Abstract
Background
The impact of maternal BMI and insulin sensitivity on bioactive components of human milk (HM) is not well understood. As the prevalence of obesity and diabetes rises, it is increasingly critical that we understand how maternal BMI and hormones associated with metabolic disease relate to concentrations of bioactive components in HM.
Methods
This longitudinal cohort design followed 48 breastfeeding mothers through the first four months of lactation, collecting fasting morning HM samples at 2-weeks and 1, 2, 3, and 4-months, and fasting maternal blood at 2-weeks and 4-months. Insulin, glucose, adipokines leptin and adiponectin, appetite regulating hormone ghrelin, marker of oxidative stress 8OHdG, and inflammatory cytokines (IL-6, IL-8, and TNF-a) were measured in HM and maternal plasma.
Results
26 normal weight (NW) (BMI=21.4±2.0 kg/m2), and 22 overweight/obese (OW/Ob) (BMI=30.4±4.2 kg/m2) were followed. Of all HM analytes measured, only insulin and leptin were different between groups - consistently higher in the OW/Ob group (leptin: p<0.001; insulin: p<0.03). HM insulin was 98% higher than maternal plasma insulin at 2-weeks and 32% higher at 4-months (p<0.001). Maternal fasting plasma insulin and HOMA-IR were positively related to HM insulin at 2-weeks (p<0.001, R2≥0.38, n=31), and 4-months (p≤0.005, R2≥0.20, n=38).
Conclusions
The concentrations of insulin in HM are higher than in maternal plasma and are related to maternal BMI and insulin sensitivity. With the exception of leptin, there were minimal other differences observed in HM composition across a wide range in maternal BMI.
Keywords: Human milk, obesity, insulin, leptin, cytokines
INTRODUCTION
The first 6 months of a newborn infant’s life are a critical period of development when nutritional exposures can exert long-lasting programming effects (1). During this time, exclusive breastfeeding is universally recommended due to the immunologic and other protective benefits of human milk (HM) that decrease infant risk for infection and chronic disease (2, 3). Exclusive breastfeeding has also been shown in most studies to impart a modest protective effect against later obesity (4, 5) and type 2 diabetes (6, 7). However, this protective effect is controversial, and may differ depending on maternal phenotype (8–10).
Human milk (HM) is a dynamic and complex substance which delivers large doses of bioactive components (hormones, cytokines, pre-and probiotics, oligosaccharides, etc.) that support infant development and optimize health. Maternal BMI may impact characteristics of HM such as % fat, leptin, adiponectin, and insulin (11–14). Obesity is characterized by chronic systemic low grade inflammation, oxidative stress, and insulin resistance (15) but how maternal metabolic phenotype impacts HM composition, particularly the oxidative stress and inflammatory profile of HM, over time is poorly understood.
Data from animal models suggest that maternal obesity and/or poor diet impacts breast milk composition, imparting deleterious postnatal programming effects to offspring, predisposing them to obesity, inflammation and components of metabolic syndrome (16–18). However, human epidemiological data are less conclusive, and have failed to show that HM from a mother with obesity is less protective against obesity than HM form a normal weight mother (19). In fact, some data suggest that the protection of breastfeeding against childhood obesity is greater from overweight vs. normal weight mothers (20).
Currently, 40% of American women are obese (21). Despite this prevalence, the impact of maternal obesity on HM composition and delivery of bioactive components to the infant remains poorly characterized. This study had several aims. First we compared bioactive components in HM that may be associated with metabolic derangement (adipokines, anabolic and appetite-regulating hormones, macronutrients, and markers of inflammation and oxidative stress) between healthy mothers vs mothers with overweight/obesity. Secondarily, we tested for relationships between specific components of maternal phenotype (BMI, circulating hormone concentrations, insulin sensitivity, and % weight loss) and HM composition. Lastly, we compared temporal trends in these HM components between groups – focusing on the first 4 months of breast feeding when infants are rapidly accreting fat and are exclusively consuming HM. Understanding the impact of maternal overweight/obesity on HM composition is critical given the rising prevalence of metabolic dysfunction among breastfeeding women.
METHODS
Study Design and Participants
This was a prospective cohort study of breastfeeding women and their infants over the first four months of lactation. The study was approved by the Colorado Multiple Institutional Review Board (clinical trials.gov: NCT01693406). Inclusion criteria included maternal age of 21 – 36 with a pre-pregnancy BMI of 17.0 – 39.9 kg/m2. Informed consent was given during pregnancy. All women were carrying a singleton fetus, planning to exclusively breastfeed for at least four months, and were otherwise healthy. Women with any chronic medical diseases requiring treatment such as cardiopulmonary, rheumatologic or renal disease or pre-existing diabetes were excluded. Women who developed gestational diabetes, preeclampsia, or who delivered their infant <37 weeks were excluded. All women delivered their infants at the University of Colorado Hospital at the Anschutz Medical Campus (Denver, CO). Maternal pre-pregnancy BMI was based on self-report of pre-pregnant weight and measured height.
Based on previously published means and standard deviations for HM leptin concentrations (22), we calculated that a sample size of 40 (20 Normal Weight (NW) and 20 Overweight/Obese (OW/Ob)) would provide us >90% power to detect a significant difference (p<0.05) in HM leptin concentrations between groups.
Study Protocol
Study personnel visited mothers in the hospital within 48 hours of delivery. Infant sex, birth weight, and mode of delivery were obtained from medical records.
At 2-weeks, 1, 2, 3, and 4-months, participants were seen for morning follow-up visits. Mothers were asked to fast from midnight the previous night. At each visit, maternal height and weight were measured and mothers were administered a shortened version of the Infant Feeding Practices II Questionnaire, which queried about current feeding practices, breastfeeding exclusivity, and breastfeeding problems (23). The Infant Feeding Practices II documents the number of breastfeeding or bottle-feeding sessions (including snacks) that occurred in the past 24 hours. Breastfeeding exclusivity was calculated at each visit as the percentage of feedings in previous day that were breast milk.
At 4-months, total breastfeeding exposure was calculated to reflect exclusivity and duration (as % over 4 months months), such that 100% indicates exclusive breastfeeding for a full 4-months. A score of 50% could indicate exclusive breastfeeding for 2 months and then no breastfeeding for months 2 – 4, or 50% of feeds from breastmilk over the 4-month study.
Sample Collection
A mid-feed breast milk sample was collected at each visit. Mothers initiated a nursing session, and approximately halfway through the feed (based on normal feeding time and maternal sensation of milk removal and breast softening), the infant was removed, the nipple and areola wiped clean, and approximately 20mL was expressed using a hand pump (Ameda, Buffalo Grove, IL). Milk was immediately placed on ice and transported to the laboratory. Skim milk was generated by spinning milk at 10,000g for 10 minutes at 4°C. Aliquots of skim and whole milk were then stored at −80°C until analysis.
At 2-weeks and 4-months a fasting venous sample of maternal blood was also collected. Plasma and serum were generated by centrifugation and frozen at −80°C until analysis.
Sample Analysis
Percent milk fat was measured by creamatocrit, and fat g/L was estimated using the following approximation: g/L = (creamatocrit [%] − 0.59)/0.146) (24). Lactose was measured by enzymatic digestion and detection of galactose (BioVision, Inc., Milpitas, CA), and protein measured by a modified version of the Bradford method (BioRad Inc., Hercules, CA). HM caloric density was calculated as the summation of fat, protein and lactose assuming 9kcal/g, 4kcal/g and 4kcal/g, respectively.
8-hydroxy-deoxyguansoine (8OHdG), a marker of oxidative stress, was measured in skim milk and maternal plasma samples using a commercial EIA (Cayman Chemical, Ann Arbor, MI). HM and plasma IL-6, IL-8, and TNF-α were measured simultaneously using a high sensitivity magnetic bead Milliplex Assay (Millipore, Billerica, MA). Insulin and free glucose were measured in the skim fraction of HM and maternal plasma using a radioimmunoassay (Millipore, Billerica MA), and a hexokinase ultraviolet assay (Beckman Coulter, Brea CA), respectively. Total adiponectin was measured in the skim fraction of HM and in plasma using a radioimmunoassay (Millipore, Billerica MA). Leptin was measured in the skim fraction of HM at a dilution between 1:2 and 1:5 using a high sensitivity ELISA (R&D, Minneapolis MN). Leptin in maternal serum was measured using an ELISA (Millipore, Billerica, MA).
Maternal hemoglobin A1C (HbA1C) was measured in whole blood (Siemens DCA Vantage, Malvern PA). HOMA-IR was calculated in maternal samples as follows: (glucose (mg/dL) * insulin (uIU/mL))/405. Maternal oxidized-LDL concentrations were measured via ELISA (Mercodia, Winston Salem, NC).
Statistical Analysis
All data are presented as mean ± SD, unless otherwise specified. All study variables were tested for normality using the Shapiro-Wilks test. Outcome variables that were not normally distributed were log transformed to correct skewness.
A linear mixed model was used to test HM analytes for overall group differences, trends over time and any potential interaction between group and time. Differences in HM analytes between groups at individual time points were tested via t-test among analytes that had a significant group difference in the linear mixed model.
Paired t-tests were used to determine if the concentration of HM hormones was significantly higher or lower than the concentration in maternal circulation.
Simple linear regression was used to determine if analyte concentrations in maternal blood were related to those in HM at 2-weeks and 4-months, individually. Simple linear regression was also used to determine if maternal BMI was related to analyte concentrations in blood or in HM (as separate outcomes) at both 2-weeks and 4 months. If a significant relationship was detected between a maternal phenotype (BMI or concentration of an analyte in maternal circulation) and the analyte concentration in HM, then multivariable regression was used to test for a potential interaction between maternal phenotype and maternal overweight status (as a dichotomous variable).
Simple linear regression was used to test if a given HM analyte at 2-weeks was related to the concentration at 4-months.
RESULTS
Cohort Characteristics
A total of 48 mother/infant pairs (26 NW and 22 OW/Ob) participated in the study. Ten women did not provide blood samples at 2-weeks, or undergo study visits at 1, 2, and 3-months. Participant characteristics are reported in Table 1. Only one woman was underweight (pre-pregnant BMI <18.5). Because exclusion of her data did not change the results, she was included in the NW group. Neither maternal age, mode of delivery, length of gestation, or total gestational weight gain differed between groups. Infant weight gain over the first 4 months of life did not differ by maternal group.
Table 1.
Mean subject Characteristics by Group.
| Characteristic1 | NW (n=26) | OW/Ob (n=22) | Whole Cohort (n=48) | p-value2 |
|---|---|---|---|---|
| Maternal Age (yr) | 30.8 ± 2.6 | 30.3 ± 3.9 | 30.6 ± 3.2 | 0.66 |
| Pre-pregnancy BMI (kg/m2) | 21.4 ± 2.0 | 30.4 ± 4.2 | 25.5 ± 5.5 | <0.0001 |
| Vaginal Delivery (%) | 81% | 68% | 75% | 0.33 |
| Gestational Age (wks) | 39.9 ± 0.7 | 39.9 ± 1.2 | 39.9 ± 0.9 | 0.99 |
| Gestational Weight Gain (lbs) | 33.0 ± 9.5 | 32.9 ± 16.0 | 33.0 ± 12.6 | 0.96 |
| Infant Sex (% male) | 42% | 78% | 58% | 0.014 |
| Infant birth weight (g) | 3227 ± 352 | 3689 ± 490 | 3439 ± 477 | 0.0053 |
| Infant weight gain (birth to 4 months) (g) |
3012 ± 584 | 3157 ± 692 | 3078 ± 633 | 0.963 |
Data presented as mean ± SD
Comparison between NW vs OW/Ob groups assessed via t-test for independent groups.
Controlled for infant sex
In the cohort as a whole, mothers had lost 84 ± 29% of their gestational weight gain by 4-months postpartum, and the % of weight loss did not differ between NW vs. OW/Ob women (Table 2).
Table 2.
Mean Subject and Blood Characteristics by Group at 2-weeks and 4-months
| 2-weeks | 4-months | p-over time3 | |||||
|---|---|---|---|---|---|---|---|
| Characteristic1 | NW (n=16) |
OW/Ob (n=22) |
p-value2 | NW (n=26) |
OW/Ob (n=22) |
p-value2 | |
| Age at Visit (weeks) | 2.2 ± 0.2 | 2.2 ± 0.3 | 0.31 | 18.5 ± 1.2 | 18.5 ± 1.4 | 0.93 | |
| Breastfeeding Exclusivity (%) | 97 ± 10 | 98 ± 6 | 0.87 | 92 ± 21 | 72 ± 45 | 0.08 | 0.003 |
| Maternal BMI (kg/m2) | 23.8 ± 2.5 | 32.3 ± 3.4 | <0.0001 | 22.6 ± 2.8 | 31.6 ± 4.4 | <0.0001 | <0.0001 |
| Maternal Weight Loss (lbs)4 | 17.8 ± 4.5 | 21.9 ± 8.0 | 0.04 | 25.5 ± 7.9 | 25.3 ±11.6 | 0.94 | <0.0001 |
| Maternal Weight Loss (% of gestational weight gain) | 57.9 ± 20.6 | 85.4 ± 54.6 | 0.04 | 80.7 ± 22.8 | 88.0 ±35.7 | 0.42 | 0.02 |
| Triglycerides (mg/dL) | 83.0 ± 39.7 | 81.8 ± 23.9 | 0.92 | 51.4 ± 16.7 | 75.3 ± 36.7 | 0.0100 | <0.0001 |
| Glucose (mg/dL) | 81.2 ± 6.8 | 82.9 ± 6.9 | 0.47 | 86.5 ± 10.2 | 87.9 ± 7.3 | 0.60 | <0.01 |
| Insulin (μIU/mL) | 7.4 ± 3.2 | 10.1 ± 3.5 | 0.035 | 8.7 ± 3.4 | 12.9 ± 5.2 | 0.0025 | 0.06 |
| HOMA-IR | 1.5 ± 0.7 | 2.1 ± 0.8 | 0.041 | 1.9 ± 1.0 | 2.8 ± 1.2 | 0.0074 | 0.03 |
| Hb-A1C (%) | 5.2 ± 0.2 | 5.3 ± 0.2 | 0.63 | 5.2 ± 0.2 | 5.2 ± 0.3 | 0.78 | 0.65 |
| Leptin (ng/mL) | 9.0 ± 10.5 | 16.3 ± 9.8 | 0.049 | 7.1 ± 7.3 | 15.5 ± 8.1 | 0.0006 | 0.33 |
| Adiponectin (μg/mL) | 8.4 ± 3.2 | 8.4 ± 4.4 | 0.99 | 10.3 ± 4.7 | 9.8 ± 4.8 | 0.748 | 0.14 |
| Ghrelin (pg/mL) | 1450 ± 547 | 1118 ± 310 | 0.038 | 1380 ± 521 | 1011 ± 300 | 0.0048 | <0.01 |
| 8OHdG (ng/mL) | 9.8 ± 2.6 | 10.3 ± 3.0 | 0.63 | 8.5 ± 2.0 | 8.6 ± 2.3 | 0.86 | <0.0001 |
| Oxidized LDL (U/L) | 61.6 ± 17.0 | 56.7 ± 18.9 | 0.43 | 44.3 ± 11.9 | 40.5 ± 10.6 | 0.27 | <0.0001 |
| IL-6 (ng/mL) | 9.4 ± 16.0 | 6.1 ± 4.0 | 0.43 | 8.8 ±15.7 | 10.6 ± 19.0 | 0.75 | 0.84 |
| IL-8 (ng/mL) | 5.8 ± 2.6 | 5.0 ± 1.9 | 0.37 | 4.6 ± 3.9 | 4.5 ± 3.7 | 0.97 | 0.49 |
| TNF-a (ng/mL) | 8.2 ± 4.0 | 7.8 ± 2.9 | 0.76 | 7.1 ± 4.0 | 6.4 ± 2.9 | 0.55 | 0.17 |
Data presented as mean ± SD
p-value for t-test comparison between NW vs OW/Ob groups at each time point, individually
p-value for paired t-test for change over time (2-weeks vs. 4-months) in the cohort as a whole.
Maternal weight loss (in lbs) from full-term weight to 2-weeks, and to 4-months, respectively.
Temporal Changes in Maternal Biochemical Phenotype
Maternal biochemical characteristics are presented for the cohort as a whole, and in the groups individually in Table 2. In the cohort as a whole, breastfeeding exclusivity decreased over time (from 2-weeks to 4-months; p=0.003). Maternal BMI and triglycerides significantly decreased over time (p<0.0001). Maternal fasting glucose and HOMA-IR significantly increased over time (p=0.005 and 0.03, respectively), whereas maternal fasting insulin and Hb-A1C did not change. Both markers of oxidative stress, 8OHdG and oxidized-LDL, decreased in maternal circulation (p<0.0001). No change was observed in maternal circulating adipokines (leptin and adiponectin) or cytokines (IL-6, IL-8, and TNF-α) over time.
Differences in Maternal Biochemical Phenotype by Overweight Status
Maternal plasma leptin, insulin, and HOMA-IR were all significantly higher among OW/Ob compared to NW mothers at both 2-weeks and 4-months. Furthermore, concurrent maternal BMI was positively associated with maternal leptin, insulin, and HOMA-IR at both 2-weeks (p<0.02, R2 > 0.17) and 4-months (p<0.002, R2 > 0.27). Maternal ghrelin concentrations were lower among OW/Ob mothers and negatively associated with concurrent maternal BMI at both time points (p=0.03, R2 = 0.15 at 2-weeks and p<0.001, R2 = 0.22 at 4-months). Maternal triglycerides were higher among OW/Ob women only at 4-months.
Differences in HM Composition Over Time, and By Group
The HM characteristics of the cohort as a whole are presented in Table 3. HM protein, insulin, leptin, adiponectin, ghrelin, 8OHdG, and the inflammatory cytokines all decreased over the course of lactation, while lactose and glucose concentrations increased. These changes over time were consistent in both groups.
Table 3.
Longitudinal HM Macronutrients, Endocrine, and Inflammatory and Oxidative Stress Biomarkers
| Characteristic1 | 2-week (n=48) |
1-month (n=34) |
2-month (n=32) |
3-month (n=30) |
4-month (n=40) |
p-value2 |
|---|---|---|---|---|---|---|
| Calories (per 100mL) | 65 ± 11 | 64 ± 15 | 66 ± 19 | 65 ± 14 | 68 ± 17 | 0.81 |
| Fat (g/100mL) | 3.5 ± 1.2 | 3.3 ± 1.6 | 3.5 ± 1.9 | 3.2 ± 1.6 | 3.5 ± 1.9 | 0.34 |
| Lactose (g/100mL) | 7.3 ± 1.0 | 7.4 ± 1.3 | 7.5 ± 1.7 | 8.1 ± 0.6 | 8.1 ± 0.7 | <0.001 |
| Protein (g/100mL) | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.6 | 0.9 ± 0.1 | 0.8 ± 0.2 | <0.001 |
| Glucose (mg/dL) | 20.7 ± 6.4 | 24.7 ± 6.9 | 25.2 ± 5.8 | 26.3 ± 5.9 | 26.2 ± 7.4 | <0.001 |
| Insulin (μIU/mL)3 | 15.8 ± 15.0d | 37.5 ± 83.8b | 16.9 ± 12.4 | 14.6 ± 9.6b | 13.6 ± 8.8 | <0.001 |
| Leptin (pg/mL) | 976 ± 1275c | 1006 ± 1410b | 1129 ± 2327d | 557 ± 599c | 475 ± 480a | <0.001 |
| Adiponectin (ng/mL)3 | 22.0 ± 14.8 | 20.2 ± 11.3 | 20.4 ± 18.2 | 20.9 ± 27.6 | 15.0 ± 7.9 | 0.001 |
| Ghrelin (pg/mL) | 510 ± 4111 | 268 ± 348 | 251 ± 283 | 0.002 | ||
| 8OHdG (ng/mL)3 | 14.5 ± 7.3 | 13.3 ± 7.3 | 12.4 ± 6.1 | 9.9 ± 4.0 | 9.3 ± 3.1 | <0.001 |
| IL-6 (pg/mL)3 | 25.1 ± 51.3 | 15.7 ± 27.1 | 16.7 ± 32.4 | 6.7 ± 8.6 | 5.2 ± 8.6 | <0.001 |
| IL-8 (pg/mL)3 | 87 ± 142 | 104 ± 145 | 145 ± 229 | 108 ± 124 | 117 ± 201 | <0.01 |
| TNF-a (pg/mL)3 | 11.1 ± 14.6 | 10.5 ± 13.0 | 8.9 ± 7.0 | 8.3 ± 7.1 | 6.0 ± 4.9 | 0.023 |
Data presented as mean ± SD; sample size is denoted in parentheses
p-value for change over time, controlling for potential differences between groups
Log Transformation used for comparison over time and between groups; means presented are not transformed.
NW group lower than the OW/Ob group;
p < 0.0001;
p < 0.004;
p < 0.02;
p < 0.05.
Controlling for the effect of time, only insulin and leptin were different in HM and were consistently higher in the OW/Ob group (leptin: p<0.001; insulin: p<0.03). Considering time-points individually, HM leptin was higher in the OW/Ob group at every time point (p<0.05) and HM insulin was higher in the OW/Ob group at 2-weeks, 1-month and 3-months (p<0.05). Temporal changes in HM insulin and leptin by group are presented in Figure 1.
Figure 1.
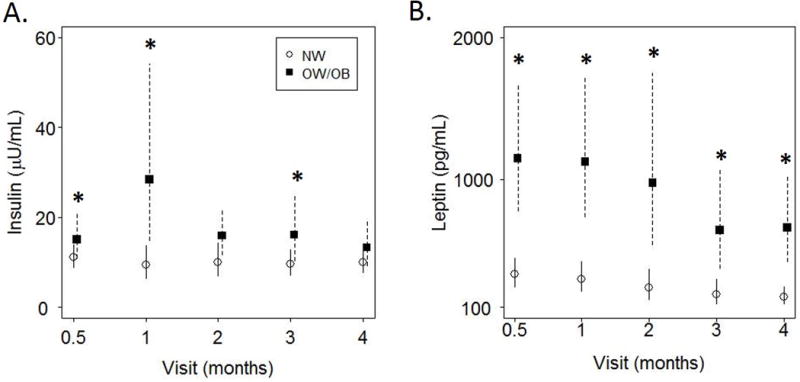
Concentrations of Human milk (HM) insulin and leptin over time are presented as mean ± 95% CI.
A) HM concentrations of insulin decreased over the first 4 months of lactation (p<0.0001) and were higher in HM from overweight/obese women at 2-weeks, 1-month, and 3-months (*p<0.05). B) HM concentrations of leptin decreased over the first 4 months of lactation (p<0.0001) and were higher in HM from overweight/obese women at every time point (*p<0.05), by t-test.
HM Insulin is Higher than Maternal Circulating Insulin
The concentrations of insulin in HM were significantly higher than those in maternal blood: HM insulin was 98% higher than concentrations in maternal blood at 2-weeks (p<0.0001), and 32% higher at 4-months (p<0.001) in the cohort as a whole (Figure 2). The HM concentrations of all other hormones measured were significantly lower than in maternal circulation (p<0.0001).
Figure 2.
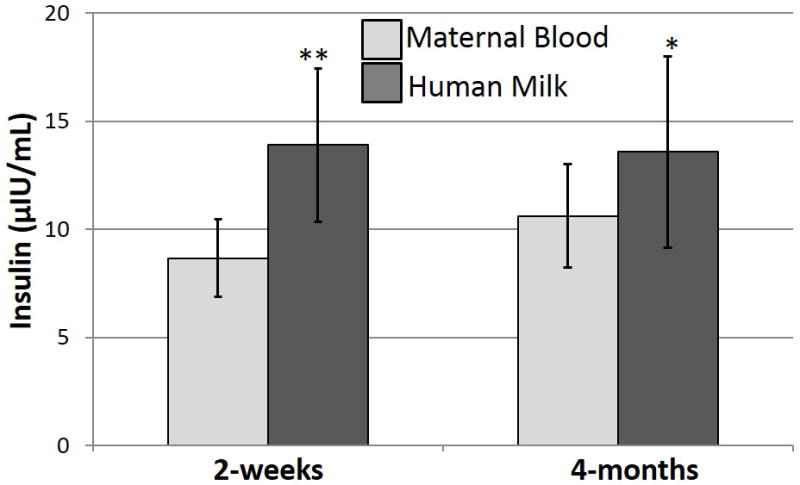
Insulin concentrations were measured in fasting maternal blood and human milk samples. Insulin concentrations in human milk were 98% higher than concentrations in maternal blood at 2-weeks (**p<0.004) and 32% higher at 4-months (*p<0.0009) (differences assessed using a paired t-test).
Maternal Phenotype is Associated with HM Characteristics
Concurrent maternal BMI was strongly positively correlated with HM leptin concentrations at both 2-weeks (p<0.0001, R2=0.28, n=43) and 4-months (p<0.0001, R2=0.70, n=41). Maternal serum leptin concentrations were also correlated with HM leptin concentrations at both 2-weeks (p=0.001, R2=0.27, n=33), and 4-months (p=0.01, R2=0.40, n=40). These relationships with HM leptin were similar in both the NW and OW/Ob group, as indicated by the non-significant interaction term.
Concurrent maternal BMI was positively correlated with HM insulin concentrations at both 2-weeks (p=0.02, R2=0.12, n=48) and 4-months (p=0.03, R2=0.12, n=40). Maternal insulin concentrations were also strongly correlated with HM insulin concentrations at both 2-weeks (p<0.0001, R2=0.42, n=32), and 4-months (p<0.001, R2=0.29, n=38). Additionally, maternal HOMA-IR was also strongly correlated with HM insulin concentrations at both 2-weeks (p<0.001, R2=0.38, n=31), and 4-months (p=0.005, R2=0.20, n=38). These relationships with HM insulin were similar in both the NW and OW/Ob group, as indicated by the non-significant interaction term.
The relationships between maternal circulating and HM insulin and leptin concentrations are presented in Figure 3.
Figure 3.
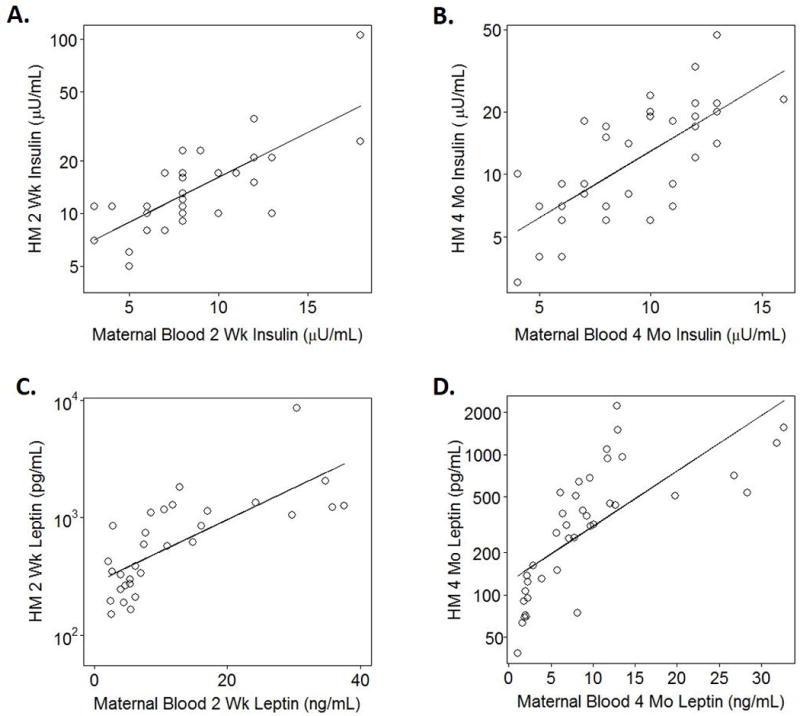
A–B) Maternal fasting plasma insulin concentrations circulation were significantly and positively related to insulin concentrations in Human Milk (HM) at 2-weeks (A: p<0.0001, R2=0.42, n=32) and 4-months (B: p<0.001, R2=0.29, n=38). C–D) Maternal plasma leptin concentrations were significantly and positively related to leptin concentrations in HM at 2-weeks (A: p=0.01, R2=0.19, n=32) and 4-months (B: p<0.001, R2=0.26, n=40).
Although HM adiponectin was not significantly different between groups, maternal BMI at 2-weeks was positively associated with HM adiponectin at 2-weeks (p=0.04, R2=0.09, n=47). Maternal circulating adiponectin concentrations were, however, positively correlated with HM adiponectin concentrations at both 2-weeks (p=0.01, R2=0.19, n=32) and 4-months (p<0.001, R2=0.26, n=40). This relationship between maternal and HM adiponectin was similar in both the NW and OW/Ob group, as indicated by the non-significant interaction term.
Neither maternal BMI, weight loss, parity, age, nor infant sex were consistently related to other HM analytes (macronutrients, cytokines, hormones, and markers of oxidative stress).
DISCUSSION
This study is unique in the prospective longitudinal design and comprehensive characterization of HM composition across a broad range of BMI. Our novel assessment of a marker of oxidative stress in HM (8OHdG), along with biomarkers in maternal circulation substantially extends our understanding of the drivers of HM composition. In addition to these novel study components, we tightly controlled our sampling protocol and collected only fasting HM and blood samples – providing our data a degree of rigor lacking in other studies of HM composition. Given the depth of our characterization, it is of significant clinical importance, and is reassuring, that we found few differences in HM composition between maternal BMI groups. Maternal BMI was consistently related to differences in HM insulin and leptin only.
Several other studies have noted that HM leptin concentrations are related to maternal BMI (25–27). Smaller studies that included assessment of maternal circulating leptin have shown tight correlations with those in HM (25, 26), as we also find. However, maternal BMI seems to explain just as much, if not more, variation in HM leptin than does maternal leptin concentrations (Figure 2).
One of the unique findings in our study is the relationship between maternal fasting insulin and HOMA-IR with HM insulin concentrations, since insulin in HM has been much less-studied. Other small studies have reported similar or slightly lower HM concentrations than those detected in our cohort (28, 29), but few studies have investigated the determinants of HM insulin concentrations, or controlled for maternal prandial state. One study reported that maternal BMI as well as hyperglycemia and insulin resistance during pregnancy were both related to elevated insulin in HM (29), similar to our findings. Neither study measured concurrent maternal insulin concentrations. Our novel data suggest that maternal plasma insulin concentration and insulin sensitivity impact HM insulin concentrations more than maternal BMI (Figure 3).
Our striking finding that insulin levels in HM are higher than maternal insulin (Figure 2) concentrations suggests that insulin must be either actively transported from maternal circulation into milk, and/or synthesized in the mammary gland. This finding is of particular note from an infant perspective. As HM insulin rises after a meal (along with maternal insulin) (30), the insulin concentrations we detect in fasting HM likely represent the nadir of infant insulin exposure via HM. Furthermore, HM insulin concentrations at 2-weeks and 4-months were tightly correlated, meaning that many infants were chronically exposed to relatively high doses of oral insulin throughout these critical early months of lactation. The concentrations of fasting HM insulin at 2-weeks were, on average, 5.6 fold higher (p<0.0001) than previously published data of circulating infant insulin concentrations at 1-week (n=366) (31). This may be particularly physiologically relevant as infant intestinal tight junctions remain open during this early neonatal period, and oral insulin impacts infant intestinal maturation and development of the microbiome (32, 33).
Adiponectin has been well-studied in HM. We did not find a difference in maternal serum or HM adiponectin between maternal BMI groups, but did detect a positive relationship between maternal BMI and HM adiponectin at 2-weeks, consistent with previous studies (22, 34). Both groups were actively losing weight throughout the course of the study which may have masked underlying differences in maternal adiponectin.
Ghrelin in HM is much less- -understood. Ghrelin has been reported to both increase (35) and decrease (36) in HM over the course of lactation. Our longitudinal data suggest that it does indeed decrease (Table 2). The drivers of HM ghrelin are equally unknown as concentrations are reported to both correlate with (37), and be unrelated to (35) circulating maternal ghrelin concentrations. We do not detect any relationship between maternal and HM ghrelin concentrations, in a fasting state. This lack of a relationship between maternal blood and HM concentrations is particularly interesting as it differs from all other hormones assessed (adiponectin, leptin, and insulin). HM ghrelin concentrations did not differ between groups, unlike maternal serum ghrelin. Thus, any potential effect of HM ghrelin on infant appetite regulation seems equivalent across maternal BMI categories.
A novelty of our study was assessment of bioactive compounds in HM that are associated with metabolic derangement – namely inflammatory cytokines and markers of oxidative stress. 8-hydroxy-2-deoxy-guanosine (8OHdG), a marker of oxidative stress, has been detected in HM (38), but never studied. TNF-α, IL-6, and IL-8 are all inflammatory cytokines found in HM at widely varied concentrations (39) and are presumed to be active and impact infant development (40).
Both 8OHdG, and all three inflammatory cytokines decreased in milk over time in this cohort. Contrary to our hypothesis, we found virtually no differences in the inflammatory or oxidative stress profiles according to maternal BMI status. This finding duplicates similar data from our lab in a different cohort of normoglycemic obese and lean women (under review). However, concentrations of these markers in maternal circulation were not different between maternal BMI groups. This may be due to the metabolic demands of lactation and peri-partum stress response, which may mask any underlying differences in chronic low grade inflammation and oxidative stress that accompany obesity.
One of the notable strengths of this study was its longitudinal design which provided serial collections of HM and blood samples at discreet time points allowing us to assess dynamic relationships over time in both maternal circulation and HM. The controlled HM sampling procedures (all morning and fasted samples) and number of analytes measured in HM set this study apart from other published reports. Furthermore, our paired phenotyping of both HM and maternal serum in addition to a wide range in maternal BMI allowed for inferences about which maternal characteristics have the greatest impact on HM composition. While our sampling schema was well-controlled, collection of mid-feed HM samples did limit our ability to precisely measure analytes that change over the course of a feed, such as milk fat and calories.
This study is the first investigation to deeply characterize milk composition longitudinally in concert with maternal metabolic phenotype. It adds to our understanding of the driving forces regulating HM composition and of infant exposure to non-nutritive bioactive compounds in HM over time. The general lack of differences in milk composition between NW and OW/Ob mothers is both striking and reassuring to obese breastfeeding women. The elevations in HM insulin in OW/OB women compared to NW women in this normoglycemic cohort may be driven by maternal insulin concentrations, or insulin sensitivity. The fact that insulin concentrations in HM surpass those in maternal circulation suggest that infants are exposed to chronic high doses of oral insulin, especially if breastfed by an obese mother. The ramifications of such exposure necessitate further study.
Acknowledgments
We wish to deeply thank the mothers and infants who participated in this research. We also acknowledge
Claire Westcott, BS; Catherine Chartier-Logan, MPH; Melanie Reece, PhD; and Regina Reynolds, MD for support with study execution.
FUNDING
National Institute of Health (NIH)/NICHD F32-HD0978068 (PI: BEY)
Thrasher Research Fund Early Career Award (PI: BEY)
Center for Women’s Health Research at the University of Colorado Anschutz Medical Campus (PI: BEY)
Colorado Clinical & Translational Sciences Institute (CCTSI) with the Development and Informatics Service Center (DISC) grant support (Colorado CTSI Grant Number NIH/NCATS UL1-TR001082; PI: BEY)
NIH/NIDDK K24-DK083772 (PI: NFK)
NIH/NIDDK T32-DK007658-21 (PI: NFK)
Footnotes
Conflict of Interest:
The Authors have no conflicts of interest to disclose.
References
- 1.Young BE, Johnson SL, Krebs NF. Biological determinants linking infant weight gain and child obesity: current knowledge and future directions. Advances in Nutrition. 2012;3(5):675–86. doi: 10.3945/an.112.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breastfeeding; AAoPSo. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e41. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 3.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. The Cochrane database of systematic reviews. 2012;8:CD003517. doi: 10.1002/14651858.CD003517.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115(5):1367–77. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 5.Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–90. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 6.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. 2006;84(5):1043–54. doi: 10.1093/ajcn/84.5.1043. [DOI] [PubMed] [Google Scholar]
- 7.Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):30–7. doi: 10.1111/apa.13133. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Kaur H, Choi WS, Huang TT, Lee RE, Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res. 2005;13(2):362–71. doi: 10.1038/oby.2005.48. [DOI] [PubMed] [Google Scholar]
- 9.Buyken AE, Karaolis-Danckert N, Remer T, Bolzenius K, Landsberg B, Kroke A. Effects of breastfeeding on trajectories of body fat and BMI throughout childhood. Obesity. 2008;16(2):389–95. doi: 10.1038/oby.2007.57. [DOI] [PubMed] [Google Scholar]
- 10.Beyerlein A, von Kries R. Breastfeeding and body composition in children: will there ever be conclusive empirical evidence for a protective effect against overweight? Am J Clin Nutr. 2011;94(6 Suppl):1772S–5S. doi: 10.3945/ajcn.110.000547. [DOI] [PubMed] [Google Scholar]
- 11.Barbosa L, Butte NF, Villalpando S, Wong WW, Smith EO. Maternal energy balance and lactation performance of Mesoamerindians as a function of body mass index. The American Journal of Clinical Nutrition. 1997;66(3):575–83. doi: 10.1093/ajcn/66.3.575. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja S, Boylan M, Hart S, Roman-Shriver C, Spallholz J, Pence B, et al. Glucose and Insulin Levels are Increased in Obese and Overweight Mothers’ Breast-Milk. Food and Nutrition Sciences. 2011;2:201–6. [Google Scholar]
- 13.Andreas NJ, Hyde MJ, Gale C, Parkinson JR, Jeffries S, Holmes E, et al. Effect of maternal body mass index on hormones in breast milk: a systematic review. PLoS One. 2014;9(12):e115043. doi: 10.1371/journal.pone.0115043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields DA, Demerath EW. Relationship of insulin, glucose, leptin, IL-6 and TNF-alpha in human breast milk with infant growth and body composition. PediatrObes. 2012;7(4):304–12. doi: 10.1111/j.2047-6310.2012.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R768–78. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- 17.Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52(6):913–20. doi: 10.1016/j.jhep.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 18.Du Y, Yang M, Lee S, Behrendt CL, Hooper LV, Saghatelian A, et al. Maternal western diet causes inflammatory milk and TLR2/4-dependent neonatal toxicity. Genes Dev. 2012;26(12):1306–11. doi: 10.1101/gad.191031.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW. Breast-feeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diabetes Care. 2006;29(10):2231–7. doi: 10.2337/dc06-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beyerlein A, Toschke AM, von Kries R. Breastfeeding and childhood obesity: shift of the entire BMI distribution or only the upper parts? Obesity (SilverSpring) 2008;16(12):2730–3. doi: 10.1038/oby.2008.432. [DOI] [PubMed] [Google Scholar]
- 21.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016;315(21):2292–9. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weyermann M, Brenner H, Rothenbacher D. Adipokines in human milk and risk of overweight in early childhood: a prospective cohort study. Epidemiology. 2007;18(6):722–9. doi: 10.1097/ede.0b013e3181567ed4. [DOI] [PubMed] [Google Scholar]
- 23.Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant Feeding Practices Study II: study methods. Pediatrics. 2008;122(Suppl 2):S28–35. doi: 10.1542/peds.2008-1315c. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence RAL, R M. Breastfeeding - A Guide for the Medical Professional. Elsevier Mosby; 2011. p. 7. [Google Scholar]
- 25.Schuster S, Hechler C, Gebauer C, Kiess W, Kratzsch J. Leptin in maternal serum and breast milk: association with infants’ body weight gain in a longitudinal study over 6 months of lactation. Pediatr Res. 2011;70(6):633–7. doi: 10.1203/PDR.0b013e31823214ea. [DOI] [PubMed] [Google Scholar]
- 26.Miralles O, Sanchez J, Palou A, Pico C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity(SilverSpring) 2006;14(8):1371–7. doi: 10.1038/oby.2006.155. [DOI] [PubMed] [Google Scholar]
- 27.Eilers E, Ziska T, Harder T, Plagemann A, Obladen M, Loui A. Leptin determination in colostrum and early human milk from mothers of preterm and term infants. Early Hum Dev. 2011;87(6):415–9. doi: 10.1016/j.earlhumdev.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Shehadeh N, Khaesh-Goldberg E, Shamir R, Perlman R, Sujov P, Tamir A, et al. Insulin in human milk: postpartum changes and effect of gestational age. Arch Dis Child Fetal Neonatal Ed. 2003;88(3):F214–6. doi: 10.1136/fn.88.3.F214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ley SH, Hanley AJ, Sermer M, Zinman B, O’Connor DL. Associations of prenatal metabolic abnormalities with insulin and adiponectin concentrations in human milk. AmJ Clin Nutr. 2012;95(4):867–74. doi: 10.3945/ajcn.111.028431. [DOI] [PubMed] [Google Scholar]
- 30.Koldovsky O. Hormones in milk. Vitam Horm. 1995;50:77–149. doi: 10.1016/s0083-6729(08)60655-x. [DOI] [PubMed] [Google Scholar]
- 31.Shields BM, Knight B, Shakespeare L, Babrah J, Powell RJ, Clark PM, et al. Determinants of insulin concentrations in healthy 1-week-old babies in the community: applications of a bloodspot assay. Early Hum Dev. 2006;82(2):143–8. doi: 10.1016/j.earlhumdev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Lemas DJ, Young BE, Baker PR, 2nd, Tomczik AC, Soderborg TK, Hernandez TL, et al. Alterations in human milk leptin and insulin are associated with early changes in the infant intestinal microbiome. Am J Clin Nutr. 2016;103(5):1291–300. doi: 10.3945/ajcn.115.126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shehadeh N, Sukhotnik I, Shamir R. Gastrointestinal tract as a target organ for orally administered insulin. J Pediatr Gastroenterol Nutr. 2006;43(3):276–81. doi: 10.1097/01.mpg.0000226377.03247.fb. [DOI] [PubMed] [Google Scholar]
- 34.Martin LJ, Woo JG, Geraghty SR, Altaye M, Davidson BS, Banach W, et al. Adiponectin is present in human milk and is associated with maternal factors. The American Journal of Clinical Nutrition. 2006;83(5):1106–11. doi: 10.1093/ajcn/83.5.1106. [DOI] [PubMed] [Google Scholar]
- 35.Ilcol YO, Hizli B. Active and total ghrelin concentrations increase in breast milk during lactation. Acta Paediatr. 2007;96(11):1632–9. doi: 10.1111/j.1651-2227.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 36.Karatas Z, Durmus Aydogdu S, Dinleyici EC, Colak O, Dogruel N. Breastmilk ghrelin, leptin, and fat levels changing foremilk to hindmilk: is that important for self-control of feeding? Eur J Pediatr. 2011;170(10):1273–80. doi: 10.1007/s00431-011-1438-1. [DOI] [PubMed] [Google Scholar]
- 37.Savino F, Benetti S, Lupica MM, Petrucci E, Palumeri E, Cordero di Montezemolo L. Ghrelin and obestatin in infants, lactating mothers and breast milk. Horm Res Paediatr. 2012;78(5–6):297–303. doi: 10.1159/000345876. [DOI] [PubMed] [Google Scholar]
- 38.Lam PM, Mistry V, Marczylo TH, Konje JC, Evans MD, Cooke MS. Rapid measurement of 8-oxo-7,8-dihydro-2'-deoxyguanosine in human biological matrices using ultra-high-performance liquid chromatography-tandem mass spectrometry. Free RadicBiolMed. 2012;52(10):2057–63. doi: 10.1016/j.freeradbiomed.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groer MW, Shelton MM. Exercise is associated with elevated proinflammatory cytokines in human milk. JObstetGynecolNeonatal Nurs. 2009;38(1):35–41. doi: 10.1111/j.1552-6909.2008.00303.x. [DOI] [PubMed] [Google Scholar]
- 40.Garofalo R. Cytokines in human milk. The Journal of Pediatrics. 2010;156(2 Suppl):S36–S40. doi: 10.1016/j.jpeds.2009.11.019. [DOI] [PubMed] [Google Scholar]


