal cord are important in mediating cardiovascular responses to acute stress. Hypertension. 2016;62:P549. [Google Scholar]
- 63.Li P, et al. Melanocortin 4 receptors in the paraventricular nucleus modulate the adipose afferent reflex in rat. PLoS One. 2013;8(11):e80295. doi: 10.1371/journal.pone.0080295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nicholson JR, et al. Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol. 2007;19(12):974–82. doi: 10.1111/j.1365-2826.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 65.Guarini S, et al. MC(3) receptors are involved in the protective effect of melanocortins in myocardial ischemia/reperfusion-induced arrhythmias. Naunyn Schmiedebergs Arch Pharmacol. 2002;366(2):177–82. doi: 10.1007/s00210-002-0572-8. [DOI] [PubMed] [Google Scholar]
- 66.Chitravanshi VC, Bhatt S, Sapru HN. Microinjections of alpha-melanocyte stimulating hormone into the nucleus ambiguus of the rat elicit vagally mediated bradycardia. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1402–11. doi: 10.1152/ajpregu.90978.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bazzani C, et al. Protective effect of melanocortin peptides in rat myocardial ischemia. J Pharmacol Exp Ther. 2001;297(3):1082–7. [PubMed] [Google Scholar]
- 68.Moller CL, et al. Melanocortin agonists stimulate lipolysis in human adipose tissue explants but not in adipocytes. BMC Res Notes. 2015;8:559. doi: 10.1186/s13104-015-1539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giuliani D, et al. Neuroprotection in focal cerebral ischemia owing to delayed treatment with melanocortins. Eur J Pharmacol. 2007;570(1–3):57–65. doi: 10.1016/j.ejphar.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 70.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88(2):389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ottani A, et al. Protective effects of the melanocortin analog NDP-alpha-MSH in rats undergoing cardiac arrest. Eur J Pharmacol. 2014;745:108–16. doi: 10.1016/j.ejphar.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 72.Jochem J, et al. The role of melanocortin peptides in the cardiovascular regulation in haemorrhagic shock. Folia Med Cracov. 2005;46(3–4):13–21. [PubMed] [Google Scholar]
- 73.Giuliani D, et al. Selective melanocortin MC4 receptor agonists reverse haemorrhagic shock and prevent multiple organ damage. Br J Pharmacol. 2007;150(5):595–603. doi: 10.1038/sj.bjp.0707115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guarini S, et al. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc Res. 2004;63(2):357–65. doi: 10.1016/j.cardiores.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 75.Versteeg DH, et al. Melanocortins and cardiovascular regulation. Eur J Pharmacol. 1998;360(1):1–14. doi: 10.1016/s0014-2999(98)00615-3. [DOI] [PubMed] [Google Scholar]
- 76.Bertolini A, et al. Alpha-MSH and other ACTH fragments improve cardiovascular function and survival in experimental hemorrhagic shock. Eur J Pharmacol. 1986;130(1–2):19–26. doi: 10.1016/0014-2999(86)90179-2. [DOI] [PubMed] [Google Scholar]
- 77.Greenfield JR, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360(1):44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 78.Kievit P, et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62(2):490–7. doi: 10.2337/db12-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuhnen P, et al. Proopiomelanocortin Deficiency Treated with a Melanocortin-4 Receptor Agonist. N Engl J Med. 2016;375(3):240–6. doi: 10.1056/NEJMoa1512693. [DOI] [PubMed] [Google Scholar]
- 80.Chen KY, et al. RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals. J Clin Endocrinol Metab. 2015;100(4):1639–45. doi: 10.1210/jc.2014-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]


