Abstract
Background
Increased incidence and prevalence of asthma have been documented for perinatally HIV-infected (HIV) youth 10–21 years of age (youth) compared to HIV-exposed uninfected (HEU) youth.
Objective
To perform objective pulmonary function tests (PFTs) in HIV and HEU youth with and without diagnosed asthma.
Method
Asthma was determined in 370 participants (218 HIV, 152 HEU) by chart review and self-report at 13 sites. Interpretable PFTs (188 HIV, 132 HEU) were classified as obstructive, restrictive, or normal and reversibility was determined after bronchodilator inhalation. HIV-1 RNA, CD4 and CD8 T-cells, eosinophils, total IgE, allergen-specific IgE, and urinary cotinine were measured. Adjusted prevalence ratios (aPR) of asthma and PFT outcomes were determined for HIV relative to HEU controlling for age, race/ethnicity and sex.
Results
Current asthma was identified in 75/218 (34%) HIV and 38/152 (25%) HEU (aPR=1.33, p=0.11). Prevalence of obstructive disease did not differ by HIV status. Reversibility was less likely in HIV youth than in HEU (17/183 (9%) vs. 21/126 (17%), (aPR=0.47, p=0.020) overall and among just those with OBS (aPR=0.46, p=0.016). Among HIV youth with current asthma, serum IgE levels were inversely correlated with CD8 T-cell, and positively correlated with eosinophil counts, and not associated with CD4 T-cells. HIV youth had lower association of specific IgE to several inhalant and food allergens compared to HEU and significantly lower CD4/CD8 T-cell ratios (suggesting immune imbalance).
Conclusion
Compared to HEU, HIV youth demonstrated decreased reversibility of obstructive lung disease, atypical of asthma. This may indicate an early stage of chronic obstructive pulmonary disease. Follow-up into adulthood is warranted to further define their pulmonary outcomes.
Keywords: Pediatric HIV infection, pulmonary complications of HIV infection, asthma, pulmonary function testing, obstructive and restrictive pulmonary disease, reversibility of obstructive air flow with bronchodilators, chronic obstruction pulmonary disease (COPD), asthma-COPD overlap syndrome, immune imbalance (TH2 Shift)
INTRODUCTION
Within the past decade, there have been several reports of a higher burden of asthma in HIV-infected (HIV) adults (1–12) and children. (13–19) Data supporting this claim in children have used asthma medication history, physician examination and diagnosis, and medical chart review by medical personnel. The diagnosis of asthma in these studies lacked objective assessments of lung function. In the absence of objective measures, there is concern that asthma may be over diagnosed in children. There may be a bias toward labeling HIV children with respiratory symptoms as asthmatics, resulting in overtreatment with bronchodilators and asthma controller medications.
The possibility of misclassification of respiratory disease in HIV was raised by Gingo et al in HIV adults. (6) They compared lung function in HIV adult patients with a diagnosis of asthma to those without asthma. They found that HIV subjects with asthma were more likely to have lung disease and less likely to have reversible airway obstruction. Non-reversible airway obstruction is not consistent with the definition of asthma, which is characterized by obstructive pulmonary airflow reversible after bronchodilator treatments. Gingo et al postulated that the abnormal pulmonary function represents a complex syndrome of asthma and chronic obstructive pulmonary disease (COPD). (8, 9) Recently, several reports and editorials have appeared that describe an asthma-COPD overlap syndrome (20–25) with mixed elements of both asthma and COPD, and lack of reversibility. The etiology of this syndrome is unknown and has been associated with adult HIV infection and lack of antiretroviral therapy (ART). (6)
In this report, we present the results of a large, multicenter study of respiratory disease and lung function in youth 10–21 years of age born to HIV-infected mothers. One distinct advantage of this pediatric observational study is that it includes the HEU cohort as a comparison group to HIV youth. We defined asthma from chart review coded using MedDRA® (Medical Dictionary for Regulatory Activities physician diagnosis, self-report asthma questionnaires, and pulmonary function tests (PFT). In addition, we evaluated immune imbalance as a possible mechanism of chronic inflammation causing this disease. (13, 15, 26) (See Supplement Introduction in online repository)
METHODS
Study Population
HIV and HEU youth enrolled in the Adolescent Master Protocol (AMP) of the Pediatric HIV/AIDS Cohort Study (PHACS) were recruited to enroll in this pulmonary substudy at 13 clinical sites. (17) Participants were excluded if they could not follow instructions for PFTs, had a known history of cardiac arrhythmia and/or hypertension, were pregnant or gave birth within the last six weeks. Based on assumptions of the prevalence of asthma in HEU, we determined a priori the sample size necessary to have 80% power to detect a 13% increase in asthma in HIV youth compared to HEU. All aspects of this research study were approved by the Baylor College of Medicine Institutional Review Board, the Harvard T.H. Chan School of Public Health, and each participating clinical site. Permission of parents or legal guardians was obtained for youth (<18 years of age) on informed consent forms approved by local site Institutional Review Boards and the Harvard T.H. Chan School of Public Health. Assent of youth <18 years of age was obtained when appropriate.
Outcome Measures
The outcome measures were a history of ever having asthma, current asthma, ever having other pulmonary diseases, current pulse oximetry results, and current PFT results. The definitions for asthma and PFT classifications were developed based on recommendations from two study pulmonologists (MK, AAC).
Asthma and other pulmonary diseases
Diagnoses of asthma and other pulmonary diseases were defined using five questionnaires administered at entry and/or clinical diagnoses abstracted from the medical chart during the previous participant’s enrollment in the Pediatric AIDS Clinical Trials Group (PACTG) 219C study and PHACS AMP.(17) The questionnaires (Q) used to define asthma status were: Q1) Questionnaire for Other Pulmonary Diseases,(27) Q2) Child Asthma Control Test for Children 4 to 11 Years Old, (28) Q3) Asthma Control Test for Teens 12 Years and Older, (29) Q4) Abbreviated NHANES Questionnaire for Asthma, (30) and Q5) Asthma history questionnaire, designed by the study pulmonologists. Clinical diagnoses were coded into MedDRA® by data management center personnel. MedDRA® coded clinical events were reviewed and all preferred terms indicative of asthma and other pulmonary complications were identified within our study population.
The definitions of “ever having asthma” and “current asthma,” provided in Supplement Table E1 (see online repository), are based on data from questionnaires, medication use, and diagnoses abstracted from the clinical chart. The definition of medication use indicative of asthma diagnosis is contained in Supplement Table E1. Based on chart review, participants were identified as “current asthma medication users” if they received montelukast with albuterol, levalbuterol, or ipratropium, OR an inhaled corticosteroid in the past year (see Table E2 in online repository).
Pulmonary Function Tests
PFTs were performed pre- and post-bronchodilation with albuterol inhalation. Flow loop (3–5 repeats) PFTs for each participant were evaluated centrally by two pediatric pulmonologists, blinded to HIV status, to determine if the PFTs were acceptable using ATS criteria.31 Based on raw spirometric data, predicted values were calculated centrally as the percent of normal values based on age-sex-race/ethnicity adjusted reference values in healthy children from the reference by Hankinson et al.(32)
The results of the PFTs allowed participants to be assigned to one of four mutually exclusive groups:
obstructive (OBS) only: FEV1 < 80% or FEV1/FVC < 80% or FEF 25–75% <65%;
restrictive (RES) only: FVC < 80% and FEV1/FVC ≥ 80%;
OBS+RES: FVC < 80% and FEV1/FVC < 80%; and
Normal: Not RES or OBS.
The 12% bronchodilator response cutoff point that best identified asthma in adults has been questioned for accuracy in children. In a series of studies, the proposed cutoff point for pediatrics ranged from 9% – to 10.7%.(33–35) We therefore chose to define reversibility as FEV1 increase greater than 10% after bronchodilation as the closest approximation.
Oxygen Saturation of Blood
Pulse oximetry (SpO2%), taken on breathing room air, was conducted to determine oxygen saturation of blood (≥ 95% O2 saturation was considered normal).
Total and specific Serum IgE
Total and allergen-specific IgE levels (15 allergens) were measured in vitro on serum samples using the FDA-cleared, Phadia ImmunoCAP® system in the CLIA-licensed, CAP-accredited Quest Diagnostics-Baltimore, MD laboratory facility.(36–38) This assay system is a fully automated fluoroenzymeimmunoassay method. (For additional description of total and specific serum IgE, see Supplement Table E3 in online repository).
Urinary Cotinine
To determine exposure to tobacco, urine specimens were assayed for nicotine, cotinine, 3-OH-cotinine, nornicotine, norcotinine and anabasine using a liquid chromatography tandem mass spectrometry method that is in routine clinical use (Quest Diagnostics Nichols Institute, Chantilly VA). For additional description of urinary cotinine, see supplement methods in online repository.
CD4 T-Cells, CD8 T-cells, Eosinophils, and HIV Viral Load
We assessed CD4 and CD8 T-cell percents and absolute CD4 T-cell counts on both HIV and HEU, and HIV-1 RNA viral load on HIV participants ± 4 months of the time of the PFT test.(17) We also determined the absolute eosinophil counts for HIV and HEU youth within the above time frame.
Covariates
Covariates included age at the time of PFT exam or at questionnaire if no PFT, race/ethnicity (non-Hispanic white/other and non-black Hispanics versus blacks, regardless of ethnicity), and sex (male versus female).
Data analysis
The distribution of sociodemographic characteristics and pulmonary outcomes were compared across HIV status and PFT categories using the Wilcoxon rank sum test/Kruskal-Wallis test for continuous variables and Fisher’s exact test/Pearson chi-square test for categorical variables.
Univariable and multivariable log-binomial regression models were fit to evaluate the associations of HIV status with pulmonary outcomes, including ever asthma (Yes/No), current asthma (Yes versus Not current or Never), obstructive vs. normal PFT, restrictive vs. normal PFT, and reversibility after bronchodilation (Yes/No). Among those with obstructive disease/normal PFT, we explored the relationship between HIV status and reversibility, using a modified Poisson regression approach.38 The log-binomial and modified Poisson regression were used to estimate prevalence rate ratios (95% confidence intervals (CI)) for current outcomes and the risk ratio (95% CI) for ever asthma for HIV versus HEU participants. We also considered potential effect modification by current asthma and ever asthma on the association between HIV status and the outcome reversibility. Multivariable models were adjusted for a priori confounders: age at the time of PFT exam, race/ethnicity, and sex.
To examine whether the CD4 and CD8 T-cell component might be associated with total non-specific levels of IgE, we fit a simple linear regression line for each HIV group. In addition, to assess the ability of the IgE immune system to respond to common allergens, we plotted the percent with high IgE levels (≥ Class III response) by HIV status and current asthma. SAS version 9.4 was used for all statistical analyses. All statistical tests were two-sided.
RESULTS
Study Population and Background Characteristics
We enrolled 370 participants (218 HIV, 152 HEU from Feb 2013 to July 2014. All participants completed at least one of the questionnaires and all but three had a PFT exam. Of the 367 with a PFT, the PFT did not meet acceptability criteria for 47 participants (28 [12.8%] HIV and 19 [12.5%] HEU). Of the 320 (188 HIV, 132 HEU) with an evaluable pre-bronchodilator PFT result, a post-bronchodilator PFT was not available for 5 HIV and 6 HEU youth. Of the 320 with evaluable PFT results, pulse oximetry results were obtained for 163 (86.7%) HIV and 120 (90.9%) HEU youth.
Table I shows the distribution of background characteristics among the 370 HIV and HEU youth. HIV youth were more likely to be older and identify as black, than HEU. HIV youth were also more likely to have lower CD4 T-cell counts, higher CD8 T-cell counts, and lower CD4/CD8 T-cell ratio and eosinophil counts than HEU (P< 0.001). They were also more likely to have been exposed to tobacco products.
Table I.
Distribution of Characteristics by HIV Status
| Characteristics | Cohort | ||
|---|---|---|---|
| HIV (N=218) | HEU (N=152) | P-Value | |
| Age (years) | 16.96 (14.36, 19.15) | 14.70 (12.82, 16.61) | <0.001 |
| Race/Ethnicity | |||
| Non-Hispanic Whites/Others and Non-Black Hispanics | 59 (27%) | 57 (38%) | 0.033 |
| Blacks, regardless of ethnicity | 159 (73%) | 95 (63%) | |
| Sex: Male | 97 (44%) | 75 (49%) | 0.36 |
| CD4 T-Cell Count (cells/μl)a | 634.50 (461.00, 788.00) | 822 (658, 1,026) | <0.001 |
| CD4 T-Cell Count Categoriesa | |||
| ≥ 750 cells/μl | 72 (34%) | 84 (62%) | <0.001 |
| 500 – 749 cells/μl | 75 (35%) | 42 (31%) | |
| 200 – 499 cells/μl | 50 (23%) | 9 (7%) | |
| < 200 cells/μl | 17 (8%) | 0 (0%) | |
| CD8 T-Cell Count (cells/μl)a | 720 (523, 950) | 522 (402, 675) | <0.001 |
| CD4/CD8 Ratioa | 0.93 (0.55, 1.26) | 1.56 (1.27, 1.98) | <0.001 |
| Eosinophil count (cells/mm3)b | 126.00 (61.50, 203.70) | 180 (102, 284) | 0.001 |
| Total Serum IgE (kU/L)c | 63 (20, 183) | 74.50 (34.00, 238.00) | 0.13 |
| Level of Exposure to Tobacco Productsd | |||
| Active Tobacco Use | 20 (10%) | 9 (7%) | 0.083 |
| Passive Smoking | 20 (10%) | 6 (4%) | |
| No Smoking | 163 (80%) | 122 (89%) | |
CD4 and CD8 T-cell count data were not available within 4 months (122 days) of PFT exam for 4 HIV and 17 HEU youth.
Eosinophil count data was not available within 4 months of PFT exam for 46 HIV and 71 HEU youth.
Total Serum IgE data was not available within 4 months of PFT exam for 7 HIV and 12 HEU youth.
Urine cotinine data for determining exposure to tobacco products was not available within 4 months of PFT exam for 15 HIV and 15 HEU youth.
Pulmonary Outcomes and Medication Use by HIV Status
Table II summarizes the distribution of pulmonary outcomes and medication use among HIV and HEU youth in unadjusted analysis. A higher percentage of HIV than HEU youth reported ever having had asthma (68/218 [31%] vs. 34/152 [22%], p=0.062) or currently having asthma (75/218 [34%] vs. 38/152 [25%], p=0.053) (criteria more stringent for current than ever asthma). HIV youth were more likely to have a history of other respiratory disorders, such as lymphoid interstitial pneumonia (LIP) (20/218 [9%] vs. 0%) and bronchitis (28/218 [13%] vs. 5/152 [3%]). HIV and HEU youth did not differ by current use of asthma medications. However, when comparing individual mediations, HIV youth were more likely to have used albuterol, levalbuterol, or ipratropium in the past year than HEU (67/218 [31%] vs. 30/152 [20%]), but they did not differ in use of montelukast or inhaled corticosteroid. In the subset with at least one evaluable PFT, there were no significant differences in FEV1 or FVC pre-bronchodilator percent predicted values between HIV and HEU youth. Among those with a pre- and post-bronchodilator PFT, HIV youth were less likely to have reversibility than HEU (17/188 [9%] vs. 21/132 [17%], p=0.052). The median (Q1, Q3) for SpO2% was 99 (98%, 100%) for both HIV and HEU youth.
Table II.
Distribution of Pulmonary Outcomes and Medication Use by HIV Status
| Pulmonary Outcomes and Medication Use | Cohort | ||
|---|---|---|---|
| HIV | HEU | P-Value | |
| Median (Q1, Q3) or N (%) | |||
| For Study Population* (N=370) | |||
| Outcomes defined by Questionnaire and Chart Review | N=218 | N=152 | |
| Ever had asthma (questionnaires & chart review) | 68 (31%) | 34 (22%) | 0.062 |
| Current asthma (questionnaires, medications, & chart review) | 75 (34%) | 38 (25%) | 0.053 |
| Is your child/teen’s asthma under control?a | |||
| Yes | 88 (92%) | 44 (94%) | 1.00 |
| No | 8 (8%) | 3 (6%) | |
| Chart Review** - LIP outcomeb | 20 (9%) | 0 (0%) | <0.001 |
| Chart Review** - Bronchitis outcome | 28 (13%) | 5 (3%) | 0.001 |
| Chart Review** - Any Respiratory outcome | 147 (67%) | 44 (29%) | <0.001 |
| Asthma Medication Use | |||
| Current asthma medication use in the past year as defined by pulmonologistsc | 24 (11%) | 16 (11%) | 0.88 |
| Albuterol, Levalbuterol, or Ipratropium use in the past year | 67 (31%) | 30 (20%) | 0.018 |
| Montelukast use in the past year | 17 (8%) | 11 (7%) | 0.84 |
| Inhaled corticosteroid use in the past year | 14 (6%) | 14 (9%) | 0.32 |
| For Subset with Evaluable PFTs* (N=320) | |||
| PFT and Pulse Oximetry Outcomes | N=188 | N=132 | |
| FEV1 percent predicted value | 98 (85, 109) | 97.25 (84.30, 107.15) | 0.44 |
| FVC percent predicted value | 100.79 (89.39, 111.64) | 99.93 (88.14, 109.92) | 0.33 |
| PFT Classification | |||
| Restrictive + obstructive | 2 (1%) | 0 (0%) | 0.64 |
| Restrictive | 18 (10%) | 17 (13%) | |
| Obstructive | 41 (22%) | 28 (21%) | |
| Normal | 127 (68%) | 87 (66%) | |
| Obstructive versus Normal PFT Classification | |||
| Normal | 127 (76%) | 87 (76%) | 0.99 |
| Obstructive | 41 (24%) | 28 (24%) | |
| Restrictive versus Normal PFT Classification | |||
| Normal | 127 (88%) | 87 (84%) | 0.38 |
| Restrictive | 18 (12%) | 17 (16%) | |
| PFT Reversibility with bronchodilatord | 17 (9%) | 21 (17%) | 0.052 |
| Pulse oximetry result (SpO2%)e | 99 (98, 100) | 99 (98, 100) | 0.63 |
In this study, 370 participants (218 HIV, 152 HEU) were enrolled and completed at least one questionnaire on asthma and/or other pulmonary complications. However, 320 (188 HIV, 132 HEU) of the 370 participants had evaluable pulmonary function test (PFT) results.
Chart review coded by MedDRA. MedDRA® the Medical Dictionary for Regulatory Activities terminology is the international medical terminology developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA® trademark is owned by IFPMA on behalf of ICH.
Participants, who answered ‘Yes’ to having been diagnosed with asthma by a physician, or having had wheezing at least once since age 3, or having received medicine by inhaler or a nebulizer for breathing trouble, wheezing, or coughing in questionnaires, were asked a series of questions to determine if their asthma was under control.
LIP – Lymphoid Interstitial Pneumonia
Definition of current asthma medication use in the past year: If participant used montelukast with albuterol, levalbuterol, or ipratropium; OR an inhaled corticosteroid in the past year.
A post-bronchodilator PFT was not available for 5 HIV and 6 HEU youth.
Of the 370 participants with at least one questionnaire completed, pulse oximetry results were obtained for 189 of the 218 HIV youth and 134 of the 152 HEU youth. Of the 320 participants with evaluable pre-bronchodilator PFT results, pulse oximetry results were obtained for 163 of the 188 HIV youth and 120 of the 132 HEU youth.
Distribution of Characteristics and Outcomes by PFT Categories
Table III shows the distribution of characteristics and outcomes by PFT classification, sequentially, for HIV and HEU youth, in unadjusted analysis. We compared those with RES and OBS to those with normal PFT classifications.
Table III.
Sociodemographic Characteristics, and Clinical and Pulmonary Outcomes by PFT Classification among HIV and HEU Youth
| Characteristics and Outcomes | PFT Classification* | ||||
|---|---|---|---|---|---|
| Restrictive1 (N=18) | Obstructive2 (N=41) | Normal3 (N=127) | P-Value13 | P-Value23 | |
| Median (Q1, Q3) or N (%) | |||||
| HIV YOUTH | |||||
| Age (years) | 16.64 (14.04, 17.69) | 17.74 (15.14, 19.17) | 16.67 (14.17, 18.84) | 0.80 | 0.20 |
| Race/Ethnicity | |||||
| Non-Hispanic Whites/Others and Non-Black Hispanics | 7 (39%) | 12 (29%) | 30 (24%) | 0.25 | 0.53 |
| Blacks, regardless of ethnicity | 11 (61%) | 29 (71%) | 97 (76%) | ||
| Sex: Male | 3 (17%) | 20 (49%) | 54 (43%) | 0.041 | 0.59 |
| Ever had asthma (questionnaires & chart review) | 8 (44%) | 22 (54%) | 31 (24%) | 0.090 | <0.001 |
| Current asthma (questionnaires, medications, & chart review) | 9 (50%) | 21 (51%) | 36 (28%) | 0.099 | 0.013 |
| Is your child/teen’s asthma under control?a | |||||
| Yes | 8 (100%) | 22 (81%) | 48 (96%) | 1.00 | 0.048 |
| No | 0 (0%) | 5 (19%) | 2 (4%) | ||
| FEV1 percent predicted value | 73.30 (65.70, 80.40) | 83.40 (78.40, 96.90) | 102.00 (95.20, 112.50) | <0.001 | <0.001 |
| FVC percent predicted value | 75.60 (68.81, 78.13) | 98.23 (87.95, 111.77) | 102.57 (93.07, 113.17) | <0.001 | 0.21 |
| PFT Reversibility with bronchodilator | 0 (0%) | 12 (31%) | 4 (3%) | 1.00 | <0.001 |
| Pulse oximetry result (SpO2%)b | 100 (99, 100) | 99 (98, 100) | 99 (98, 100) | 0.073 | 0.68 |
| CD4 T-Cell Count (cells/μl)c | 613 (462, 713) | 536 (373, 717) | 653 (464, 796) | 0.48 | 0.13 |
| CD4 T-Cell Count Categoriesc | |||||
| ≥ 750 cells/μl | 4 (22%) | 10 (24%) | 46 (37%) | 0.47 | 0.25 |
| 500 – 749 cells/μl | 9 (50%) | 14 (34%) | 41 (33%) | ||
| 200 – 499 cells/μl | 4 (22%) | 11 (27%) | 29 (23%) | ||
| < 200 cells/μl | 1 (6%) | 6 (15%) | 8 (6%) | ||
| CD8 T-Cell Count (cells/μl)c | 824 (550, 925) | 793 (549, 1,050) | 707 (499, 910) | 0.30 | 0.26 |
| CD4/CD8 Ratioc | 0.72 (0.51, 1.04) | 0.77 (0.46, 1.20) | 0.99 (0.69, 1.26) | 0.078 | 0.055 |
| Log10 HIV-1 RNA (copies/mL)d | 1.92 (1.30, 2.72) | 2.15 (1.60, 3.97) | 1.52 (1.30, 2.76) | 0.49 | 0.002 |
| Total Serum IgE (kU/L)e | 65 (27, 237) | 70 (20, 333) | 60.50 (19.00, 159.50) | 0.52 | 0.50 |
| Eosinophil count (cells/mm3)f | 138 (68, 329) | 140 (72, 246) | 108 (59, 185) | 0.28 | 0.20 |
| Level of Exposure to Tobacco Products?g | |||||
| Active Tobacco Use | 0 (0%) | 6 (15%) | 10 (8%) | 0.76 | 0.43 |
| Passive Smoking | 2 (13%) | 3 (8%) | 13 (11%) | ||
| No Smoking | 14 (88%) | 31 (78%) | 97 (81%) | ||
| Characteristics and Outcomes | PFT Classification* | ||||
|---|---|---|---|---|---|
| Restrictive1 (N=17) | Obstructive2 (N=28) | Normal3 (N=87) | P-Value13 | P-Value23 | |
| Median (Q1, Q3) or N (%) | |||||
| HEU YOUTH | |||||
| Age (years) | 14.70 (12.77, 17.32) | 13.80 (12.64, 15.85) | 14.81 (12.95, 16.58) | 0.67 | 0.24 |
| Race/Ethnicity | |||||
| Non-Hispanic Whites/Others and Non-Black Hispanics | 12 (71%) | 7 (25%) | 30 (34%) | 0.007 | 0.49 |
| Blacks, regardless of ethnicity | 5 (29%) | 21 (75%) | 57 (66%) | ||
| Sex: Male | 5 (29%) | 16 (57%) | 45 (52%) | 0.12 | 0.67 |
| Ever had asthma (questionnaires & chart review) | 1 (6%) | 5 (18%) | 23 (26%) | 0.11 | 0.45 |
| Current asthma (questionnaires, medications, & chart review) | 0 (0%) | 8 (29%) | 25 (29%) | 0.010 | 1.00 |
| Is your child/teen’s asthma under control?a | |||||
| Yes | 1 (100%) | 7 (88%) | 30 (94%) | 1.00 | 0.50 |
| No | 0 (0%) | 1 (13%) | 2 (6%) | ||
| FEV1 percent predicted value | 71.10 (63.50, 81.10) | 86.35 (79.00, 96.85) | 101.40 (95.00, 110.60) | <0.001 | <0.001 |
| FVC percent predicted value | 70.12 (61.88, 76.42) | 99.61 (85.55, 110.23) | 104.08 (91.67, 110.89) | <0.001 | 0.22 |
| PFT Reversibility with bronchodilator | 2 (13%) | 13 (50%) | 6 (7%) | 0.34 | <0.001 |
| Pulse oximetry result (SpO2%)b | 100 (99, 100) | 100 (98, 100) | 99 (98, 100) | 0.11 | 0.35 |
| CD4 T-Cell Count (cells/μl)c | 700 (565, 1,109) | 943 (807, 1,115) | 826 (656, 987) | 0.61 | 0.033 |
| CD4 T-Cell Count Categoriesc | |||||
| ≥ 750 cells/μl | 5 (45%) | 20 (77%) | 49 (61%) | 0.45 | 0.26 |
| 500 – 749 cells/μl | 5 (45%) | 6 (23%) | 25 (31%) | ||
| 200 – 499 cells/μl | 1 (9%) | 0 (0%) | 6 (8%) | ||
| CD8 T-Cell Count (cells/μl)c | 479 (380, 549) | 569 (458, 675) | 521 (403, 685) | 0.27 | 0.43 |
| CD4/CD8 Ratioc | 1.52 (1.24, 2.20) | 1.78 (1.42, 2.00) | 1.52 (1.25, 1.88) | 0.58 | 0.096 |
| Total Serum IgE (kU/L)e | 153 (34, 334) | 105 (39, 185) | 68 (31, 207) | 0.31 | 0.68 |
| Eosinophil count (cells/mm3)f | 340 (192, 429) | 169 (81, 304) | 133 (99., 251) | 0.002 | 0.38 |
| Level of Exposure to Tobacco Products?g | |||||
| Active Tobacco Use | 1 (8%) | 1 (4%) | 7 (9%) | 1.00 | 0.15 |
| Passive Smoking | 0 (0%) | 3 (11%) | 2 (2%) | ||
| No Smoking | 11 (92%) | 23 (85%) | 72 (89%) | ||
2 HIV youth that had both restrictive and obstructive PFT classification were not included in this table.
P-value for the comparison of those with restrictive to those with normal PFT classification
P-value for the comparison of those with obstructive to those with normal PFT classification.
Participants, who answered ‘Yes’ to having been diagnosed with asthma by a physician, or having had wheezing at least once since age 3, or having received medicine by inhaler or a nebulizer for breathing trouble, wheezing, or coughing in questionnaires, were asked a series of questions to determine if their asthma was under control.
Among HIV youth, 4, 2, and 19 participants with restrictive, obstructive, and normal PFT classification, respectively, are missing pulse oximetry results. Among HEU youth, 3, 2, and 7 participants with restrictive, obstructive, and normal PFT classification, respectively, are missing pulse oximetry results.
CD4 T-cell count data was not available within 4 months of PFT exam for 4 HIV youth with normal PFT classification. CD4 T-cell count data was not available within 4 months of PFT exam for 6, 2, and 7 participants with restrictive, obstructive, and normal PFT classification, respectively.
Log10 HIV-1 RNA data was not available within 4 months of PFT exam for 3 HIV youth with normal PFT classification.
Total Serum IgE data was not available within 4 months of PFT exam for 1 and 3 HIV youth with restrictive and normal PFT classification, respectively. Total Serum IgE data was not available within 4 months of PFT exam for 6 and 4 HEU youth with restrictive and normal PFT classification, respectively.
Eosinophil count data was not available within 4 months of PFT exam for 5, 13, and 19 HIV youth with restrictive, obstructive, and normal PFT classification, respectively. Eosinophil count data was not available within 4 months of PFT exam for 7, 15, and 39 HEU youth with restrictive, obstructive, and normal PFT classification, respectively.
Urine cotinine data was not available within 4 months of PFT exam for 2, 1, and 7 HIV youth with restrictive, obstructive, and normal PFT classification, respectively. Urine cotinine data was not available within 4 months of PFT exam for 5, 1, and 6 HEU youth with restrictive, obstructive, and normal PFT classification, respectively.
HIV Youth
Among HIV youth, there were no significant differences in age or race/ethnicity by PFT classification, but those with RES disease were less likely to be male than those with normal PFT (p=0.041). Those with normal classification were less likely to ever have had asthma (31/127 (24%)) than those with RES (8/18 [44%], p=0.09) or OBS (22/41 [54%], p<0.001). Similar findings were observed for current asthma; those with normal classification (36/127 [28%] were less likely to currently have asthma than those with RES (9/18 [50%], p=0.099) or OBS (21/41 [51%], p=0.013). HIV youth with OBS were less likely to have asthma under control. PFT reversibility was more likely in those with OBS (12/39 [31%]) than in those with normal classification (4/124 [3%], p<0.001), but none of those with RES had reversibility. The median log HIV-1 RNA viral load was lower in those with normal classification compared to those with OBS (2.15 vs. 1.52, p=0.002). We observed no significant differences in the distribution of pulse oximetry values, CD4 and CD8 T-cell counts, total serum IgE, eosinophil count, or level of exposure to tobacco products across PFT groups. However, median CD4/CD8 ratio is higher in those with normal classification (0.99) than in those with RES (0.72, p=0.078) or OBS (0.77, p=0.058).
HEU Youth
Among HEU youth, we observed no significant differences in age or sex by PFT, but those with RES were more likely to identify as Non-Hispanic whites/others or Non-black Hispanics and have higher eosinophil counts than those with normal classification. Those with OBS (13/26 [50%]) were more likely to have reversibility than those with normal classification (6/85 [7%]). We observed no significant differences in asthma under control, pulse oximetry values, CD8 T-cell counts, total serum IgE, and level of exposure to tobacco products among these groups. However, CD4/CD8 T-cell ratio tended to be higher in those with OBS (1.78) compared to normal classification (1.52, p=0.096).
Adjusted Associations of Pulmonary Outcomes with HIV Status
The unadjusted and adjusted models for either prevalence ratio (PR) or risk ratio (RR) of pulmonary outcomes in HIV compared to HEU youth are shown in Table IV. We describe the adjusted models (which include age, race/ethnicity, and sex). HIV youth were 53% less likely to have reversibility than HEU youth (PR=0.47, 95%CI 0.25, 0.89, p=0.02). Among those with only OBS, HIV youth were 54% less likely to have reversibility than HEU (PR=0.46, 95%CI 0.24, 0.87, p=0.016). Among those with a normal PFT, there was no significant difference in reversibility between HIV and HEU youth. The relationship between HIV status and reversibility did not differ between those with and without current asthma, or between those who have ever and those who have never had asthma (interaction term p=0.84 and p=0.12, respectively). When tobacco exposure was added to the above models, the conclusions remained the same.
Table IV.
Prevalence Ratio/Relative Risk of Pulmonary Outcomes in HIV vs. HEU Adolescents
| N | Unadjusted* | Adjusted** | ||||
|---|---|---|---|---|---|---|
| Pulmonary Outcomes | HIV | HEU | PR\RR of HIV vs. HEU Adolescents (95% CL) | P-value | PR\RR of HIV vs. HEU Adolescents (95% CL) | P-value |
| Ever had asthma 1 | 218 | 152 | 1.39 (0.98,1.99) | 0.067 | 1.32 (0.90,1.94) | 0.16 |
| Currently has asthma 1 | 218 | 152 | 1.38 (0.99,1.92) | 0.059 | 1.33 (0.93,1.89) | 0.11 |
| Obstructive Disease vs Normal 1 | 168 | 115 | 1.00 (0.66,1.52) | 0.99 | 0.97 (0.62,1.51) | 0.89 |
| Restrictive Disease vs Normal 1 | 145 | 104 | 0.76 (0.41,1.40) | 0.38 | 0.75 (0.41,1.39) | 0.37 |
| Reversibility 1 | 183 | 126 | 0.56 (0.31,1.01) | 0.055 | 0.47 (0.25,0.89) | 0.020 |
| Reversibility among those with Obstructive Disease 2 | 39 | 26 | 0.62 (0.34,1.13) | 0.12 | 0.46 (0.24,0.87) | 0.016 |
| Reversibility among those with Normal PFT results 2 | 124 | 85 | 0.46 (0.13,1.57) | 0.21 | 0.39 (0.11,1.37) | 0.14 |
| Reversibility (Interaction term: HIV status by current asthma) 3 | 183 | 126 | 1.05 (0.32,3.47) | 0.93 | 1.13 (0.34,3.76) | 0.84 |
| Reversibility (Interaction term: HIV status by ever asthma) 3 | 183 | 126 | 2.49 (0.70,8.89) | 0.16 | 2.78 (0.77,10.06) | 0.12 |
Unadjusted models estimate the prevalence ratio/relative risk of pulmonary outcomes in HIV versus HEU adolescents. Relative risk (RR) was obtained for ‘Ever had asthma,’ while prevalence ratio (PR) was obtained for all other pulmonary outcomes.
Adjusted models estimate the association between pulmonary outcomes and HIV status when adjusted for age, race/ethnicity, and sex.
Model fit using log-binomial regression approach.
Model fit using modified Poisson regression approach.
Estimate for the interaction term
Total serum IgE level: Association with CD4 and CD8 T-Cell, CD4/CD8 Ratio and absolute eosinophil counts in youth with asthma
Among those with current asthma, there was an inverse association between CD4 T-cell count and total serum IgE levels in HEU youth (regression coefficient (β) = −0.076 per 100 cells/μl, p=0.08), but no correlation in HIV youth (β = −0.026 per 100 cells/μl, p=0.34) (Fig. 1A). In contrast, we observed an inverse correlation between CD8 T-cells and total serum IgE levels in HIV youth (β = −0.04 per 100 cells/μl, p=0.03) , but none in HEU youth (β = 0.021 per 100 cells/μl, p=0.71) (Fig. 1B). There was no association of CD4/CD8 ratio and total serum IgE in HIV youth (p=6.39 per cells/μl, p=0.66), but there was an inverse association in HEU youth (β =39.25 per cells/μl, p=0.06) (Fig. 1C). Also, there was a significant positive association between total serum IgE and absolute eosinophil counts for HIV youth with current asthma (β = 0.16 per 100 cells/mm3, p=0.003), whereas no association was found in HEU youth with current asthma (β = 0.022, p=0.79) (Fig. 1D).
Figure 1.
Relationship between: CD4 T-cell count and Eosinophils with and without asthma
A. Scatterplot of CD4 T-cell count and Log10 total serum IgE by HIV status among youth with current asthma.
B. Scatterplot of CD8 T-cell count and Log10 total serum IgE by HIV status among youth with current asthma.
C. Scatterplot of CD4/CD8 Ratio and Log10 Total Serum IgE by HIV Status among those with Current Asthma
D. Scatterplot of eosinophils and Log10 total serum IgE by HIV status among youth with asthma status.
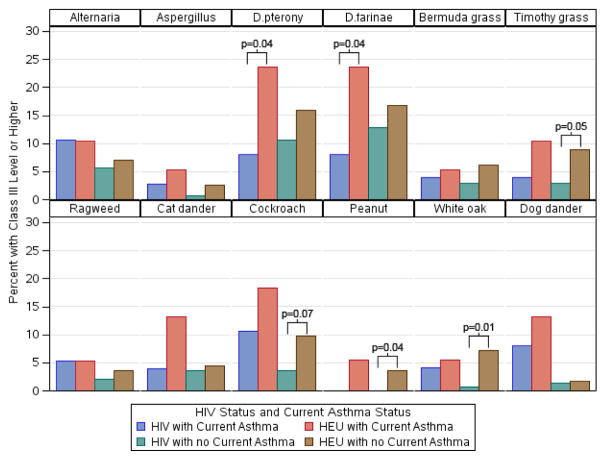
IgE responses to allergens
In Fig 2, we showed the percent with high IgE by HIV status and current asthma (see Table E4 in the online repository for values). Several comparisons showed that HIV youth with asthma were less likely to have a Class III or higher antibody response to D. pternony and D. farinae than HEU youth with asthma. Similarly, HIV youth without asthma were less likely to have a Class III or higher response to Timothy grass, cockroach, peanut and white oak than HEU youth without asthma.
Figure 2.
Percent of youth with high levels of specific IgE to allergens by HIV status among those with and without current asthma. Allergens with no significant immune reactivity were omitted.
The eosinophil count was higher in those with D.pterony (house dust mite) Class III or higher reactivity than in those with D.pterony Class II or lower among HIV youth with current asthma (p=0.18) and without current asthma (p<0.001) (Table E5 in online repository). The eosinophil count was not significantly different between those with and without reversibility among HIV youth (data not shown).
DISCUSSION
Using several well-accepted criteria for asthma based on family history, physician diagnosis, and asthma medication use (2, 6, 15, 17), we found a higher prevalence of asthma in HIV versus HEU youth as previously observed. (13, 15, 17) Assessment of pulmonary function and bronchodilator response revealed that despite the higher prevalence in HIV youth, they were less likely than HEU youth to demonstrate reversibility. This observation raises doubts as to the accuracy of clinical diagnosis alone in this population. The findings suggest that respiratory signs and symptoms in HIV youth are more likely to be labeled as asthma and treated with asthma medication than in those who are uninfected. These results in youth are consistent with those of Gingo et al (6), who reported a lack of reversibility in HIV adults diagnosed with asthma.
The prevalence of asthma in HEU youth is higher than in the general population but not as high as has been reported in some inner-city minority populations. (40, 41) Some HEU children with airway obstruction did not demonstrate reversibility. This may indicate that maternal HIV infection affected fetal lung tissue or be related to medications taken during pregnancy. Inflammatory mediators and HIV drugs could cross the placenta, injure the developing lung and create diffuse chronic inflammation. The neonatal HEU group studied by Colin et al (42) demonstrated an unexpected mean 20% decrease in expiratory airflow in HEU neonates compared to age-matched neonatal controls supports the possibility that maternal factors may adversely affect the fetal lung. However, in the absence of a control group without exposure to maternal HIV in utero, we are unable to conclude from our data that HEU youth have a higher prevalence of fixed airway obstruction than normal adolescents or that they have a higher risk for lung disease in the future.
Studies of childhood origins of COPD outside the HIV context support the possibility of early immune injury to the lung causing future immune dysregulation. (43, 44) Infectious viral, bacterial, or fungal pathogens could lodge in the lung and intensify the level of inflammation with recruitment of activated CD4 and CD8 T-cells, and eosinophils. This mechanism of early lung injury would apply to HIV neonates as well, in which the scenario of chronic inflammation of lung tissue is even more likely.
The finding that HIV youth have more fixed airway obstruction than HEU youth in late childhood-adolescence suggest that HIV infection may be another factor that poses a risk for future respiratory disease or COPD. Longitudinal studies of lung function in healthy infants (44) and in patients with asthma (45, 46) conclusively show that low pulmonary function in early childhood does not correct over time. Hence, the possibility that our study population, who are showing symptoms and reduced PFT in late childhood-adolescence, may represent the sequelae of infants that started with PFT deficits in early life.(47, 48) Our findings raise the possibility that the abnormal lung functions in early life constitute an increased risk for lung morbidity in later life, in the same way that other conditions associated with decreased pulmonary function in early life predispose such individuals to a worse long term pulmonary outcome. (49) It is also intriguing to speculate that HEUs who have deficits in lung function are at risk of COPD in the future; a question that should be further studied longitudinally.
Our investigation of the possible role of immune imbalance of the asthma-COPD role in HIV and HEU patients is revealing. The IgE system is a protective mechanism against parasitic and fungal infection, but it also serves as a signal for allergic disease: e.g., allergic rhinitis, atopic dermatitis, and asthma. IgE responses to common allergens provide sensitive assessments of abnormal immune control, e.g. as seen in patients with primary hyper-IgE syndrome.(50) or abnormal B-cell switching to IgE production.(51) In our study, CD4 T-cell counts were not associated with rising level of serum IgE, but CD8 T-cells were inversely correlated with total serum IgE levels. The lack of CD4 T-cells correlation and the inverse correlation of CD8 T-cell counts with total serum IgE levels supports the concept of the TH1-TH2 T-cell imbalance. Also, the strong positive association of absolute eosinophil counts and total serum IgE possibly indicates an immune response shifting from a TH1 cytotoxic and inflammatory response (IFN-γ, IL2) to an altered IgE TH2 response (IL4, IL13, and IL5 [eosinophil growth factor]).
In conclusion, our study may have uncovered a new pulmonary complication of perinatal HIV infection that begins in infancy, is detected in adolescence, and possibly causes progressive loss of pulmonary health. The finding of non-reversible obstructive lung disease needs to be studied over time so that the importance of lung function trajectory can be better understood in this vulnerable population.
Supplementary Material
KEY MESSAGE.
HIV-infected youth are less likely to demonstrate reversibility on pulmonary function testing suggesting that the clinical symptoms may reflect an early stage of chronic obstructive lung disease rather than typical asthma. Immune dysfunction of CD4 T- cells (TH2 immune imbalance) may influence total non-specific and specific serum levels of IgE.
Acknowledgments
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS.
William A. Meyer III, PhD facilitated the laboratory measurements, total and specific IgE, and cotinine. Janice Hopkins prepared the manuscript.
Grant support:
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigators: Kenneth Rich, Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc. (PI: Julie Davidson).
In addition, the Baylor College of Medicine-University of Texas Center for AIDS Research (AI 36211), PI: Janet S. Butel supported this study.
ABBREVIATIONS
- AMP
Adolescent Master Protocol
- ART
antiretroviral therapy
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- HEU
HIV-exposed uninfected
- HIV
human immunodeficiency virus
- IgE
Immunoglobulin E
- MedDRA®
Medical Dictionary for Regulatory Activities. MedDRA® trademark is owned by IFPMA on behalf of ICH
- NHLBI
National Heart Lung and Blood Institute
- NICHD
National Institute of Child Health and Human Development
- P2C2 HIV Study
Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted human immunodeficiency virus infection study
- PFT
pulmonary function test
- PHACS
Pediatric HIV/AIDS Cohort Study
- OBS
obstructive
- RES
restrictive
- REV, reversibility
reversible
- RS
Spearman correlation
- WITS
Women and Infants Transmission Study
Footnotes
The following institutions, clinical site investigators and staff participated in conducting PHACS AMP and AMP Up in 2015, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Baig, Anna Cintron; Children’s Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Patricia A. Garvie, James Blood; Children’s Hospital, Boston: Sandra K. Burchett, Nancy Karthas, Betsy Kammerer; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Nozyce; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica; St. Christopher’s Hospital for Children: Janet S. Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University School of Medicine: Margarita Silio, Medea Gabriel, Patricia Sirois; University of California, San Diego: Stephen A. Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Juliana Darrow, Emily Barr, Paul Harding; University of Miami: Gwendolyn Scott, Grace Alvarez, Anai Cuadra.
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
Presented in part at the 2015 Conference on Retroviruses and Opportunistic Infections (CROI).
Disclosures: None to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4:e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gingo MR, George MP, Kessinger CJ, Lucht L, Rissler B, Weinman R, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui Q, Carruthers S, McIvor A, Smaill F, Thabane L, Smieja M. Effect of smoking on lung function, respiratory symptoms and respiratory diseases amongst HIV-positive subjects: a cross-sectional study. AIDS Res Ther. 2010;7:6. doi: 10.1186/1742-6405-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond MB, Kirk GD, Astemborski J, Marshall MM, Mehta SH, McDyer JF, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012;67(4):309–14. doi: 10.1136/thoraxjnl-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristoffersen US, Lebech AM, Mortensen J, Gerstoft J, Gutte H, Kjaer A. Changes in lung function of HIV-infected patients: a 4.5-year follow-up study. Clin Physiol Funct Imaging. 2012;32(4):288–95. doi: 10.1111/j.1475-097X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 6.Gingo MR, Wenzel SE, Steele C, Kessinger CJ, Lucht L, Lawther T, et al. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J Allergy Clin Immunol. 2012;129:708–714. e708. doi: 10.1016/j.jaci.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris A, Gingo MR, George MP, Lucht L, Kessinger C, Singh V, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS. 2012;26:731–740. doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gingo MR, Morris A, Crothers K. Human immunodeficiency virus-associated obstructive lung diseases. Clin Chest Med. 2013;34:273–282. doi: 10.1016/j.ccm.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gingo MR, Morris A. Pathogenesis of HIV and the lung. Curr HIV/AIDS Rep. 2013;10:42–50. doi: 10.1007/s11904-012-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clausen E, Wittman C, Gingo M, Fernainy K, Fuhrman C, Kessinger C, et al. Chest computed tomography findings in HIV-infected individuals in the era of antiretroviral therapy. PLoS One. 2014;9:e112237. doi: 10.1371/journal.pone.0112237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick ME, Singh V, Bertolet M, Lucht L, Kessinger C, Michel J, et al. Relationships of pulmonary function, inflammation, and T-cell activation and senescence in an HIV-infected cohort. AIDS. 2014;28:2505–2515. doi: 10.1097/QAD.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond MB, Huang L, Diaz PT, Kirk GD, Kleerup EC, Morris A, et al. Factors associated with abnormal spirometry among HIV-infected individuals. AIDS. 2015;29:1691–1700. doi: 10.1097/QAD.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster SB, Paul ME, Kozinetz CA, Macias CG, Shearer WT. Prevalence of asthma in children and young adults with HIV infection. J Allergy Clin Immunol. 2007;119:750–752. doi: 10.1016/j.jaci.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Gutin F, Butt A, Alame W, Thomas R, Secord E. Asthma in immune-competent children with human immunodeficiency virus. Ann Allergy Asthma Immunol. 2009;102:438. doi: 10.1016/S1081-1206(10)60518-2. [DOI] [PubMed] [Google Scholar]
- 15.Foster SB, McIntosh K, Thompson B, Lu M, Yin W, Rich KC, et al. Increased incidence of asthma in HIV-infected children treated with highly active antiretroviral therapy in the National Institutes of Health Women and Infants Transmission Study. J Allergy Clin Immunol. 2008;122:159–165. doi: 10.1016/j.jaci.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster SB, Lu M, Thompson B, Rich KC, Matukas LM, Mason R, et al. Association between HLA inheritance and asthma medication use in HIV positive children. AIDS. 2010;24:2133–2135. doi: 10.1097/QAD.0b013e32833cba08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siberry GK, Leister E, Jacobson DL, Foster SB, Seage GR, 3rd, Lipshultz SE, et al. Increased risk of asthma and atopic dermatitis in perinatally HIV-infected children and adolescents. Clin Immunol. 2012;142:201–208. doi: 10.1016/j.clim.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bunupuradah T, Hansudewechakul R, Kosalaraksa P, Ngampiyaskul C, Kanjanavanit S, Wongsawat J, et al. HLA-DRB1454 and predictors of new-onset asthma in HIV-infected Thai children. Clin Immunol. 2015;157:26–29. doi: 10.1016/j.clim.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Shearer WT, Leister E, Siberry GK, Jacobson DL, Van Dyke RB, Peavy H, et al. for the Pediatric HIV/AIDS Cohort Study (PHACS) Pulmonary complications of HIV-1 in Children/adolescents: The PHACS AMP Study. Conference on Retroviruses and Opportunistic Infections (CROI); February 20, 2015; Seattle, WA. [Google Scholar]
- 20.Postma DS, Weiss ST, van den Berge M, Kerstjens HA, Koppelman GH. Revisiting the Dutch hypothesis. J Allergy Clin Immunol. 2015;136(3):521–9. doi: 10.1016/j.jaci.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Barnes PJ. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol. 2015;136(3):531–45. doi: 10.1016/j.jaci.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 22.Reddel HK. Treatment of overlapping asthma-chronic obstructive pulmonary disease: Can guidelines contribute in an evidence-free zone? J Allergy Clin Immunol. 2015;136(3):546–52. doi: 10.1016/j.jaci.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 23.Gelb AF, Nadel JA. Understanding the pathophysiology of the asthma-chronic obstructive pulmonary disease overlap syndrome. J Allergy Clin Immunol. 2015;136(3):553–5. doi: 10.1016/j.jaci.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136(3):556–68. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 25.Postma DS, Rabe KF. The Asthma-COPD overlap syndrome. N Engl J Med. 2015;373(13):1241–9. doi: 10.1056/NEJMra1411863. [DOI] [PubMed] [Google Scholar]
- 26.Bacot BK, Paul ME, Navarro M, Abramson SL, Kline MW, Hanson IC, et al. Objective measures of allergic disease in children with human immunodeficiency virus infection. J Allergy Clin Immunol. 1997;100:707–711. doi: 10.1016/s0091-6749(97)70177-5. [DOI] [PubMed] [Google Scholar]
- 27.Recommended Respiratory Disease Questionnaire for use with Adults and Children in Epidemiological Research. British Medical Council; London, England: 1978. pp. 48–52. [Google Scholar]
- 28.Childhood Asthma Control Test for Children 4–11 years. American Asthma Association; Quality Metrics, Incorporated; 2008. [Google Scholar]
- 29.Asthma Control Test. American Asthma Association; Quality metrics, incorporated; 2002. [Google Scholar]
- 30.National health and nutrition examination survey 2004–2006: Respiratory Disorders. 2007 Dec; [Google Scholar]
- 31.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of Spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 32.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. AM J Respir Crit Care Med. 1999;159: 179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 33.Dundas I, Chan EY, Bridge PD, McKenzie SA. Diagnostic accuracy of bronchodilator responsiveness in wheezy children. Thorax. 2005;60(1):13–6. doi: 10.1136/thx.2004.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galant SP, Morphew T, Amaro S, Liao O. Value of the bronchodilator response in assessing controller naïve asthmatic children. J Pediatr. 2007;151(5):457–62. 462.e1. doi: 10.1016/j.jpeds.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Tse SM, Gold Dr, Sordillo JE, Hoffman EB, Gillman MW, Rifas-Shiman SL, et al. Diagnostic accuracy of the bronchodilator response in children. J Allergy Clin Immunol. 2013;132(3):554–559.e5. doi: 10.1016/j.jaci.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolen WK. IgE antibody in the serum-detection and diagnostic significance. Allergy. 2003;58:717–23. doi: 10.1034/j.1398-9995.2003.00281.x. [DOI] [PubMed] [Google Scholar]
- 37.Yunginger JW, Ahlstedt S, Eggleston PA, Homburger HA, Nelson HS, Ownby DR, et al. Quantitative IgE antibody assays in allergic diseases. J Allergy Clin Immunol. 2000;105(6 Pt 1):1077–84. doi: 10.1067/mai.2000.107041. [DOI] [PubMed] [Google Scholar]
- 38.Immuno CAP IgE Package Insert. Phadia Inc; Baltimore, MD: published March 2015. [Google Scholar]
- 39.Zou GA. Modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 40.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: Rethinking the inner-city asthma epidemic. J Allergy Clin Immunol. 2015;135: 655–62. doi: 10.1016/j.jaci.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teach SJ, Gergen PJ, Szefler SJ, Mitchell HE, Calatroni A, Wildfire J, et al. Seasonal risk factors for asthma exacerbations among inner-city children. J Allergy Clin Immunol. 2015;135: 1465–73. doi: 10.1016/j.jaci.2014.12.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colin AA, Sunil Rao J, Chen XC, Hunter JM, Hanrahan J, Hiatt P, et al. Pediatric pulmonary and cardiovascular complications of vertically transmitted human immunodeficiency virus study group, NHLBI. Forced expiratory flow in uninfected infants and children born to HIV-infected mothers. Am J Respir Crit Care Med. 2001;163:865–873. doi: 10.1164/ajrccm.163.4.9901040. [DOI] [PubMed] [Google Scholar]
- 43.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–64. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tai A, Tran H, Roberts M, Clarke N, Gibson AM, Vidmar S, Wilson J, Robertson CF. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol. 2014 Jun;133(6):1572–8.e3. doi: 10.1016/j.jaci.2013.12.1033. Epub 2014 Feb 2. [DOI] [PubMed] [Google Scholar]
- 45.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003 Oct 9;349(15):1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 46.Savanes C, Sunyer J, Plana E, Dharmage S, Heinrich J, Jarvis D, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65:14–20. doi: 10.1136/thx.2008.112136. [DOI] [PubMed] [Google Scholar]
- 47.Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015 Mar 7;385(9971):899–909. doi: 10.1016/S0140-6736(14)60446-3. [DOI] [PubMed] [Google Scholar]
- 48.Sly PD, Bush A. From the cradle to the grave: The early-life origins of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193(1):1–2. doi: 10.1164/rccm.201509-1801ED. [DOI] [PubMed] [Google Scholar]
- 49.Bolton CE, Bush A, Hurst JR, Kotech S, McGarvey L. Lung consequences in adults born prematurely. Thorax. 2015 Jun;70(6):574–80. doi: 10.1136/thoraxjnl-2014-206590. [DOI] [PubMed] [Google Scholar]
- 50.Ozcan E1, Notarangelo LD, Geha RS. Primary immune deficiencies with aberrant IgE production. J Allergy Clin Immunol. 2008;122:1054–62. doi: 10.1016/j.jaci.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Wesemann DR, Magee JM, Boboila C, Calado DP, Gallagher MP, Portuguese AJ, et al. Immature B cells preferentially switch to IgE with increased direct Sμ to Sε recombination. J Exp Med. 2011;208:2733–46. doi: 10.1084/jem.20111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.