Abstract
Protruding from the apical surfaces of epithelial cells are specialized structures, including cilia, microplicae, microvilli, and stereocilia. These contribute to epithelial function by cushioning the apical surface, by amplifying its surface area to facilitate nutrient absorption, and by promoting sensory transduction and barrier function. Despite these important roles, and the diseases that result when their formation is perturbed, there remain significant gaps in our understanding of the biogenesis of apical protrusions, or the pathways that promote their organization and orientation once at the apical surface. Here, I review some general aspects of these apical structures, and then discuss our current understanding of their formation and organization with respect to proteins that specify apicobasolateral polarity and planar cell polarity.
The apical surface of epithelial cells, which lines the lumen of sac- and tube-shaped organs and the inner surfaces of the body cavities, forms the interface between the extracellular milieu and underlying tissues. It has numerous functions, including absorption and secretion, immunological surveillance, sensory transduction, and barrier formation. These functions are made possible by several specializations, including the glycocalyx, which cushions and hydrates the apical membrane, the lipids of the apical plasma membrane, which limit water and solute flux, and channels and receptors, which regulate active and passive transport and sense the presence of growth factors, cytokines, and mechanical stimuli (e.g., stretch and shear stress). Finally, projecting from the apical domain of epithelial cells is one or more of the following apical protrusions: cilia, microplicae, microvilli, or stereocilia (Fig. 1). Below, I review the form and function of these apical protrusions, I summarize the polarity proteins that specify apical identity and that organize the apical surfaces of epithelial cells, and then I describe our current understanding of how these polarity proteins promote the biogenesis and organization of these structures. An expanded review of epithelial polarity is available elsewhere (Apodaca and Gallo 2013).
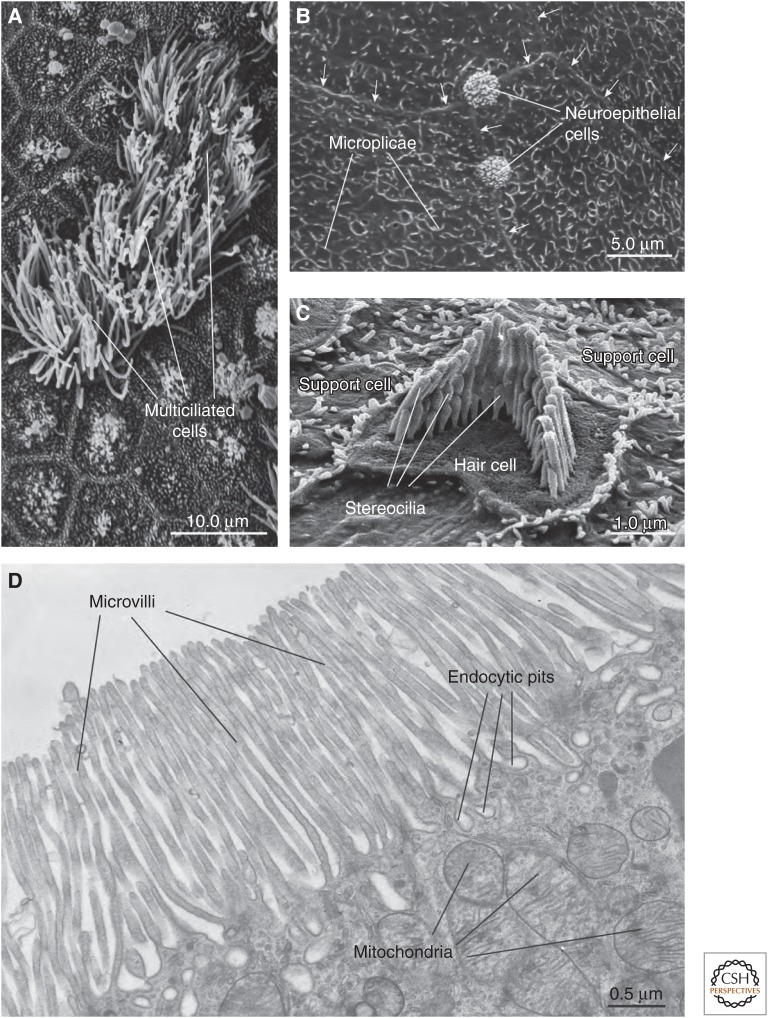
Figure 1.
Survey of apical surface protrusions found in epithelial cells. (A) Scanning electron micrograph (SEM) of the apical surface of the rat trachea showing multiciliated cells. Adjacent cells are nonciliated or have rudimentary cilia. (B) SEM of the mucosal surface of the rat proximal urethra. Microplicae are observed at the apical surface of umbrella cells found in this region. Arrows mark the junctional ring of adjacent cells. The apical surfaces of neuroepithelial cells, which are covered by an apical tuft of short microvilli, are interspersed between adjacent cells. (C) SEM of the cochlea of the adult mouse showing a hair cell with associated “hair bundle,” which is comprised of stereocilia arranged in a stair-step configuration. The apical surfaces of adjacent support cells are studded with microvilli. (D) Transmission electron micrograph of microvilli at the apical surfaces of the rat proximal tubule epithelial cells. Endocytic pits and mitochondria are marked. (Electron micrograph in panel C was kindly provided by Jonathan Franks, Center for Biological Imaging, University of Pittsburgh; and micrographs in panels A, B, and D were kindly provided by Wily G. Ruiz, Kidney Imaging Core, University of Pittsburgh.) (Figure is from Apodaca and Gallo 2013; adapted, with permission, from the authors.)
APICAL MEMBRANE PROTRUSIONS
Cilia
These are projections, 2–20 µm in length, that extend from the apical surface of epithelial cells (Fig. 1A) (Ishikawa and Marshall 2011). They have been the focus of intense research as of late, because defects in cilia formation and function lead to a variety of human diseases known as ciliopathies (Waters and Beales 2011). Cilia are the only apical protrusion that contain microtubules at their core, and motile cilia in particular have a peculiar 9+2 organization in which nine peripheral doublets of microtubules surround two single centrally localized ones (Fig. 2A,B). These microtubules are cross-linked by axonemal dyneins, which promote ciliary beating as a result of their intrinsic ATPase activity. Some cells such as the epithelial cells lining the airways (e.g., tracheal epithelial cells) and the ependymal cells lining the brain ventricles have multiple, motile cilia. However, the majority of cells in the body contain a single, “primary” cilium some time during their development (Ishikawa and Marshall 2011). Primary cilia play important roles in development and sensory perception (Tasouri and Tucker 2011). The latter function is thought to be mediated in part by the ability of cilia to initiate Ca2+ signaling in response to mechanical deflection, although this role has recently been questioned (Delling et al. 2013, 2016). The microtubules in primary cilia are arranged in a 9+0 pattern: Nine outer doublets are found at the periphery, but the two central microtubules are absent (Fig. 2A). Because they lack dynein arms, 9+0 cilia are most often immotile; however, the 9+0 cilia of nodal cells are motile, undergo clockwise rotation, and play a role in generating left–right asymmetry in the developing embryo (Hirokawa et al. 2009).
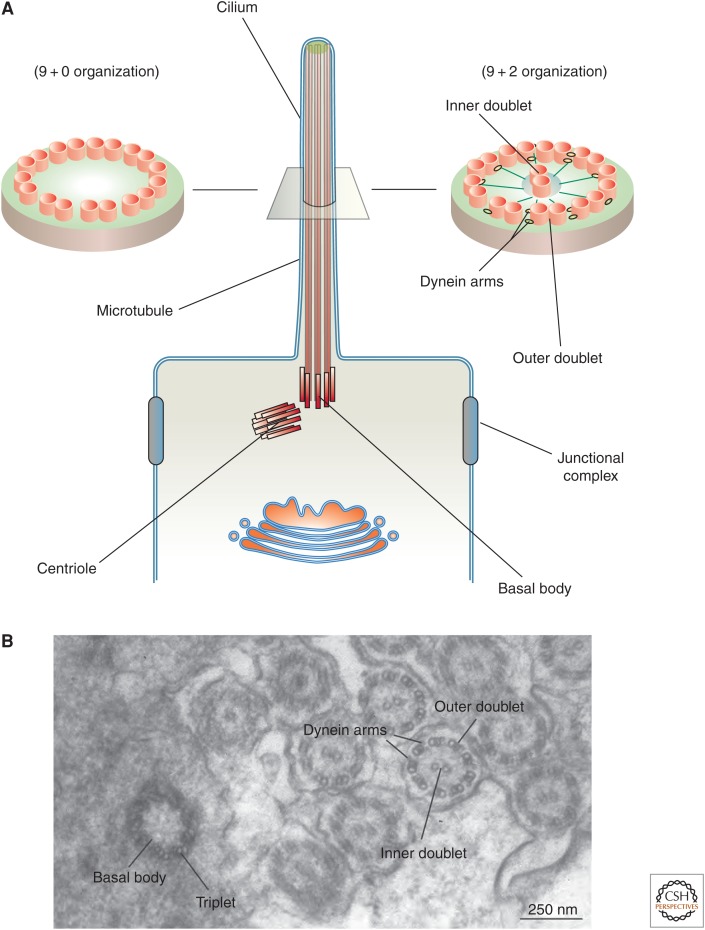
Figure 2.
Structure of cilia. (A) Cilia, which project from the apical surface of epithelial cells, have two types of organization. Motile cilia have a 9+2 organization in which a ring of nine microtubule doublets surrounds an inner microtubule doublet. Inner rays and dynein motor arms are present. Immotile cilia, such as so-called “primary cilia” have an outer ring of nine doublets, but lack the inner doublet, dynein arms, and rays. In both types of cilia, a basal body, derived from the centriole, is present and in cross section is comprised of nine bundles of microtubules arranged as triplets (see panel B). (B) Transmission electron micrograph of the zebrafish larval pronephros, which contains multiciliated cells that project cilia into the lumen of this tubular organ. A cross section through a basal body and through the axoneme highlights the structures described above. (Electron micrograph in panel B was kindly provided by Wily Ruiz, Kidney Imaging Core, University of Pittsburgh.) (Panel A from Apodaca and Gallo 2013; adapted, with permission, from the authors.)
Structurally, the base of each cilium is anchored by a cytoplasmic basal body, which in the case of primary cilia is derived from the older, “mother centriole” (Figs. 2 and 3) (Kobayashi and Dynlacht 2011). Basal bodies are composed of nine triplets of microtubules but have no central microtubules. Above this structure is a transition zone that leads to the main body of the cilium called the axoneme. This zone controls the entry and exit of proteins from the cilium and is comprised of transitional fibers, Y-shaped links, and the ciliary necklace (Fig. 3). Transitional fibers link the basal body to the ciliary membrane. Septins regulate the assembly of the nine-subunit B9 complex, which along with other proteins form the Y-shaped links that couple the microtubules to the ciliary necklace that surrounds the transition zone (Fig. 3) (Hu et al. 2010; Chih et al. 2012).
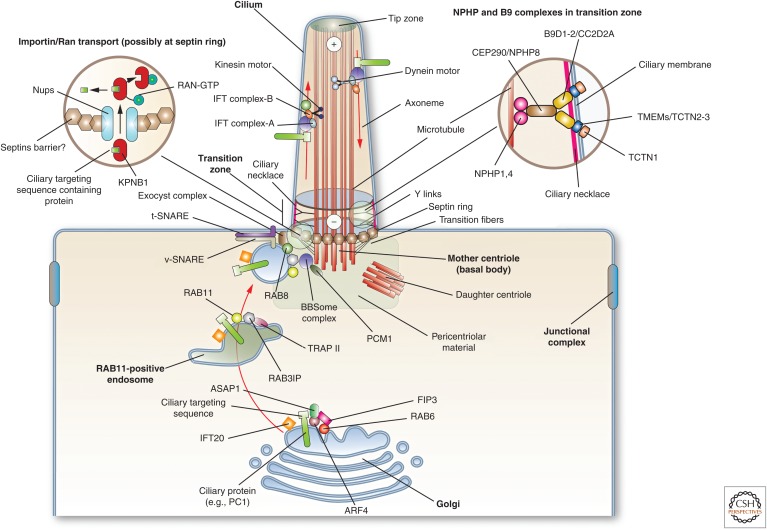
Figure 3.
Mechanisms of protein transport into cilia. At the Golgi, cilia resident proteins are recognized and packaged for delivery. In the case of PC1, its ciliary targeting sequence is recognized by ARF4, and is then delivered to RAB11-positive endosomes by way of a RAB6-, ARF4-, ASAP1-, and FIP3-dependent mechanism. The only intraflagellar transport (IFT) protein localized to the Golgi, IFT20, may have a role in promoting protein delivery to the cilium. At RAB11-positive endosomes, RAB11 recruits the TRAPII complex and the RAB8 GEF RAB3IP. The latter interacts with the basal body–localized BBSome complex (through the BBS1 subunit), and also recruits and activates RAB8. The BBSome is recruited by PCM1. RAB8 along with the exocyst complex and SNAREs, promotes the docking and fusion of the endocytic vesicle at the base of the cilium. Entry into the cilium is regulated at the transition zone by a septin ring, and the Y-links formed by the NPHP and B9 protein complexes. In addition, import into the cilium may depend on a RAN-GTP/GDP gradient (cytoplasmic RAN-GDP is not shown), which regulates KPNB1 binding to ciliary proteins and their subsequent transport across cilia-associated nucleoporins. Once in the axoneme, the ciliary proteins move along the microtubules by interactions with IFT particles and motor proteins. Anterograde traffic (directed toward the tip of the cilium) is mediated by IFT-B and KIF3 motor complex, whereas retrograde is mediated by IFT-A and the dynein-2 motor complex. The BBSome regulates the IFT complexes assembly at the basal body and its turnaround from anterograde to retrograde transport. (Figure from Apodaca and Gallo 2013; adapted, with permission, from the authors.)
All ciliary proteins must be transported from the cell proper to the axoneme (Fig. 3). These events require ciliary targeting sequences (e.g., V-X-P-X) (Wang and Deretic 2014). The GTPase requirements for the ciliary traffic of PC1 (alias polycystin-1) are defined: Its exit from the trans-Golgi network (TGN) depends on ARF4A (which recognizes the ciliary targeting sequence in PC1), the Arf GTPase-activating protein ASAP1, and RAB6 (Mazelova et al. 2009a; Ward et al. 2011; Wang et al. 2012), and its subsequent transport to the cilia occurs by way of endosomes that are RAB11A, RAB3IP (alias Rabin8), RAB8A, and TRAPPII complex positive (Knodler et al. 2010; Westlake et al. 2011). Apparently, this pathway is not shared by all cilia-localized proteins as Kim1 uses a pathway dependent on RAB5/RAB8 and Smoothened requires RAB8/RAB23 (Boehlke et al. 2010). Final fusion is likely mediated by the eight-subunit exocyst “tethering factor,” subunits of which are found in the cilium, along with SNAP25 and STX3 (alias syntaxin3) (Mazelova et al. 2009b; Zuo et al. 2009; Fogelgren et al. 2011; Feng et al. 2012). Additional components implicated in transport into the cilium include the BBSome, a family of eight conserved proteins mutated in patients with Bardet–Biedl syndrome (Nachury et al. 2007; Knodler et al. 2010), other centrosomal components (e.g., PCM1 and CEP290) (Kim et al. 2008), as well as the Ran GTPase, KPNB1 (alias β−importin), and nucleoporins (Lechtreck et al. 2009; Dishinger et al. 2010; Fan et al. 2011; Hurd et al. 2011; Kee et al. 2012; Wei et al. 2012). Once delivered to the axoneme, intraflagellar transport (IFT) particles, comprised of IFT-A and IFT-B protein complexes, move ciliary components from the basal body to the tip of the cilia by interaction with the heterotrimeric motor protein KIF3 (alias Kinesin-2) (Fig. 3). Movements in the reverse direction are mediated by IFT-dynein 1b complexes. Only one IFT protein, IFT20 (a component of IFT-B), associates with RAB8A and traffics from the Golgi to the base of the cilium (Follit et al. 2006; Omori et al. 2008).
Microplicae
Whereas cilia are well-studied microtubule-based apical protrusions, much less is known about actin-rich microplicae. These are ridge-like folds of the plasma membrane and are found at the apical surfaces of stratified epithelia including those lining the cornea, alimentary tract (e.g., oral mucosa, fungiform, and circumvallate papillae of the tongue, esophagus, anal canal), reproductive tract (e.g., vagina, vulva, and the fallopian tubes of postmenopausal women), and lower urinary tract including the ureters, bladder, and upper urethra (Fig. 1B) (Andrews 1976; Collin and Collin 2006; Correr et al. 2006; Ovalle and Nahirney 2008; Khandelwal et al. 2009; Julio et al. 2010; Asikainen et al. 2012). Even nonstratified, “simple” epithelia (e.g., the intercalated cells of the kidney) can have microplicae (Evan et al. 1991). When cross-sectioned and examined by transmission electron microscopy, microplicae appear as short microvilli; however, when examined by scanning electron microscopy, they form extensive ridge-like structures that are approximately 100–200 nm in width and 200–800 nm in height (Andrews 1976). They can be straight or curved, and oftentimes show branching giving the impression of a labyrinthine organization. Whether microplicae are found at the basolateral surface of cells remains unclear, although based solely on transmission electron microscopy this was proposed to be the case (Andrews 1976). The extracellular surface of microplicae are studded with large, O-glycosylated, transmembrane mucins (Blalock et al. 2007; Asikainen et al. 2012), whereas the cytoplasmic surface is electron dense and contains a loosely organized cytoskeleton comprised of randomly oriented actin filaments (Anderson 1977). Despite their relative abundance in the body, the function of microplicae is not well understood. One possibility is that the associated mucins help to cushion the apical surface, protecting it from abrasion and mechanical damage.
Microvilli and the Apical Brush Border
Unlike microplicae, which are ridge-like and lack an organized actin cytoskeleton, microvilli are finger-like protrusions (1–2 µm long) that contain a core of highly organized bundled actin microfilaments (Figs. 1D and 4). The latter are anchored in the subjacent terminal web. Microvilli are commonly found on epithelia that recover nutrients or water and solutes from the adjacent lumen, such as the enterocytes in the small intestine and the proximal tubule cells of the kidney (Crawley et al. 2014a). In these later two cell types, the microvilli are densely packed and uniform in length and appearance, forming a “brush border” that increases the apical surface area by 15- to 30-fold (Crawley et al. 2014a). The brush border of proximal tubule epithelial cells contains a specialized intermicrovillar domain, less apparent in enterocytes, which contains coated pits and associated endocytic machinery (see Fig. 1D) (Larsson 1975; Biemesderfer et al. 1992).
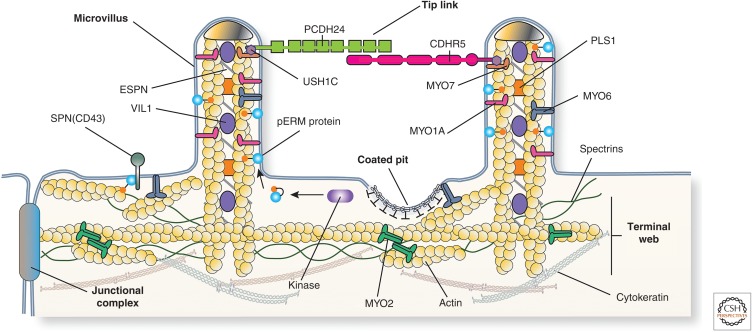
Figure 4.
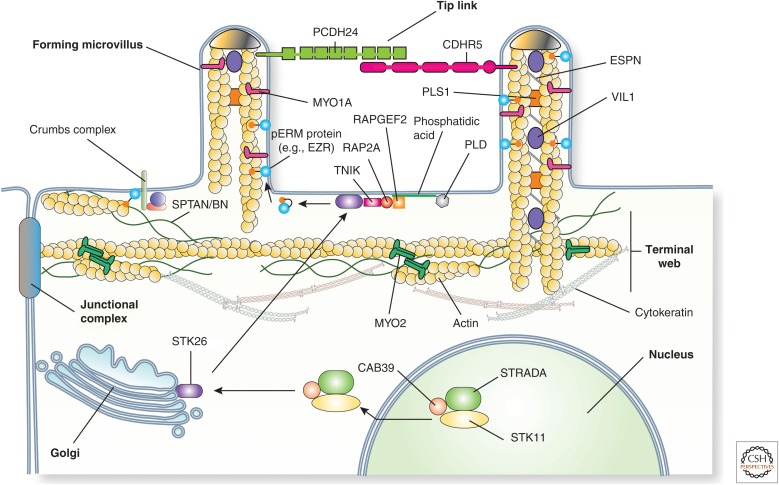
Protein constituents of the epithelial cell brush border. The brush border of enterocytes and kidney proximal tubule epithelial cells is comprised of a regular array of microvilli. Each microvillus contains a core of actin, which is associated with MYO1A, ESPN, PLS1, VIL1, and ERM proteins such as EZR. In their active, phosphorylated state, ERM proteins link the actin cytoskeleton to the membrane, in some cases via the membrane protein SPN (alias CD43). The microvillus actin terminates in “rootlets” that associate with actin, spectrins, MYO2, and cytokeratins to form the terminal web. Adjacent microvilli are linked to one another by tip links, which are comprised of the protocadherins PCDH24 and CDHR5. These proteins are anchored to the actin filaments by way of MYO7 and the adaptor protein USH1C. (Figure from Apodaca and Gallo 2013; adapted, with permission, from the authors.)
Each microvillus contains a core of bundled and cross-linked actin filaments, which are associated with VIL1 (alias villin), PLS1 (alias plastin-1/fimbrin), and ESPN (alias espin) (Fig. 4) (Heintzelman and Mooseker 1992; Revenu et al. 2012; Crawley et al. 2014a). The actin filaments terminate as “rootlets,” which are embedded in the terminal web by MYO2 and spectrins (Fig. 4). Other components of the terminal web include TPM (alias tropomyosins), CALD1 (alias caldesmon), and cytokeratins (Takemura et al. 1988; Heintzelman and Mooseker 1992). Brush-border MYO1A forms a helically arranged series of cross bridges that connect the actin bundles of the microvillus to the plasma membrane (Tyska et al. 2005). Other associated myosins include MYO6, which links the apical surface with the underlying actin cytoskeleton (Ameen and Apodaca 2007; Hegan et al. 2015a), and MYO7B, which is associated with the electron dense distal tips of microvilli (Wolfrum et al. 1998). The latter also forms interactions between the cytoplasmic scaffolding protein USH1C (alias harmonin-a) and the transmembrane protocadherins PCDH24 (alias protocadherin-24) and CDHR5 (alias the mucin-like protocadherin) (Fig. 4) (Reiners et al. 2005, 2006; Crawley et al. 2014b). By forming trans-heterophilic interactions, these protocadherins generate the thread-like links that adhere adjacent microvilli in the brush border to one another in a manner analogous to the tip links observed in stereocilia (vide infra). An additional major component of microvilli is the EZR (alias ezrin)-RDZ (alias radixin)-MSN (alias moesin) (ERM) family of proteins (Bretscher et al. 2002). In the presence of PtdIns(4,5)P2, and on phosphorylation of a conserved threonine residues (T567 in EZR), ERM proteins leave their autoinhibited state and form interactions with membrane proteins (via an amino-terminal FERM domain), regulatory molecules, and the microvillar bundles of actin (by way of a carboxy-terminal actin-binding domain) (Bretscher et al. 2002). In addition to amplifying apical surface area, microvilli are also important for sensory perception, flow sensation, ion channel regulation, Ca2+ signaling, and immune system modulation (McConnell et al. 2009; Lange 2011).
Stereocilia
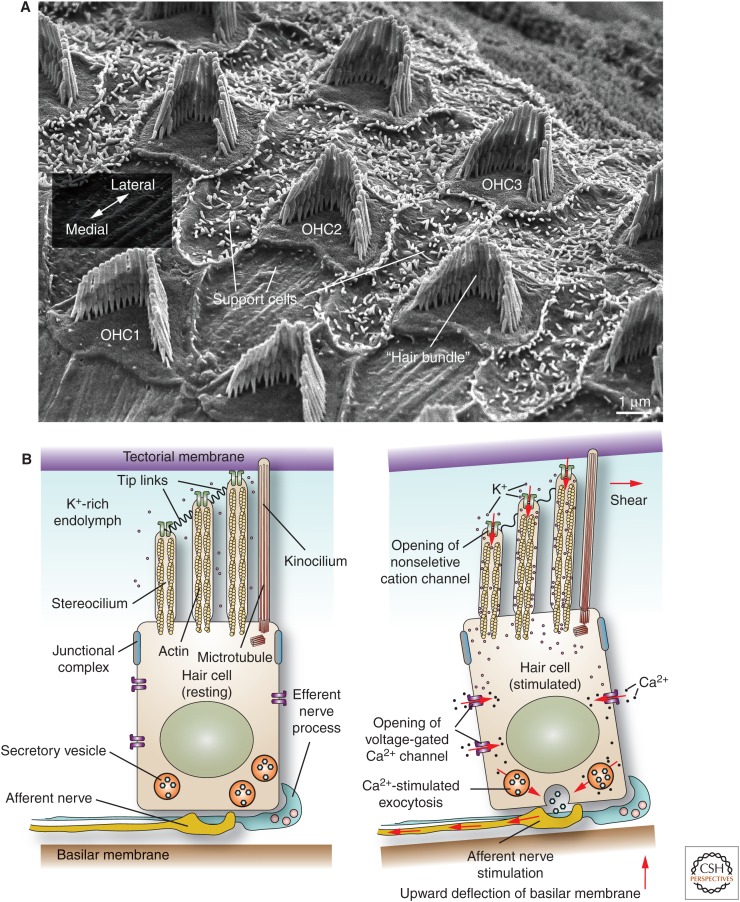
The final actin-based structure discussed are the stereocilia, which are long structures (10–50 µm in length) that protrude from the apical surfaces of the pseudostratified columnar cells that line the epididymis and the vas deferens, and the apical surface of the hair cells of the middle ear (Figs. 1C and 5A) (Ovalle and Nahirney 2008; Drummond et al. 2012; Barr-Gillespie 2015). Although their function in the male reproductive tract is not well delineated, it is hypothesized that the epididymal stereocilia may act to increase surface area and thus promote fluid resorption as developing sperm move from the seminiferous tubules to the epididymis. In contrast, the stereocilia of the middle ear are required for hearing (Drummond et al. 2012; Barr-Gillespie 2015). Sound vibrations, captured by the pinnae, are transmitted by small bones within the inner ear (malleus, incus, and stapes) across the oval window, creating hydraulic waves in the fluid that fills the cochlea. The latter is a spiral canal covered by rows of one inner and three outer epithelial hair cells arranged in a mediolateral orientation, as well as interdigitating support cells (see outer hair cells in Fig. 5A). Hair cells rest on the basilar membrane, which fluctuate as the sound waves pass through the cochlear fluid (Fig. 5B). The epithelium is innervated from below by afferent and efferent nerve processes.
Figure 5.
Organization and function of hair cells in the inner ear. (A) Scanning electron micrograph of mucosal surface of adult mouse cochlea after removal of the overlying tectorial membrane. The position of the three rows of outer hair cells (OHC1-3) with associated hair bundle is indicated. The single row of inner hair cells is not shown. Hair cells are surrounded by support cells. The orientation of the lateromedial axis is shown. (B) (Left panel) The basal surface of the hair cell rests on the basilar membrane, while its apical stereocilia, surrounded by K+-rich endolymph fluid, insert into the tectorial membrane. Stereocilia are attached to one another via tip links. (Right panel) Sound waves, propagated through the cochlear fluid, cause an upward deflection of the basilar membrane, generating a shear force between the tectorial membrane and the stereocilia. The bending of the stereocilia promotes the opening of a mechanosensitive, nonselective cation channel. The inward movement of K+ ions through this channel depolarizes the cell, stimulating the opening of voltage-gated Ca2+ channels, which increase cytosolic Ca2+. The subsequent release of neurotransmitters triggers an action potential in the afferent neuronal processes. (Panel B from Apodaca and Gallo 2013; adapted, with permission, from the authors.)
Protruding from the apical surfaces of the hair cells is a “hair bundle,” comprised of 100–200 stereocilia, which are organized in a staircase-like manner that forms a “V” pattern (Figs. 1C and 5A) (Drummond et al. 2012; Barr-Gillespie 2015). The stereocilia are connected to one another by fine tip links, which are comprised of CDH23 (alias cadherin-23) and PCDH15 (alias protocadherin-15) (Nayak et al. 2007). Other links are found at the base of the stereocilia (Nayak et al. 2007). Just behind the apex of the stereocilia bundle lies a single microtubule-rich kinocilium, which in cochlear hair cells disappears soon after birth (Barr-Gillespie 2015). The tips of the stereocilia are embedded within the overlying tectorial membrane. In response to sound waves, upward movements of the basilar membrane causes the stereocilia to bend, gating open a mechanically sensitive ion channel, which allows for the influx of potassium ions into the cell (Fig. 5B) (Richardson et al. 2011). The resulting depolarization opens voltage-gated calcium channels that conduct calcium inward, triggering the release of neurotransmitters that stimulate action potentials in nearby sensory nerve processes.
POLARITY PROTEINS AND THEIR FUNCTIONS
Temporally, apical protrusions develop once the epithelial cell establishes distinct apical and basolateral plasma membrane domains separated by a junctional complex. The generation of this apicobasolateral polarity is a complex process that requires membrane trafficking events, the cytoskeleton, signaling cascades, and polarity proteins, which specify and reinforce cortical membrane domain identity (Bryant and Mostov 2008; Datta et al. 2011). In addition to apicobasolateral polarization, which occurs in an orientation that is orthogonal to the tissue plane, epithelia also show polarity in an axis that runs parallel to the apical surface of the tissue. This latter type of polarity, called “planar cell polarity” (PCP), accounts for the orientation of hairs, feathers, scales, insect bristles, and cilia, as well as polarized cell division, coordinated beating of cilia, and collective cell migration (Devenport 2014, 2016; Sebbagh and Borg 2014). The latter includes convergent extension, a process in which sheets of cells converge in one axis and then extend in the other and is important during gastrulation and neurulation. In the following section, a brief overview of the proteins involved in apicobasolateral polarity and PCP is provided; however, those readers seeking additional information about these pathways are directed to other reviews in the literature (Suzuki and Ohno 2006; Assemat et al. 2008; Schluter and Margolis 2012; Devenport 2014, 2016; Sebbagh and Borg 2014).
Apicobasolateral Polarity
The development of apicobasolateral polarity depends on the activity of at least three highly conserved polarity complexes, originally described in Drosophila and Caenorhabditis elegans (Suzuki and Ohno 2006; Assemat et al. 2008; Bryant and Mostov 2008). These include the following (see Table 1 for summary of these and other polarity genes described in this review) (Fig. 6A): (1) The Drosophila Crumbs complex is comprised of Crumbs (CRB1-3 isoforms in vertebrates), Stardust (MPP5 in vertebrates; alias PALS1), and Discs lost (INADL and MUPP1 isoforms in vertebrates; alias PATJ). In vertebrates, this complex is localized to the apical side of the junctional complex and is required for tight junction formation; (2) the Drosophila Par complex is comprised of atypical protein kinase cells (aPKCs) (PRKCI and Z isoforms in vertebrates), Bazooka (PARD3 and 3B isoforms in vertebrates), and Par-6 (PARD6A, B, G isoforms in vertebrates), as well as the Rho family GTPase Cdc42. The Par complex, like the Crumbs complex, is localized near the junctional complex and modulates its assembly; (3) the Drosophila Scribble complex includes Scribble (SCRIB in vertebrates), Discs large (DLG1-5 isoforms in vertebrates), and Lethal giant larvae (LLGL1-2 isoforms in vertebrates). In vertebrates, the Scribble complex is found along the lateral membrane domain (Schluter and Margolis 2012; Tepass 2012). Although genetically and functionally linked, polarity complexes can form subcomplexes. For example, in Drosophila and vertebrates, Bazooka recruits an aPKC-Par-6 subcomplex to the adherens junction (the apical-most junction in flies), whereas PARD3 recruits the PRKC-PARD6 subcomplex to the tight junction of vertebrates. aPKC-Par-6 and PRKC-PARD6 are released to the apical domain as polarity establishment completes (Suzuki et al. 2002; Harris and Peifer 2005; Horikoshi et al. 2009). It is worth emphasizing that the vertebrate orthologs of these apicobasolateral polarity proteins have a large number of isoforms and splice variants, which can be expressed in a tissue-specific manner (Assemat et al. 2008). However, their tissue-specific functions are often unknown.
Table 1.
Drosophila and vertebrate polarity proteins described in this review
| Drosophila protein | Vertebrate ortholog/paralog | Alias |
|---|---|---|
| Apicobasolateral polarity proteins | ||
| Crumbs | CRB1- 3 | |
| Stardust | MPP5 | PALS1 |
| Discs lost | INADL; MUPP1 | PATJ |
| Atypical protein kinase C | PRKCi; PRKCz | aPKC |
| Bazooka | PARD3, 3B | Par3 |
| Par-6 | PARD6A,B,G | |
| Cdc42 | CDC42 | |
| Scribble | SCRIB | |
| Discs large | DLG1- 5 | |
| Lethal giant larvae | LLGL1-2 | |
| Par-1 | MARK3; MARK2 | |
| 14-3-3ɛ/ζ | YWHAZ; YWHAE | Par5 |
| Yurt | EPB41L5 | Band 4.1 like 5 |
| Coracle | EPB41 | Band 4.1 |
| ATPα/β | ATP1A1-4; ATP1B1 | Sodium potassium ATPase |
| Nrx-IV | CNTNAP1 | Neurexin-4 |
| Lkb1 kinase | STK11 | Lkb1/Par4 |
| Planar cell polarity proteins | ||
| Flamingo | CELSR1-3 | |
| Van Gogh | VANGL1-2 | |
| Prickle | PRICKLE1-4 | |
| Frizzled | FZD | |
| Diego | INVS; DIV | Inversin; diversin |
| Dishevelled | DVL1-3 | |
| - | PTK7 | Protein tyrosine kinase 7 |
| Secretory 24AB | SEC24B | |
| Scribble | SCRIB | |
| Fat | FAT1-4 | |
| Daschous | DCHS1-2 | Protocadherin-16; protocadherin-23 |
| Four jointed | FJX1 | |
| Inturned | INTU | |
| Fuzzy | FUZ | |
| Fritz | WDPCP | BBS15 |
| Flattop | CFAP126 | |
| Inscuteable | INSC | |
| Partner of Inscuteable | GPSM2 | LGN |
| Gαi | GNAI | |
Figure 6.
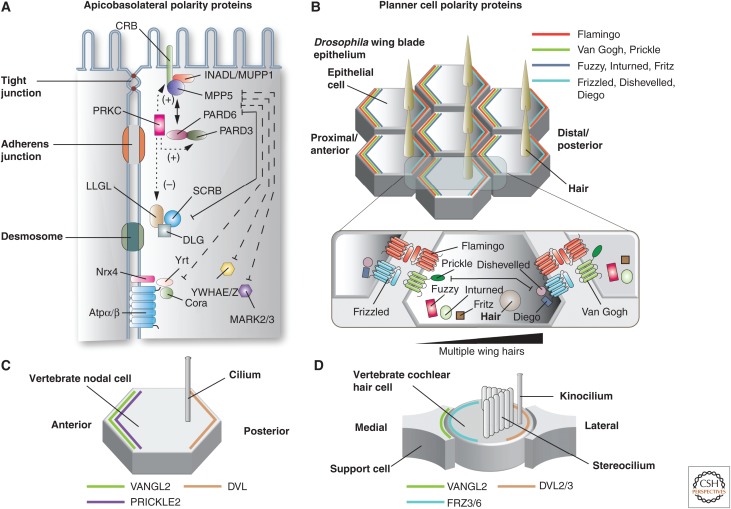
Polarity proteins in epithelial cells. (A) Apicobasolateral polarity proteins. The apical-most Crumbs complex includes the transmembrane proteins CRB1-3 and the cytoplasmic proteins INADL/MUPP1 and MPP5. The other apical complex is the Par complex, which is comprised of PRKC, PARD3, and PARD6. PRKC phosphorylates CRB and PARD3, promoting apical identity. In contrast, PRKC-dependent phosphorylation of LLGL stimulates its degradation, and thus represses basolateral identity. Basolateral polarity is promoted by the Scribble complex, comprised of SCRB, DLG, and LLGL. Additional basolateral polarity proteins include YWHAE/Z and MARK2/3, and the Drosophila Yurt (Yrt)/Coracle (Cora) complex, which includes Yrt, Cora, neurexin4 (Nrx4), and Na+/K+-ATPase α-subunit (NaKα). (Panel A from Apodaca and Gallo 2013; adapted, with permission, from the authors.) (B) Planar cell polarity (PCP) proteins in Drosophila wing blade epithelium. An actin-based “hair” extends from the apical surface of each epithelial cell. The localization of the core PCP anterior complex (Flamingo, Van Gogh, Prickle), the core PCP posterior complex (Frizzled, Dishevelled, and Diego), and PCP effector proteins (Fuzzy, Inturned, and Fritz) is shown at the apical surfaces of wing epithelial cells. A portion of the interface between adjacent cells is magnified in the inset, which depicts the interaction between opposing PCP protein subcomplexes and PCP effectors. The gradient of the PCP effector protein Multiple wing hairs is indicated (black indicates higher expression). (C) Localization of core PCP proteins in vertebrate nodal cells. (D) Localization of core PCP proteins in the vertebrate cochlear hair cell and associated support cells.
One important function of polarity complexes is to bring about the formation and expansion of their associated membrane domain: In vertebrates and Drosophila, the Crumbs and Par complexes promote apical membrane formation, whereas, in Drosophila, the Scribble complex promotes basolateral surface expansion (Bryant and Mostov 2008). Whether SCRIB functions identically in mammalian cells remains to be established (Schluter and Margolis 2012), and as noted below SCRIB appears to play an important role in PCP (Montcouquiol et al. 2003). Polarity complexes can function cooperatively or in an antagonistic fashion. An example of the former is the interaction between the Par and Crumbs complexes, which is mediated by direct binding of MPP5 or CRB3 to PARD6 in vertebrates, or Discs lost to Par-6 in Drosophila (Hurd et al. 2003; Nam and Choi 2003; Lemmers et al. 2004). In contrast, in Drosophila, binding of Par-6 (in a complex with aPKC) to Lethal giant larvae leads to its phosphorylation by aPKC, triggering the dissociation of Lethal giant larvae from the membrane (Plant et al. 2003; Laprise and Tepass 2011), reinforcing apical domain identity.
Finally, there are additional polarity proteins involved in basolateral membrane formation including vertebrate orthologs of the C. elegans proteins PAR-1 and PAR-5 (MARK2-3 and YWHAE/YWHAZ in vertebrates) (Benton and St Johnston 2003; Cohen et al. 2004), RAC1 and phosphoinositide-3-kinase (Chartier et al. 2011), DLG5, which binds to STX4 (alias Syntaxin-4) and regulates basolateral trafficking of CDH1 (alias E-cadherin) (Nechiporuk et al. 2007), and the Drosophila Yurt/Coracle complex, which is comprised of Yurt (EPB41L5 in vertebrates), Coracle (EPB41 in vertebrates), Atpα/β (ATP1A1-4 and ATP1B1 in vertebrates; alias Na+, K+-ATPase), and Nrx-4 (CNTNAP1 in vertebrates; alias neurexin-4) (Fig. 6) (Laprise et al. 2009). In mammalian cells, the Yurt ortholog, EPB41L5, is similarly required for lateral membrane formation (Laprise et al. 2009).
Planar Cell Polarity
Similar to apicobasolateral polarity, PCP requires a cluster of highly conserved polarity proteins, which were originally described in invertebrates (Fig. 6B). The “core complex” is required for the establishment of local polarity within and between adjacent cells, and mutation of any one if its components alters the localization of the others (Devenport 2014, 2016; Sebbagh and Borg 2014). The name of these and other PCP proteins are summarized in Table 1. Early in polarization, the core complex proteins are intermixed at the apical surface, but then become segregated to opposite sides of the apical surface (i.e., distal or proximal), with the exception of the Drosophila seven-transmembrane domain atypical cadherin Flamingo (CELSR1-3 in vertebrates), which is found at both locations (Fig. 6B). Segregation appears to depend on negative repulsive interactions, E3-ligase-dependent degradation of core components, positive stabilization of clusters, and directed vesicular traffic (Devenport 2014; Sebbagh and Borg 2014). In addition to Flamingo, the distal complex members in Drosophila include the receptor Van Gogh (vertebrate isoforms are VANGL1-2) and the cytoplasmic protein Prickle (PRICKLE1-4 in vertebrates) (Fig. 6B). The proximal complex is comprised of Flamingo, the receptor Frizzled (FZD3&6 in vertebrates), and the cytoplasmic proteins Diego (INVS and DIV in vertebrates: aliases inversin and diversin) and Dishevelled (DVL1-3 in vertebrates) (Fig. 6B). The receptors Frizzled and Van Gogh, in conjunction with Flamingo, are thought to bridge the two complexes at sites of cell–cell contact, and in doing so propagate polarity from cell to cell.
Although conserved across species, and required for the organization of apical protrusions, our knowledge of the distribution of core PCP proteins in vertebrates is limited and is not always as expected. For example, in vertebrate node cells only VANGL2, PRICKLE2, and DVL have been localized (Fig. 6C) (Antic et al. 2010; Hashimoto et al. 2010). Likewise, in vertebrate hair cells only the distribution of VANGL2, FRZ3/6, and DVL2/3 are known (Fig. 6D). Surprisingly, hair cells FRZ3/6 and DVL2/3 are localized on opposite poles of the cell, and VANGL2 is apparently localized to support cells and the not the hair cell itself (Wang et al. 2005; Giese et al. 2012). Although the number of isoforms of core PCP proteins is expanded in vertebrates, knowledge about their location and function awaits further study.
In addition to the six members of the core module, PCP proteins in vertebrates have been expanded to include several additional ones, including PTK7, a member of the receptor tyrosine kinase family (that apparently lacks kinase activity), SEC24B, a component of the COPII vesicular coat, and SCRIB, which as described above plays an important role in establishing apicobasolateral polarity (Lu et al. 2004; Merte et al. 2010; Wansleeben et al. 2010). All three of these proteins show genetic interactions with VANGL2, and COPII is required for VANGL2’s exit from the endoplasmic reticulum (Wansleeben et al. 2010). Moreover, biochemical studies have confirmed a direct protein–protein interaction between the SCRIB PDZ3-4 domains and the VANGL2 carboxyl terminus (Kallay et al. 2006). The requirement for additional PCP components likely reflects the need of vertebrates to meet the specialized needs of having multiple tissue types, all requiring some form of PCP to function.
The core module promotes asymmetry within and between cells, but it does not have the ability to orient its action to the plane of the tissue. This tissue-level alignment is transmitted by way of the evolutionarily conserved “global module” (Devenport 2014; Sebbagh and Borg 2014). The exact relationship of the global and core modules remains unclear, but the core module must have a mechanism to sense the output of the global module. In Drosophila, the global module is comprised of the Golgi-associated ecto-kinase Four-jointed (FJX1 in vertebrates), and two atypical cadherins: Fat (FAT1-4 in vertebrates) and Daschous (DCHS1-2 in vertebrates) (Matis and Axelrod 2013). In Drosophila, Four-jointed phosphorylates the extracellular domains of Daschous and Fat, which alters their affinity for one another. Four-jointed and Daschous form opposing gradients across various tissues, possibly in response to a morphogenic gradient, which leads to subtle gradients of Fat at the cellular level. Although the Fat-Daschous pathway is conserved in vertebrates (Matis and Axelrod 2013), and appears to regulate PCP in mammals (Saburi et al. 2008; Zakaria et al. 2014), it may not be regulated by the vertebrate homolog of Four-jointed, FJX1 (Sadeqzadeh et al. 2014). At present, our understanding of how individual tissues in vertebrates translate global orientation cues to planar polarity at the cellular level is very much a work in progress.
To end this subsection, I touch on the “PCP effector proteins,” which are thought to function downstream of the core PCP proteins. In general, PCP effectors regulate the cytoskeleton, which as noted above plays a major role in generating apical surface protrusions (Devenport 2014; Sebbagh and Borg 2014). In Drosophila wing blade epithelium, the PCP effectors Inturned (INTU in vertebrates), Fuzzy (FUZ in vertebrates), and Fritz (WDPCP in vertebrates) apparently act downstream of Van Gogh/Prickle to regulate the formation of the single actin-based hair that projects from the apical surface of each cell that comprises the wing blade epithelium (Fig. 6B) (Wong and Adler 1993; Adler et al. 2004; Collier et al. 2005; Strutt and Warrington 2008). Although their function remains somewhat mysterious, all three proteins interact with one another and they segregate to the anterior portion of the apical domain. Mutations in the genes that encode these proteins results in the presence of multiple hairs on wing epithelial cells. Thus, they appear to be negative regulators of the actin cyotoskeleton. These proteins are thought to act in part by modulating the activity of the protein called Multiple wing hairs, a formin-like actin-binding protein that is enriched on the posterior side of the cell (Fig. 6B) (Wong and Adler 1993; Collier et al. 2005). Interestingly, Multiple wing hairs also antagonize actin formation, indicating its primary role may be to modulate the extent of actin formation at the apical surface (Yan et al. 2008; Lu et al. 2015b). Although our understanding of PCP effector proteins in vertebrates is currently deficient, they are implicated in the formation of apical protrusions, a topic broached in more detail below.
Interactions between Apicobasolateral Polarity Determinants and PCP Proteins
As noted at the start of this section, apical protrusions only develop once the apical surface is established. As such, there is likely to be important cross talk between proteins that promote apicobasolateral polarity and those that specify PCP. Indeed, work in Drosophila shows that Frizzled’s activity and stability are regulated by aPKC-dependent phosphorylation, and aPKC’s activity is, in turn, antagonized by Bazooka (Djiane et al. 2005). Moreover, Dishevelled, possibly acting downstream of Frizzled8, regulates the localization and activity of Lethal giant larvae in the Xenopus ectoderm and the Drosophila follicular epithelium (Dollar et al. 2005). Because Lethal giant larvae antagonizes the action of the Par complex, and Frizzled8 overexpression leads to dissociation of Lethal giant larvae from the cortex, Frizzled/Dishevelled may promote apical domain formation. Finally, in Drosophila, the apicobasolateral polarity determinant Scribble binds directly to Van Gogh and acts as an effector of PCP establishment (Lee et al. 2003). Obviously, there is still much to be learned about how these two polarity-generating systems modulate each other’s activity.
POLARITY PROTEINS AND THE BIOGENESIS AND ORGANIZATION OF CILIA, MICROVILLI, AND STEREOCILIA
This section examines the role of polarity proteins in the generation and organization of apical membrane protrusions. The focus here is on vertebrate systems, but when relevant, invertebrate biology is also presented. What quickly becomes apparent is that our current understanding is limited, and there is a need for both mechanistic- and observation-based studies in multiple cell types to fully comprehend the links between apical projections and the broad diversity of polarity proteins and their isoforms. Such information would not only increase our understanding of how cells and tissues are formed, but also provide insights into why defects in the generation and function of apical projections leads to diseases that include blindness, deafness, sterility, and a raft of other developmental and adult afflictions (Muller et al. 2008; Sang et al. 2011; Waters and Beales 2011; Sebbagh and Borg 2014). I note that despite their presence on numerous epithelia and their fascinating structure there is almost nothing known about the biogenesis of microplicae or the role of polarity proteins in their formation or function. Thus, the following section is focused on cilia, microvilli, and stereocilia.
Cilia
This subsection begins with a brief overview of the process that builds cilia in vertebrates, and ends with a description of potential steps in which polarity proteins may intervene. Ciliogenesis is somewhat different in invertebrates and the reader is directed to the following review for additional insights into ciliogenesis in these organisms (Lee and Chung 2015).
Cilia Biogenesis
Depending on cell type, ciliogenesis occurs by one of two pathways (Fig. 7) (Sorokin 1962, 1968; Reiter et al. 2012; Chang et al. 2015): (1) In “intracellular ciliogenesis” a “ciliary vesicle” forms on the surface of the basal body and the forming axoneme starts to extend before docking with the apical plasma membrane. This pathway is important for the formation of primary cilia (Sorokin 1962); (2) In “extracellular ciliogenesis,” the centriole is delivered below the apical plasma membrane, a ciliary vesicle is formed, but extension of the axoneme occurs at the surface. This latter pathway is observed in vertebrate epithelial cells with multiple motile cilia (Sorokin 1968).
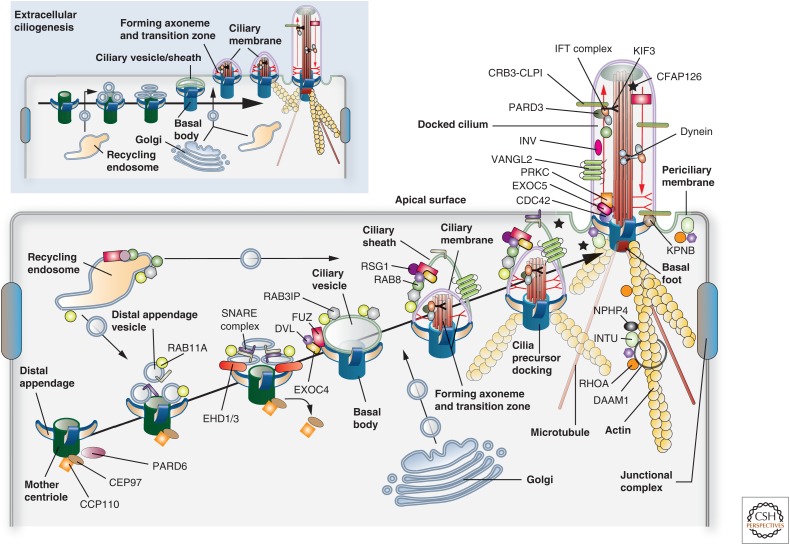
Figure 7.
Models for ciliogenesis. Cilia are formed by one of two mechanisms: “intracellular ciliogenesis” or “extracellular ciliogenesis.” The former is depicted in the larger panel and the latter in the smaller panel found in the upper left of the figure. Both should be viewed from left to right. The proteins and associated binding partners depicted in this figure are culled from numerous studies, and may vary depending on organism, cell type, and mechanism of ciliogenesis. (Larger panel) In “intracellular ciliogenesis,” a cilia precursor is formed in the cytoplasm and is subsequently delivered to the apical surface where it undergoes docking. Initially, a mother centriole with attached distal appendages accumulates RAB11A-positive distal appendage vesicles, which subsequently recruit EHD1/3, triggering a fusion event that results in the formation of a “ciliary vesicle.” Concomitantly, the centriole discharges CEP90 and CCP110, marking the conversion of the centriole to the basal body. Either before, or subsequent to ciliary vesicle formation, FUZ recruits DVL, the exocyst subunit EXOC4, and eventually RSG1 to the vesicle surface. In a similar fashion, RAB11 recruits the RAB8 exchange factor RAB3IP. In the next step, protein components from the Golgi (e.g., IFT20, ciliary membrane proteins, and PCP core proteins such as VANGL2), from recycling endosomes (e.g., RAB8), and from the cytoplasm (e.g., IFT and transition zone proteins) arrive at the ciliary vesicle and begin to assemble the axoneme and transition zone. This step is marked by the presence of the ciliary sheath, which will ultimately give rise to the periciliary membrane, and covers the inner ciliary membrane. Expansion of both membranes likely requires continued input from endosomes and Golgi. The ciliary precursor undergoes fusion in a process called “docking,” followed by further building of the axoneme and transition zone. The cilium is associated with both microtubules and actin filaments. (Smaller panel) Extracellular ciliogenesis is observed in cells with multiple cilia. Here, the centriole is delivered to a position just below the apical surface, undergoes maturation, and a ciliary vesicle/sheath is formed. The latter undergoes fusion with the apical plasma membrane, a step that is followed by assembly of the transition zone and axoneme at the cell surface.
Intracellular ciliogenesis begins with maturation of the mother centriole, a process that includes the addition of a pinwheel-shaped structure, consisting of at least five proteins, that forms at the distal ends of the centriole (Kobayashi and Dynlacht 2011; Lee and Chung 2015). It is called the “distal appendage” at this stage, and will ultimately sit at the interface between the apical plasma membrane and the ciliary membrane, forming a key component of the transition fibers observed within the transition zone (see Fig. 3; not depicted in Fig. 7). Some centrioles also contain subdistal appendages and a “basal foot,” which anchors microtubules (Garcia and Reiter 2016). The distal appendages serve as a docking site for “distal appendage vesicles,” which are likely derived from RAB11A-positive recycling endosomes (Westlake et al. 2011). In a process that requires the activity of the membrane-tubulating proteins EHD1 and EHD3 (Lu et al. 2015a), distal appendage vesicles undergo a fusion process that culminates in the formation of the ciliary vesicle. Further maturation of this vesicle and the forming basal body requires the recruitment of IFT components, other components of the transition zone, and release of the centriolar protein CCP110 and its binding partner CEP97, both of which negatively regulate centriole-to-basal body conversion (Spektor et al. 2007). In addition, the RAB11A effector and guanine nucleotide-exchange factor RAB3IP recruits RAB8 (Knodler et al. 2010), which further promotes maturation of the ciliary vesicle to a structure called the “ciliary sheath.” Thus, RAB11A and its effector cascade not only function after cilia are established (see Fig. 3), but are also key components of the pathway that builds cilia (Fig. 7). Encapsulated within this sheath region is the emerging “ciliary membrane,” generating a “cilia precursor” that contains a double membrane structure. After further modification, including axoneme elongation and building of the transition zone, the cilia precursor undergoes docking, which results in fusion of the ciliary sheath with the apical membrane (Fig. 7). The sheath gives rise to the “periciliary membrane” (Fig. 7).
Vertebrate multiciliated cells undergo extracellular ciliogenesis. In these cells, the centrioles are duplicated from within a specialized structure called the deuterostome, which is derived from the daughter centriole (Al Jord et al. 2014). The duplicated centrosomes are delivered to just below the surface, and then undergo differentiation to basal bodies as a ciliary vesicle is formed. Sheath formation is either reduced or absent. The ciliary vesicle/sheath then appears to directly fuse with the apical plasma membrane before axoneme and transition zone assembly (Fig. 7) (Sorokin 1968).
As a final note, the centriole is embedded within the actin-rich cortex underlying the apical surface, and short interconnecting actin filaments connect neighboring centrioles (Sandoz et al. 1988; Chailley et al. 1989). Thus, it should not be surprising that the actin cytoskeleton plays an important but somewhat poorly understood role in docking and ciliary movements.
Role of Apicobasolateral Polarity Proteins in Ciliogenesis
In this and the following subsection, it is presumed that many of the proteins and signaling events will be conserved, but this may depend on organism, cell type, and pathway for ciliogenesis. Both Crumbs and Par complexes are localized to cilia (Fan et al. 2004; Sfakianos et al. 2007; Zuo et al. 2011). Depletion of CRB3-CLPI, a splice variant of CRB3 that ends in the amino acid sequence C-L-P-I (single amino acid code) instead of the more common sequence E-R-L-I, leads to cilia loss, indicating that CRB3-CLPI is an important contributor to cilia formation (Fan et al. 2007). Similarly, depletion of PARD3 or inactivation of PRKC by way of inhibitors leads to defects in cilia formation and elongation (Sfakianos et al. 2007; Pruliere et al. 2011).
Crumbs and Par complexes are also important for the formation of the photoreceptor outer segment, a modified cilium that projects from the apical region of photoreceptor cells, and which contains stacks of membrane packed with RHO (alias rhodopsin) photopigment. A basal body sits at the interface between the outer and inner segments, which are bridged by a connecting cilium. In flies, a structure analogous to the outer segment is called the rhabdomere (an actin-rich structure), which forms a subdomain distinct from the rest of the apical domain. Mutations in CRB1 lead to photoreceptor degeneration in patients with retinitis pigmentosa type 12 and Leber congenital amaurosis (den Hollander et al. 1999, 2001). Moreover, there is a strong body of genetic evidence in Drosophila and evidence in zebrafish that both the Crumbs and the Par complexes are important for outer segment/rhabdomere formation and growth (Izaddoost et al. 2002; Pellikka et al. 2002; Nam and Choi 2003; Omori and Malicki 2006; Pocha et al. 2011; Krock and Perkins 2014). In the case of the fly, Bazooka is found at the adherence junction of the photoreceptor cell and is required for apical targeting of Crumbs and aPKC/Par-6, and loss of any of the Crumbs complex member leads to mislocalization of the Par complex proteins (Nam and Choi 2003). This loss likely reflects an interaction between Discs lost and Par-6, which is proposed to provide cross talk between these two complexes in the fly eye (Nam and Choi 2003).
How the Crumbs and Par complexes promote ciliogenesis remains an open question. A key step in ciliogenesis is the maturation of the centriole (Kobayashi and Dynlacht 2011). In this regard, PAR6G is associated with the mother centriole (Fig. 7), and it regulates the recruitment of proteins to this organelle, including DCTN1 (p150-Glued subunit of dynactin) and PAR6A, steps that are important for proper ciliogenesis (Dormoy et al. 2013). There are also some indications that one role of apicobasolateral polarity proteins is to promote vesicle traffic and protein entry into the cilium. For example, an association between CDC42-PARD6 with the exocyst subunit EXOC5 (alias Sec10) is observed at the cilium, indicating that the Par complex may recruit proteins at active sites of fusion (Fig. 7) (Zuo et al. 2011). In addition, CRB3 interacts with KPNB, an association that, as noted above, is likely to have a role in regulating the entry of proteins to the cilia (Fan et al. 2007). Furthermore, PARD3 interacts with the motor protein KIF3 (Fig. 7), and PRKC activity is required for microtubule organization during ciliogenesis (Fan et al. 2004; Sfakianos et al. 2007). Therefore, the Par complex may regulate the stability of the ciliary axoneme and also direct the polarized traffic of proteins along the ciliary microtubules by way of IFT. Obviously, much work remains to understand whether or how apicobasolateral polarity proteins regulate critical steps in ciliogenesis, including the positioning, docking, and fusion of the cilia precursor with the apical membrane, whether they regulate formation and subsequent transport through the ciliary barrier (e.g., transition zone), and what functions they play to promote the import of proteins into the forming axoneme.
Role of PCP Proteins in Cilia Formation
In addition to the Crumbs and Par complexes, PCP proteins are also implicated in ciliary biogenesis and function. INVS and VANGL2 are localized to the ciliary axoneme, whereas DVL is asymmetrically positioned at the base of the basal body (Fig. 7) (Otto et al. 2003; Ross et al. 2005; Park et al. 2008). Moreover, several PCP proteins are associated with the centrosome, and PCP proteins are known to be important in orienting the mitotic spindle during cell division (Sebbagh and Borg 2014).
There is a growing body of evidence that implicates PCP proteins in early steps of ciliogenesis, docking in particular (Fig. 7). For example, in the multiciliated cells of the Xenopus mucociliary epithelium, Vangl2 is required for docking, as is Dvl (Park et al. 2008; Mitchell et al. 2009). The latter not only regulates docking of basal bodies, but also modulates polarized ciliary beating in an anterior-to-posterior direction (Park et al. 2006). In Xenopus, Dvl may function in at least two ways. First, it activates RhoA (Park et al. 2008), which is implicated in ciliogenesis (Pan et al. 2007), and is a well-known modulator of the actin cytoskeleton. As noted above, actin is enriched at the apical surfaces of multiciliated cells and is required for the apically directed movement and docking of basal bodies (Boisvieux-Ulrich et al. 1990; Dawe et al. 2009; Bershteyn et al. 2010). Second, in Xenopus, Dvl regulates the recruitment of vesicles and the exocyst subunit Exoc4 (alias Sec8) to the maturing centriole/basal body (Park et al. 2008). In vertebrates, Dvl may act at a similar step as it appears to associate with (or even recruit) Rab8-positive vesicles as ciliary sheath formation progresses (Zilber et al. 2013). Thus, DVL may regulate ciliogenesis by way of modulating membrane trafficking pathways and the actin cytoskeleton. Finally, in Celsr2/3 knockout (KO) mice, there is a pronounced defect in ciliogenesis, including a failure to dock basal bodies (Tissir et al. 2010). The reason for these defects is not known, but disruption of FZD3 and VANGL2 expression in these mice indicates a general disorganization of the PCP machinery.
Several PCP effectors, described above, have also been implicated in ciliogenesis (Fig. 7). In Xenopus, Intu is localized to basal bodies, recruits RhoA to the apical domain, and regulates the formation of the subapical cytoskeleton in multiciliated cells by way of interactions with the cilia-associated protein NPHP4 and the formin DAAM1 (Park et al. 2006, 2008; Yasunaga et al. 2015). In mice, FUZ is essential for proper ciliogenesis, apical accumulation of the microtubule-bundling protein CLAMP in the tips of cilia, and apical secretion (Gray et al. 2009). In vertebrate primary cilia, FUZ controls ciliogenesis by recruiting DVL and RAB8 to basal bodies (Zilber et al. 2013). FUZ also forms interactions with a potential regulator of membrane traffic called RSG1 (alias Rem/Rab-similar GTPase 1) (Gray et al. 2009). Although not much else is known about RSG1, if it is a bona fide regulator of membrane traffic then it would further implicate PCP pathways in trafficking and ciliogenesis. An additional PCP effector protein called CFAP126 (Flattop in Drosophila) is also localized to the cilium, basal body, and apical membrane, and is required for basal body docking and ciliogenesis in multiciliated cells and the kinocilium of mouse hair cells (Gegg et al. 2014).
On docking to the apical membrane, basal bodies become asymmetrically oriented, specifying the beat direction of their associated cilia. PCP proteins contribute to basal body/cilia polarity in three ways (Kishimoto and Sawamoto 2012; Devenport 2016). “Translational” polarity refers to any positional displacement of cilia/basal bodies within an individual cell, an example of which is the kinocilium in hair cells or the posterior clustering of cilia in ependymal epithelial cells or their equivalent in the Xenopus gastrocoel roof plate. In the latter, depletion of Prickle3 leads to defects in translational polarity as well as defects in cilia growth and basal body organization (Chu et al. 2016). In mice, translational polarity in ependymal cells is affected by the loss of MYO1D expression (Hegan et al. 2015b), an ortholog of the Drosophila Myo1A protein that was previously implicated in left–right visceral asymmetry (Hozumi et al. 2006; Speder et al. 2006). Although Myo1d KO mice do not have situs inversus (reversed organ asymmetry), they do show defects in the clustering of cilia at the posterior region of ependymal cells (Hegan et al. 2015b). Lack of Myo1d expression has a more pronounced effect on “rotational polarity,” which describes the alignment of basal bodies within an individual cell, and is important in those cells where cilia beat or rotate in a regular pattern. In ependymal cells, loss of MYO1D protein leads to disrupted cilia beating (Hegan et al. 2015b). Likewise, decreasing Dvl expression similarly affects rotational polarity in Xenopus epidermal cells and in mouse ependymal cells (Park et al. 2008; Hashimoto et al. 2010). In Celsr1 KO mice, the multiciliated cells that line the oviduct appear to form normally, but transport along this structure is impaired as a result of defects in ciliary beating (Shi et al. 2014).
Finally, “tissue polarity” (i.e., global polarity) refers to the coordination of translational and/or rotational polarity across the tissue (Kishimoto and Sawamoto 2012; Devenport 2016). How tissue polarity comes about in vertebrates and any role for the Fat-Daschous pathway are important but unanswered questions. What is known is that Celsr2, Celsr3, and Vangl2 are all required for establishment of polarized fluid flow across mouse ependymal cells (Mitchell et al. 2009; Tissir et al. 2010), and Celsr1 appears to have a similar role in the oviduct (Shi et al. 2014). And, as noted above, Dvl and Rhoa appear to govern the anterior-to-posterior beating of cilia in the Xenopus epidermis (Park et al. 2008). Importantly, this function for Dvl appears to be distinct from its role in cilia docking, as cells expressing the Xdd1 mutant of Dvl form motile cilia, but beat irregularly (Park et al. 2008).
One possible mechanism that may promote ciliary tissue polarity is fluid flow. When Xenopus epidermal tissue is explanted, the orientation of ciliary beat is governed by a previous exposure to an imposed directional flow (Mitchell et al. 2007). Interestingly, when proteins that affect cilia motility (but do not affect ciliogenesis) are downregulated, including the ciliary proteins Spag6, Dnai1, or Tekt2a, the cilia fail to reorient when exposed to directional flow (Mitchell et al. 2007). Thus, early patterning of basal bodies in Xenopus likely occurs as they dock at the membrane. In contrast, polarized ciliary flow requires a positive feedback loop in which the cilia produce flow, sense this flow, and then further refine their orientation at the tissue level to optimize this flow. Somewhat like the Xenopus system, maturing mouse ependymal cells will also orient their beating when exposed to directional fluid flow (Guirao et al. 2010). In this system, a nonfunctional Vangl2 mutant called “looptail” has no effect on ependymal cell ciliogenesis (and the frequency and amplitude of ciliary beating in these animals is normal); however, the alignment of cilia to flow is significantly perturbed both ex vivo and in vivo.
Besides the requirements for the actin cytoskeleton described above, ciliary beating also depends on the microtubule cytoskeleton, which connects adjacent basal bodies and ensures their spacing and rotational polarity (Sandoz et al. 1988). Interestingly, a pool of polarized microtubules emanate from the “basal foot” (Fig. 7), a nucleation center that extends from the basal body in a polarized orientation that often correlates with the axis of PCP asymmetry and cilia beat (Clare et al. 2014). The plus ends of these microtubules are oriented toward the FRZ-DVL pole of the cell (Vladar et al. 2012). When coupled with laminar flow, this could help orient cilia position and beating across the tissue.
Microvilli
The majority of studies to date have focused on exploring the roles for the major structural and regulatory proteins in formation of microvilli. In cultured cells, these proteins appear to have important roles (Takeuchi et al. 1994; Bretscher et al. 2002; Bonilha et al. 2006a; Wald et al. 2008; LaLonde et al. 2010); however, in vivo studies using KO mice paint a much more complicated picture. Mice with mutations in Vil1, Pls1, Myo1a, ERM proteins, Slc9a3r1 (EBP-50), or a spontaneous Espn mutant mouse (the deaf jerker mouse) still form some microvilli (Pinson et al. 1998; Doi et al. 1999; Morales et al. 2004; Saotome et al. 2004; Tyska et al. 2005; Bonilha et al. 2006b; Grimm-Gunter et al. 2009; Casaletto et al. 2011; Revenu et al. 2012). Although the observed phenotypes may reflect redundancy of proteins (Tyska et al. 2005), it is also likely that brush-border formation is the coordinated product of multiple regulatory and structural proteins, which cannot be appreciated by single gene knockouts. In the following section, the formation of the apical brush border is examined, followed by a discussion of those studies that implicate polarity proteins in microvilli/brush-border biogenesis.
Formation of Microvilli and the Apical Brush Border
The broad outlines of brush-border formation have been documented in kidney and intestine (Larsson 1975; Shibayama et al. 1987; Heintzelman and Mooseker 1990, 1992; Biemesderfer et al. 1992; Peterson et al. 1993; Peterson and Mooseker 1993), primarily using chicken and rodent models. Although the length of time it takes to form the brush border is species dependent, the process is similar. In brief, enterocytes, which have undergone apicobasolateral polarization, have a bulbous apical surface from which extend sparse microvillar-like structures. Over the next several days, the microvilli become denser and self-organize into clusters, which as described above are dependent on PCDH24 and CDHR5 (Crawley et al. 2014b). Higher order clustering continues until one observes consolidation of clusters into a packed brush border in which microvilli show a sixfold hexagonal symmetry. Concomitant with clustering is the formation of “rootlets,” which emerge from the base of the nascent microvilli and elongate into the forming terminal web. On completion of terminal web biogenesis, the microvilli undergo further elongation, while maintaining a highly regular and uniform length and width.
Role of Polarity Proteins in Brush-Border Formation
The events that trigger microvilli formation are not well understood and, despite the uniform packing of microvilli in the brush border and the important role of actin in their formation, there are few if any studies that implicate PCP in the formation of microvilli or an assembled brush border. In Drosophila, Crumbs contains a FERM-binding site, which interacts with Moe (alias moesin), the single ERM protein in the fly (Medina et al. 2002). The complex also includes Bheavy isoform of Spec (alias spectrin), a component of the cortical actin cytoskeleton and terminal web. One possibility is that Crumbs recruits Moe/Bheavy Spec, and in doing so triggers the formation of the brush border at the apical pole of the epithelial cell (Fig. 8). An additional experimental system is the pronephros of the zebrafish embryo. When expression of the zebrafish homolog of UPK3a (originally called Upk3l but since renamed to Upk3b) is down-regulated in these embryos, they show edema as a result of pronephros dysfunction. One striking defect in these embryos is the complete disappearance of the brush border normally found at the luminal surfaces of the epithelial cells that line the pronephros (Mitra et al. 2012). Interestingly, these cells also lose expression of phosphorylated ERM proteins, lose expression of Prkcz, and show a redistribution of Pard3. Whether Upk3l interacts with ERM or polarity proteins is currently unknown, but if so it could act to promote brush-border formation by scaffolding proteins at the apical membrane domain.
Figure 8.
Biogenesis of the epithelial brush border. In Drosophila, Crumbs may trigger brush-border morphogenesis by interactions with Moesin, the single ERM protein in the fly, as well as the βheavy isoform of spectrin (SPTAN/BN in vertebrates). An additional pathway for brush-border formation may require the activity of the STK11 kinase. In the presence of the pseudokinase STRADA and the cofactor CAB39, STK11 exits the nucleus and promotes the translocation of the kinase STK26 from the Golgi apparatus to the apical pole of the cell. Here, STK26 phosphorylates T567 in EZR, which could act to further promote brush-border formation by stabilizing the actin cytoskeleton and proteins at the apical cell surface. The translocation and activation of STK26 is dependent on PLD-induced production of phosphatidic acid, which promotes association of RAPGEF, a GEF for the small Ras-family GTPase RAP2A, with the cell surface. In turn, RAP2A recruits the TNIK kinase, which promotes translocation of STK26 to the plasma membrane, presumably as a result of TNIK-induced STK26 phosphorylation. Tip links are described in Figure 4. (Figure from Apodaca and Gallo 2013; adapted, with permission, from the authors.)
The final experimental system uses an intestinal cell line that links microvilli formation to STK11 (aliases Lkb1/Par4), a master regulator of cell polarity (Morton et al. 1992; Watts et al. 2000). In the presence of the pseudokinase STRADA (STRAD) and the cofactor CAB39 (Mo25), STK11 exits the nucleus and promotes the translocation of the kinase STK26 (Mst4) from the Golgi apparatus to the apical pole of the cell where it phosphorylates T567 in EZR (Fig. 8) (ten Klooster et al. 2009). Even in single intestinal epithelial cells, expression of STK11/STRADA stimulates the formation of an apical domain, studded with microvilli, that is separated from a basolateral domain by junctional proteins (Baas et al. 2004). More recent studies show that STK26 recruitment requires a signaling cascade that initiates with PLD (alias phospholipase D) binding to apical membrane-associated PtdIns(4,5)P2 (Fig. 8) (Gloerich et al. 2012). PLD-dependent generation of phosphatidic acid promotes recruitment of RAPGEF2 (alias PDZGEF), a GEF for RAP2A. Active, GTP-bound RAP2A then recruits the TNIK kinase, which presumably phosphorylates STK26, stimulating STK26 localization to the apical membrane. Although these above results are exciting, additional work, using genetically tractable organisms, is needed to determine whether these findings can be translated into more physiologically relevant systems.
Stereocilia
Although stereocilia are also found in the male reproductive tract, there is limited available information about their function, or the mechanisms of their formation. In contrast, the cochlear epithelium is an ideal vertebrate model to study polarity because its anatomy and development are well described, the orientation and organization of the stereocilia and kinocilium are peculiar, predictable, and quantifiable, and genetically tractable model systems are available for study including mice. Despite these features, there remain many open questions about how polarity proteins contribute to stereociliogenesis in hair cells.
Stereociliogenesis
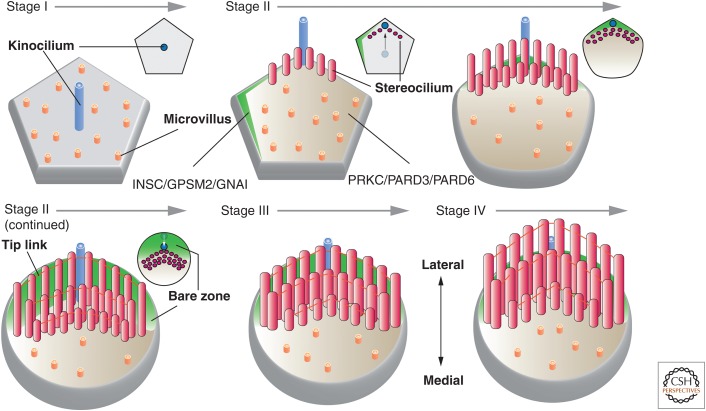
Stereociliogenesis occurs in four stages (Fig. 9) (Nayak et al. 2007). In stage I (∼E14.5 in mice), the hair cell undergoes terminal division and establishes apicobasolateral polarity. At this time, the hair cells have a pentagonal or hexagonal shape and numerous short microvilli emerge, which surround a single centrally located kinocilium. In stage II (∼E16.5), the kinocilium elongates and moves to one side of the cell. Those microvilli nearest the kinocilium undergo lengthening first, followed by those in rows further away, eventually resulting in the formation of the characteristic staircase configuration. During this stage, the kinocilium begins to initiate its inward relocalization, eventually taking up a position halfway between the outer edge of the hair cell and its center (Tarchini et al. 2013). This generates a “bare zone,” which lacks microvilli and is found between the lateral edge of the hair cell and the kinocilium (Fig. 9). In addition, the number of actin filaments increases in each stereocilium, these filaments become cross-linked, and adjacent stereocilia are linked to one another by tip links. As the cochlear epithelium matures, the hair cells become progressively ovoid in shape and are surrounded by support cells. By stage III, the stereocilia temporarily stop lengthening and instead become wider as the number of actin filaments per stereocilium increases and the filaments become more cross-linked. The base of each stereocilium begins to taper and the cuticular floor at the base of each cilium begins to form. Finally, in stage IV, the stereocilia lengthen again until they reach their final height (by E18.5 in mice).
Figure 9.
Stages of stereociliogenesis. Stage I: After the hair cell precursor establishes its apicobasolateral axis of polarity, a single kinocilium extends from the center of the cell. At this stage, the hair cell has a pentagonal or hexagonal shape. Stage II: The kinocilium undergoes a centrifugal displacement, positioning it at the periphery of the cell next to the tip of a polarized surface domain marked by INSC/GPSM2/GNAI and opposite of a domain enriched in PRKC/PARD3/PARD6. Stereocilia are seen to emerge in this stage, forming a “V”-shaped, staircase-like pattern as additional rows of cilia form. The vertices of each row point toward the kinocilium. Subsequently, the kinocilium undergoes a second migration, placing it midway between the cell center and cell periphery. The INSC/GPSM2/GNAI domain is now positioned in the lateral portion of the apical surface, establishing a “bare zone,” which derives its name from the lack of microvilli in this region. Stereocilia track the kinocilium and end up positioned at the border of the bare zone and the PRKC/PARD3/PARD6 domain. By the end of stage II, the stereocilia are connected by tip links and the hair cell apical surface has achieved an ovoid-shaped morphology. Stage III: The stereocilia stop growing in height and instead grow wider as additional actin filaments are packed into each stereocilium and cross-linked. Stage IV: The stereocilia continue to lengthen until they reach their final height. The small images at the upper right of each stage show an en face view of the position of the kinocilium and stereocilia at the indicated stage of development.
Role of Polarity Proteins in Stereociliogenesis
The organization of stereocilia in the cochlea is one of the first to be linked to the core PCP pathway in mammals (Montcouquiol et al. 2003). Polarization of the core PCP proteins occurs early in mice, before the migration of the kinocilium and formation of the stereocilia (Vladar et al. 2012). As noted above, the expression patterns of those PCP proteins that have been localized do not fit the pattern predicted from studies in Drosophila. In particular, although VANGL2 is on a membrane domain across from FRZ3/6, it appears to be localized to adjacent support cells and not the hair cell itself (Fig. 6D) (Giese et al. 2012). This would fit with recent analysis showing that mutations in Nectin3 disrupt supporting hair cell junctions and in doing so alters the polarity of stereocilia bundles (Fukuda et al. 2014). Largely because of the lack of isoform-specific antibodies, the localization of the other PCP isoforms in the vertebrate cochlea remains to be described.
Regardless of their localization patterns, mutants in the core PCP genes Vangl2, Frz3/6, Celsr1, Dvl1/2, and Dvl3, as well as Scrib all lead to misorientation of the hair bundle (Curtin et al. 2003; Montcouquiol et al. 2003; Wang et al. 2005, 2006; Etheridge et al. 2008). However, the hair bundles still retain their stereotypical V-shaped organization. Moreover, the kinocilium is still formed and is found near the cell periphery, but its position is no longer uniformly in the lateral region of the apical surface. Thus, in hair cells, core PCP proteins do not appear to be required for formation of the kinocilium or the stereocilia bundles per se, but instead they are required to orient the kinocilium and the vertices of their associated hair bundles along the mediolateral axis. If not core PCP proteins, then what allows hair cells to form V-shaped hair bundles, which remain polarized with respect to the kinocilium?
At the cellular level, the PCP of stereocilia bundles appears to depend on the centrifugal migration of the kinocilium and associated basal body from the cell center to the cell periphery, followed by its secondary relocalization to a position between the cell center and lateral end of the hair cell (Fig. 9) (Tarchini et al. 2013). Data in support of this hypothesis include the following. First, after hair bundle development is complete the kinocilium degenerates (in mice this occurs ∼8 d after birth). Second, when kinocilium formation and/or function is perturbed by mutations in the genes encoding the IFT transport proteins KIF3A and IFT88, or Bardet–Biedl syndrome genes, stereocilia bundles are misoriented (in some cases appearing in straight rows instead of V-shaped ones), some stereocilia bundles appear flattened, and the basal bodies are often randomly placed in the lateral half of the cell (Ross et al. 2005; Jones et al. 2008; Sipe and Lu 2011). Third, in those hair cells with mutations in Ift88 that lack cilia but maintain a centralized basal body (∼10% of mutant hair bundles), circular bundles of stereocilia are formed (Jones et al. 2008). Finally, a pathway has recently been described that may regulate the migration of the kinocilium and the PCP of stereocilia at the cellular level. This pathway is described further below.
Strikingly, the localization of core PCP proteins is not affected in Kif3a and Ift88 mutants, or mutations in several other genes that regulate ciliogenesis, possibly indicating that directed kinocilium migration may be occurring by way of a PCP pathway parallel to the core PCP signaling pathway (or that acts downstream of it) (Jones et al. 2008; May-Simera et al. 2015). However, it is worth noting that mutations in Ift20 and Bbs8 do alter the localization of VANGL2 (and BBS8 co-immunoprecipitates with VANGL2), which may indicate a more complex relationship between kinocilia formation and core PCP proteins (May-Simera et al. 2015). The nature of the putative, alternate PCP pathway has recently been described and is a repurposing of one originally described to regulate the orientation of the mitotic spindle during asymmetric cell divisions. In Drosophila and C. elegans, mitotic spindle orientation requires the Par complex (which is localized to the posterior side of the cell) and an anterior module comprised of the adaptor protein Inscuteable (INSC in vertebrates), the Partner of Inscuteable (GPSM2 in vertebrates), and the heterotrimeric G protein Gαi (GNAI in vertebrates). Acting together, these proteins couple mitotic spindle orientation to cortical polarity and ensure asymmetric inheritance of fate determinants between sister cells (Gonczy 2008). In a manner analogous to proteins in invertebrates, INSC and GPSM2 regulate planar divisions in vertebrate systems (Lancaster and Knoblich 2012).
During hair cell development in mice, the INSC/GPSM2/GNAI module are initially localized at the medial side of the hair cell, and by E15.5 are positioned at the microvilli-free “bare zone” that lies between the kinocilium and the distal/lateral surface of the hair cell (see green-colored zones in Fig. 9) (Tarchini et al. 2013). Mutants in Gpsm2 disrupt the organization of the hair cell bundles, and the kinocilium position becomes dispersed within the lateral half of the hair cell apical surface (Ezan et al. 2013; Tarchini et al. 2013). A similar phenotype is observed when GNAI is inhibited with pertussis toxin or in mice with mutations in Gnai3 (Ezan et al. 2013). The Gpsm2 mutant does not affect the initial lateral migration of the kinocilium, but instead appears to affect its shift closer to the cell center (Tarchini et al. 2013). As a result, the kinocilium assumes a position in the lateral domain that appears random. The localization of DVL2 and FZ6 is not obviously affected by mutations in Insc or Gpsm2, nor is VANGL2 localization affected by inhibition of GNAI (Ezan et al. 2013; Tarchini et al. 2013). Thus, the INSC/GPSM2/GNAI module appears to act in a manner distinct from the core PCP machinery. At present, there is no information about the receptor(s) acting upstream of GNAI, or the proteins that act downstream of INSC and GPSM2.
Finally, insights into the function of Crumbs/Par/Stribble complexes in stereociliogenesis have lagged those of PCP proteins considerably. In Drosophila, asymmetric cell division depends on the partitioning of Inscuteable/Partner of Inscuteable/Gαi in a membrane domain distinct from the Par complex (Gonczy 2008). In turn, these two modules exert differential forces on the mitotic spindle, orienting it before cell division. The INSC/GPSM2/GNAI and PRKC/PARD6/CDC42 modules may act in an analogous manner in hair cells (Fig. 9). In support of this hypothesis are the following data. Whereas INSC/GPSM2/GNAI are localized to the bare zone, PRKC/PARD6/CDC42 are enriched in the opposite region of apical surface (PARD3 has been variously localized to either zone) (Ezan et al. 2013; Tarchini et al. 2013). The INSC/GPSM2/GNAI domain may limit the PRKC zone, maintaining the asymmetry at the apical surface of the hair cell (Tarchini et al. 2013). Furthermore, mutations in Cdc42 leads to misorientation of the stereocilia bundles similar to the reported effects for the kinocilia mutants described above (Kirjavainen et al. 2015). Finally, in Cdc42 mutants, those microtubules surrounding and emanating from the kinocilium basal body are disorganized and malformed (Kirjavainen et al. 2015). Although the underlying mechanism of how INSC/GPSM2/GNAI and the Par complex work, it is possible that these two modules may be exerting opposing pulling forces on basal body nucleated microtubules, helping to direct the movement and final positioning of the kinocilium, and by extension the stereociliary bundle. Interestingly, and as noted above, these effects happen independently of kinocilium assembly or initial migration to the lateral edge. What is responsible for this initial migration is unknown. Nor is it clear how the core PCP module interfaces with the cell autonomous INSC/GPSM2/GNAI module to generate stereocilia PCP.
FINAL WORDS
Apical projections, which are found on the luminal surface of all vertebrate epithelial cells, play critical roles in epithelial cell functions. Moreover, defects in their formation/function leads to numerous diseases, including blindness, deafness, infertility, microvillar inclusion disease, and other developmental disorders including spina bifida and cranorachischisis (Waters and Beales 2011; van der Velde et al. 2013; Sebbagh and Borg 2014). Yet, we have a limited understanding of the early steps in their biogenesis and organization. In the case of microplicae, which are found throughout our body, we know nothing about their biogenesis or function. And although studied for decades, there is limited understanding of the early steps in the biogenesis of the brush border. Even in the case of cilia and stereocilia, in which we understand significantly more about early steps in their formation, there remains much to be learned.
Because apical protrusions are specialized regions of the apical surface, and because polarity proteins function to generate asymmetric membrane domains, it is not surprising that polarity proteins are required for their biogenesis and organization. However, there are still unanswered questions. For example, why are there so many isoforms of polarity proteins in vertebrates (see Table 1)? Although likely to contribute to tissue-specific organization and function of apical projections, currently there are limited insights into the expression patterns and functional differences between the multitude of polarity protein isoforms that have been described. Furthermore, what accounts for differences in polarity protein requirements between vertebrate tissues? For example, in multiciliated cells, cilia formation depends on core PCP proteins for docking, but these same proteins do not play an obvious role in the generation of the kinocilium. Apparently, there is an unidentified mechanism that controls kinocilium docking in the inner ear, the nature of which has not been described.
Because apical protrusions develop after apicobasolateral polarity is established, there must be a mechanism to signal the completion of the apical surface domain, before initiation of PCP. But the nature of this signal is unknown. Even after the apical surface is established, both ciliogenesis and stereociliogenesis require apicobasolateral and PCP proteins. But how these polarity complexes communicate and the hierarchy of this information exchange is not well understood. Based on the partitioning of the INSC module and Par complex observed in hair cells (Ezan et al. 2013; Tarchini et al. 2013), one paradigm is that the PCP proteins and apicobasolateral proteins define distinct membrane domains that ensure partitioning of effectors. Which leads to the next question: What are the effectors in vertebrates that act downstream of the polarity proteins to modulate the biogenesis and organization of apical projections? Considering that all apical projections have cytoskeletal elements at their core, regulators of the cytoskeleton are obvious candidates and ones previously implicated in PCP signaling events (Devenport 2014; Sebbagh and Borg 2014). This fits with the finding that CRB3 interacts with KIF3, and roles for INTU and FUZ in ciliogenesis (Fan et al. 2004; Gray et al. 2009; Zilber et al. 2013). However, a complete understanding will require further study using different animal and tissue models. Finally, how is polarity at the cellular level governed at the tissue level? As noted above, the Fat-Daschous plays an important role in Drosophila, but the roles of these proteins in vertebrates is less clear, and in the case of hair cells there remains limited understanding of what the core PCP proteins are doing to orient hair cell bundles.
ACKNOWLEDGMENTS
G.A. is supported by NIH Grants R01DK104287, R01DK099196, and the Kidney Imaging Core of the Pittsburgh Center for Kidney Research (P30DK079307).
Footnotes
Editor: Keith E. Mostov
Additional Perspectives on Cell Polarity available at www.cshperspectives.org
REFERENCES
- Adler PN, Zhu C, Stone D. 2004. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr Biol 14: 2046–2051. [DOI] [PubMed] [Google Scholar]
- Al Jord A, Lemaitre AI, Delgehyr N, Faucourt M, Spassky N, Meunier A. 2014. Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nature 516: 104–107. [DOI] [PubMed] [Google Scholar]
- Ameen N, Apodaca G. 2007. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998–1006. [DOI] [PubMed] [Google Scholar]
- Anderson RA. 1977. Actin filaments in normal and migrating corneal epithelial cells. Invest Ophthalmol Vis Sci 16: 161–166. [PubMed] [Google Scholar]
- Andrews PM. 1976. Microplicae: Characteristic ridge-like folds of the plasmalemma. J Cell Biol 68: 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. 2010. Planar cell polarity enables posterior localization of nodal cilia and left–right axis determination during mouse and Xenopus embryogenesis. PLoS ONE 5: e8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Gallo LI. 2013. Epithelial polarity. In Colloquim series on building blocks of the cell: Cell structure and function (ed. Nabi IR). Morgan & Claypool Life Sciences, Williston, VT. [Google Scholar]
- Asikainen P, Ruotsalainen TJ, Mikkonen JJ, Koistinen A, Ten Bruggenkate C, Kullaa AM. 2012. The defence architecture of the superficial cells of the oral mucosa. Med Hypotheses 78: 790–792. [DOI] [PubMed] [Google Scholar]
- Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. 2008. Polarity complex proteins. Biochim Biophys Acta 1778: 614–630. [DOI] [PubMed] [Google Scholar]
- Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. 2004. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116: 457–466. [DOI] [PubMed] [Google Scholar]
- Barr-Gillespie PG. 2015. Assembly of hair bundles, an amazing problem for cell biology. Mol Biol Cell 26: 2727–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, St Johnston D. 2003. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115: 691–704. [DOI] [PubMed] [Google Scholar]
- Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE. 2010. MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell 19: 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemesderfer D, Dekan G, Aronson PS, Farquhar MG. 1992. Assembly of distinctive coated pit and microvillar microdomains in the renal brush border. Am J Physiol 262: F55–F67. [DOI] [PubMed] [Google Scholar]
- Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, Gipson IK. 2007. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci 48: 4509–4518. [DOI] [PubMed] [Google Scholar]
- Boehlke C, Bashkurov M, Buescher A, Krick T, John AK, Nitschke R, Walz G, Kuehn EW. 2010. Differential role of Rab proteins in ciliary trafficking: Rab23 regulates smoothened levels. J Cell Sci 123: 1460–1467. [DOI] [PubMed] [Google Scholar]
- Boisvieux-Ulrich E, Laine MC, Sandoz D. 1990. Cytochalasin D inhibits basal body migration and ciliary elongation in quail oviduct epithelium. Cell Tissue Res 259: 443–454. [DOI] [PubMed] [Google Scholar]
- Bonilha VL, Rayborn ME, Bhattacharya SK, Gu X, Crabb JS, Crabb JW, Hollyfield JG. 2006a. The retinal pigment epithelium apical microvilli and retinal function. Adv Exp Med Biol 572: 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha VL, Rayborn ME, Saotome I, McClatchey AI, Hollyfield JG. 2006b. Microvilli defects in retinas of ezrin knockout mice. Exp Eye Res 82: 720–729. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. 2002. ERM proteins and merlin: Integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Mostov KE. 2008. From cells to organs: Building polarized tissue. Nat Rev Mol Cell Biol 9: 887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto JB, Saotome I, Curto M, McClatchey AI. 2011. Ezrin-mediated apical integrity is required for intestinal homeostasis. Proc Natl Acad Sci 108: 11924–11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chailley B, Nicolas G, Laine MC. 1989. Organization of actin microfilaments in the apical border of oviduct ciliated cells. Biol Cell 67: 81–90. [DOI] [PubMed] [Google Scholar]
- Chang CF, Schock EN, Attia AC, Stottmann RW, Brugmann SA. 2015. The ciliary baton: Orchestrating neural crest cell development. Curr Top Dev Biol 111: 97–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier FJ, Hardy EJ, Laprise P. 2011. Crumbs controls epithelial integrity by inhibiting Rac1 and PI3K. J Cell Sci 124: 3393–3398. [DOI] [PubMed] [Google Scholar]
- Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, Sandoval W, Peterson AS. 2012. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol 14: 61–72. [DOI] [PubMed] [Google Scholar]
- Chu CW, Ossipova O, Ioannou A, Sokol SY. 2016. Prickle3 synergizes with Wtip to regulate basal body organization and cilia growth. Sci Rep 6: 24104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare DK, Magescas J, Piolot T, Dumoux M, Vesque C, Pichard E, Dang T, Duvauchelle B, Poirier F, Delacour D. 2014. Basal foot MTOC organizes pillar MTs required for coordination of beating cilia. Nat Commun 5: 4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Rodriguez-Boulan E, Musch A. 2004. Par-1 promotes a hepatic mode of apical protein trafficking in MDCK cells. Proc Natl Acad Sci 101: 13792–13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S, Lee H, Burgess R, Adler P. 2005. The WD40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the Drosophila epidermis. Genetics 169: 2035–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin SP, Collin HB. 2006. The corneal epithelial surface in the eyes of vertebrates: Environmental and evolutionary influences on structure and function. J Morphol 267: 273–291. [DOI] [PubMed] [Google Scholar]
- Correr S, Makabe S, Heyn R, Relucenti M, Naguro T, Familiari G. 2006. Microplicae-like structures of the fallopian tube in postmenopausal women as shown by electron microscopy. Histol Histopathol 21: 219–226. [DOI] [PubMed] [Google Scholar]
- Crawley SW, Mooseker MS, Tyska MJ. 2014a. Shaping the intestinal brush border. J Cell Biol 207: 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley SW, Shifrin DA Jr, Grega-Larson NE, McConnell RE, Benesh AE, Mao S, Zheng Y, Zheng QY, Nam KT, Millis BA, et al. 2014b. Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell 157: 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, et al. 2003. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol 13: 1129–1133. [DOI] [PubMed] [Google Scholar]
- Datta A, Bryant DM, Mostov KE. 2011. Molecular regulation of lumen morphogenesis. Curr Biol 21: R126–R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe HR, Adams M, Wheway G, Szymanska K, Logan CV, Noegel AA, Gull K, Johnson CA. 2009. Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J Cell Sci 122: 2716–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. 2013. Primary cilia are specialized calcium signalling organelles. Nature 504: 311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, Clapham DE. 2016. Primary cilia are not calcium-responsive mechanosensors. Nature 531: 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, ten Brink JB, de Kok YJ, van Soest S, van den Born LI, van Driel MA, van de Pol DJ, Payne AM, Bhattacharya SS, Kellner U, et al. 1999. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat Genet 23: 217–221. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Heckenlively JR, van den Born LI, de Kok YJ, van der Velde-Visser SD, Kellner U, Jurklies B, van Schooneveld MJ, Blankenagel A, Rohrschneider K, et al. 2001. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet 69: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D. 2014. The cell biology of planar cell polarity. J Cell Biol 207: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D. 2016. Tissue morphodynamics: Translating planar polarity cues into polarized cell behaviors. Semin Cell Dev Biol 55: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishinger JF, Kee HL, Jenkins PM, Fan S, Hurd TW, Hammond JW, Truong YN, Margolis B, Martens JR, Verhey KJ. 2010. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-β2 and RanGTP. Nat Cell Biol 12: 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djiane A, Yogev S, Mlodzik M. 2005. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell 121: 621–631. [DOI] [PubMed] [Google Scholar]
- Doi Y, Itoh M, Yonemura S, Ishihara S, Takano H, Noda T, Tsukita S. 1999. Normal development of mice and unimpaired cell adhesion/cell motility/actin-based cytoskeleton without compensatory up-regulation of ezrin or radixin in moesin gene knockout. J Biol Chem 274: 2315–2321. [DOI] [PubMed] [Google Scholar]
- Dollar GL, Weber U, Mlodzik M, Sokol SY. 2005. Regulation of Lethal giant larvae by Dishevelled. Nature 437: 1376–1380. [DOI] [PubMed] [Google Scholar]
- Dormoy V, Tormanen K, Sutterlin C. 2013. Par6γ is at the mother centriole and controls centrosomal protein composition through a Par6α-dependent pathway. J Cell Sci 126: 860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MC, Belyantseva IA, Friderici KH, Friedman TB. 2012. Actin in hair cells and hearing loss. Hearing Res 288: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, et al. 2008. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet 4: e1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan AP, Satlin LM, Gattone VH II, Connors B, Schwartz GJ. 1991. Postnatal maturation of rabbit renal collecting duct. II: Morphological observations. Am J Physiol 261: F91–F107. [DOI] [PubMed] [Google Scholar]
- Ezan J, Lasvaux L, Gezer A, Novakovic A, May-Simera H, Belotti E, Lhoumeau AC, Birnbaumer L, Beer-Hammer S, Borg JP, et al. 2013. Primary cilium migration depends on G-protein signalling control of subapical cytoskeleton. Nat Cell Biol 15: 1107–1115. [DOI] [PubMed] [Google Scholar]
- Fan S, Hurd TW, Liu CJ, Straight SW, Weimbs T, Hurd EA, Domino SE, Margolis B. 2004. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol 14: 1451–1461. [DOI] [PubMed] [Google Scholar]
- Fan S, Fogg V, Wang Q, Chen XW, Liu CJ, Margolis B. 2007. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin β interactions. J Cell Biol 178: 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Whiteman EL, Hurd TW, McIntyre JC, Dishinger JF, Liu CJ, Martens JR, Verhey KJ, Sajjan U, Margolis B. 2011. Induction of Ran GTP drives ciliogenesis. Mol Biol Cell 22: 4539–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Knodler A, Ren J, Zhang J, Zhang X, Hong Y, Huang S, Peranen J, Guo W. 2012. A Rab8 guanine nucleotide exchange factor–effector interaction network regulates primary ciliogenesis. J Biol Chem 287: 15602–15609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelgren B, Lin SY, Zuo X, Jaffe KM, Park KM, Reichert RJ, Bell PD, Burdine RD, Lipschutz JH. 2011. The exocyst protein Sec10 interacts with Polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet 7: e1001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, Tuft RA, Fogarty KE, Pazour GJ. 2006. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell 17: 3781–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Kominami K, Wang S, Togashi H, Hirata K, Mizoguchi A, Rikitake Y, Takai Y. 2014. Aberrant cochlear hair cell attachments caused by Nectin-3 deficiency result in hair bundle abnormalities. Development 141: 399–409. [DOI] [PubMed] [Google Scholar]
- Garcia G III, Reiter JF. 2016. A primer on the mouse basal body. Cilia 5: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg M, Bottcher A, Burtscher I, Hasenoeder S, Van Campenhout C, Aichler M, Walch A, Grant SG, Lickert H. 2014. Flattop regulates basal body docking and positioning in mono- and multiciliated cells. eLife 3: e03842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese AP, Ezan J, Wang L, Lasvaux L, Lembo F, Mazzocco C, Richard E, Reboul J, Borg JP, Kelley MW, et al. 2012. Gipc1 has a dual role in Vangl2 trafficking and hair bundle integrity in the inner ear. Development 139: 3775–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloerich M, ten Klooster JP, Vliem MJ, Koorman T, Zwartkruis FJ, Clevers H, Bos JL. 2012. Rap2A links intestinal cell polarity to brush border formation. Nat Cell Biol 14: 793–801. [DOI] [PubMed] [Google Scholar]
- Gonczy P. 2008. Mechanisms of asymmetric cell division: Flies and worms pave the way. Nat Rev Mol Cell Biol 9: 355–366. [DOI] [PubMed] [Google Scholar]
- Gray RS, Abitua PB, Wlodarczyk BJ, Szabo-Rogers HL, Blanchard O, Lee I, Weiss GS, Liu KJ, Marcotte EM, Wallingford JB, et al. 2009. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol 11: 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm-Gunter EM, Revenu C, Ramos S, Hurbain I, Smyth N, Ferrary E, Louvard D, Robine S, Rivero F. 2009. Plastin 1 binds to keratin and is required for terminal web assembly in the intestinal epithelium. Mol Biol Cell 20: 2549–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, et al. 2010. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol 12: 341–350. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. 2005. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol 170: 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, et al. 2010. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol 12: 170–176. [DOI] [PubMed] [Google Scholar]
- Hegan PS, Kravtsov DV, Caputo C, Egan ME, Ameen NA, Mooseker MS. 2015a. Restoration of cytoskeletal and membrane tethering defects but not defects in membrane trafficking in the intestinal brush border of mice lacking both myosin Ia and myosin VI. Cytoskeleton (Hoboken) 72: 455–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegan PS, Ostertag E, Geurts AM, Mooseker MS. 2015b. Myosin Id is required for planar cell polarity in ciliated tracheal and ependymal epithelial cells. Cytoskeleton (Hoboken) 72: 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzelman MB, Mooseker MS. 1990. Assembly of the brush border cytoskeleton: Changes in the distribution of microvillar core proteins during enterocyte differentiation in adult chicken intestine. Cell Motil Cytoskeleton 15: 12–22. [DOI] [PubMed] [Google Scholar]
- Heintzelman MB, Mooseker MS. 1992. Assembly of the intestinal brush border cytoskeleton. Curr Top Dev Biol 26: 93–122. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Tanaka Y, Okada Y. 2009. Left–right determination: Involvement of molecular motor KIF3, cilia, and nodal flow. Cold Spring Harb Perspect Biol 1: a000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi Y, Suzuki A, Yamanaka T, Sasaki K, Mizuno K, Sawada H, Yonemura S, Ohno S. 2009. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J Cell Sci 122: 1595–1606. [DOI] [PubMed] [Google Scholar]
- Hozumi S, Maeda R, Taniguchi K, Kanai M, Shirakabe S, Sasamura T, Speder P, Noselli S, Aigaki T, Murakami R, et al. 2006. An unconventional myosin in Drosophila reverses the default handedness in visceral organs. Nature 440: 798–802. [DOI] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. 2010. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329: 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. 2003. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol 5: 137–142. [DOI] [PubMed] [Google Scholar]
- Hurd TW, Fan S, Margolis BL. 2011. Localization of retinitis pigmentosa 2 to cilia is regulated by Importin β2. J Cell Sci 124: 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. 2011. Ciliogenesis: Building the cell’s antenna. Nat Rev Mol Cell Biol 12: 222–234. [DOI] [PubMed] [Google Scholar]
- Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. 2002. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature 416: 178–183. [DOI] [PubMed] [Google Scholar]
- Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. 2008. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet 40: 69–77. [DOI] [PubMed] [Google Scholar]
- Julio G, Merindano D, Canals M, Rallo M. 2010. Image processing techniques in a preliminary morphometric characterization of corneal epithelium by scanning electron microscopy. Microsc Res Tech 73: 1059–1066. [DOI] [PubMed] [Google Scholar]
- Kallay LM, McNickle A, Brennwald PJ, Hubbard AL, Braiterman LT. 2006. Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J Cell Biochem 99: 647–664. [DOI] [PubMed] [Google Scholar]
- Kee HL, Dishinger JF, Blasius TL, Liu CJ, Margolis B, Verhey KJ. 2012. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat Cell Biol 14: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal P, Abraham SN, Apodaca G. 2009. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297: F1477–F1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Krishnaswami SR, Gleeson JG. 2008. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet 17: 3796–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirjavainen A, Laos M, Anttonen T, Pirvola U. 2015. The Rho GTPase Cdc42 regulates hair cell planar polarity and cellular patterning in the developing cochlea. Biol Open 4: 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto N, Sawamoto K. 2012. Planar polarity of ependymal cilia. Differentiation 83: S86–S90. [DOI] [PubMed] [Google Scholar]
- Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. 2010. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci 107: 6346–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Dynlacht BD. 2011. Regulating the transition from centriole to basal body. J Cell Biol 193: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krock BL, Perkins BD. 2014. The Par-PrkC polarity complex is required for cilia growth in zebrafish photoreceptors. PLoS ONE 9: e104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLonde DP, Garbett D, Bretscher A. 2010. A regulated complex of the scaffolding proteins PDZK1 and EBP50 with ezrin contribute to microvillar organization. Mol Biol Cell 21: 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA. 2012. Spindle orientation in mammalian cerebral cortical development. Curr Opin Neurobiol 22: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K. 2011. Fundamental role of microvilli in the main functions of differentiated cells: Outline of an universal regulating and signaling system at the cell periphery. J Cell Physiol 226: 896–927. [DOI] [PubMed] [Google Scholar]
- Laprise P, Tepass U. 2011. Novel insights into epithelial polarity proteins in Drosophila. Trends Cell Biol 21: 401–408. [DOI] [PubMed] [Google Scholar]
- Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, Beitel GJ, McGlade CJ, Tepass U. 2009. Yurt, Coracle, Neurexin IV and the Na+,K+-ATPase form a novel group of epithelial polarity proteins. Nature 459: 1141–1145. [DOI] [PubMed] [Google Scholar]
- Larsson L. 1975. The ultrastructure of the developing proximal tubule in the rat kidney. J Ultrastruct Res 51: 119–139. [DOI] [PubMed] [Google Scholar]
- Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB. 2009. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol 187: 1117–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Chung YD. 2015. Ciliary subcompartments: How are they established and what are their functions? BMB Rep 48: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee OK, Frese KK, James JS, Chadda D, Chen ZH, Javier RT, Cho KO. 2003. Discs-Large and Strabismus are functionally linked to plasma membrane formation. Nat Cell Biol 5: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Medina E, Arsanto JP, Le Bivic A. 2004. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell 15: 1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. 2004. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 430: 93–98. [DOI] [PubMed] [Google Scholar]
- Lu Q, Insinna C, Ott C, Stauffer J, Pintado PA, Rahajeng J, Baxa U, Walia V, Cuenca A, Hwang YS, et al. 2015a. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol 17: 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Schafer DA, Adler PN. 2015b. The Drosophila planar polarity gene multiple wing hairs directly regulates the actin cytoskeleton. Development 142: 2478–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis M, Axelrod JD. 2013. Regulation of PCP by the Fat signaling pathway. Genes Dev 27: 2207–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Simera HL, Petralia RS, Montcouquiol M, Wang YX, Szarama KB, Liu Y, Lin W, Deans MR, Pazour GJ, Kelley MW. 2015. Ciliary proteins Bbs8 and Ift20 promote planar cell polarity in the cochlea. Development 142: 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. 2009a. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J 28: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J, Ransom N, Astuto-Gribble L, Wilson MC, Deretic D. 2009b. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci 122: 2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell RE, Higginbotham JN, Shifrin DA Jr, Tabb DL, Coffey RJ, Tyska MJ. 2009. The enterocyte microvillus is a vesicle-generating organelle. J Cell Biol 185: 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina E, Williams J, Klipfell E, Zarnescu D, Thomas G, Le Bivic A. 2002. Crumbs interacts with moesin and β(Heavy)-spectrin in the apical membrane skeleton of Drosophila. J Cell Biol 158: 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merte J, Jensen D, Wright K, Sarsfield S, Wang Y, Schekman R, Ginty DD. 2010. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol 12: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B, Jacobs R, Li J, Chien S, Kintner C. 2007. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature 447: 97–101. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. 2009. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol 19: 924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Lukianov S, Ruiz WG, Cianciolo Cosentino C, Sanker S, Traub LM, Hukriede NA, Apodaca G. 2012. Requirement for a uroplakin 3a-like protein in the development of zebrafish pronephric tubule epithelial cell function, morphogenesis, and polarity. PLoS ONE 7: e41816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. 2003. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423: 173–177. [DOI] [PubMed] [Google Scholar]
- Morales FC, Takahashi Y, Kreimann EL, Georgescu MM. 2004. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci 101: 17705–17710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DG, Roos JM, Kemphues KJ. 1992. par-4, a gene required for cytoplasmic localization and determination of specific cell types in Caenorhabditis elegans embryogenesis. Genetics 130: 771–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Hess MW, Schiefermeier N, Pfaller K, Ebner HL, Heinz-Erian P, Ponstingl H, Partsch J, Rollinghoff B, Kohler H, et al. 2008. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat Genet 40: 1163–1165. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. 2007. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129: 1201–1213. [DOI] [PubMed] [Google Scholar]
- Nam SC, Choi KW. 2003. Interaction of Par-6 and Crumbs complexes is essential for photoreceptor morphogenesis in Drosophila. Development 130: 4363–4372. [DOI] [PubMed] [Google Scholar]
- Nayak GD, Ratnayaka HS, Goodyear RJ, Richardson GP. 2007. Development of the hair bundle and mechanotransduction. Int J Dev Biol 51: 597–608. [DOI] [PubMed] [Google Scholar]
- Nechiporuk T, Fernandez TE, Vasioukhin V. 2007. Failure of epithelial tube maintenance causes hydrocephalus and renal cysts in Dlg5−/− mice. Dev Cell 13: 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Malicki J. 2006. oko meduzy and related crumbs genes are determinants of apical cell features in the vertebrate embryo. Curr Biol 16: 945–957. [DOI] [PubMed] [Google Scholar]
- Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, Malicki J. 2008. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol 10: 437–444. [DOI] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, et al. 2003. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left–right axis determination. Nat Genet 34: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovalle WK, Nahirney PC. 2008. Netter’s essential histology. Saunders Elsevier, Philadelphia, PA. [Google Scholar]
- Pan J, You Y, Huang T, Brody SL. 2007. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J Cell Sci 120: 1868–1876. [DOI] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. 2006. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet 38: 303–311. [DOI] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. 2008. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 40: 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, Ready DF, Tepass U. 2002. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature 416: 143–149. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Mooseker MS. 1993. An in vitro model for the analysis of intestinal brush border assembly. I: Ultrastructural analysis of cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci 105: 445–460. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Bement WM, Mooseker MS. 1993. An in vitro model for the analysis of intestinal brush border assembly. II: Changes in expression and localization of brush border proteins during cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci 105: 461–472. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Dunbar L, Samuelson L, Gumucio DL. 1998. Targeted disruption of the mouse villin gene does not impair the morphogenesis of microvilli. Dev Dyn 211: 109–121. [DOI] [PubMed] [Google Scholar]
- Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T. 2003. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol 5: 301–308. [DOI] [PubMed] [Google Scholar]
- Pocha SM, Shevchenko A, Knust E. 2011. Crumbs regulates rhodopsin transport by interacting with and stabilizing myosin V. J Cell Biol 195: 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruliere G, Cosson J, Chevalier S, Sardet C, Chenevert J. 2011. Atypical protein kinase C controls sea urchin ciliogenesis. Mol Biol Cell 22: 2042–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiners J, van Wijk E, Marker T, Zimmermann U, Jurgens K, te Brinke H, Overlack N, Roepman R, Knipper M, Kremer H, et al. 2005. Scaffold protein harmonin (USH1C) provides molecular links between Usher syndrome type 1 and type 2. Hum Mol Genet 14: 3933–3943. [DOI] [PubMed] [Google Scholar]
- Reiners J, Nagel-Wolfrum K, Jurgens K, Marker T, Wolfrum U. 2006. Molecular basis of human Usher syndrome: Deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp Eye Res 83: 97–119. [DOI] [PubMed] [Google Scholar]
- Reiter JF, Blacque OE, Leroux MR. 2012. The base of the cilium: Roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep 13: 608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenu C, Ubelmann F, Hurbain I, El-Marjou F, Dingli F, Loew D, Delacour D, Gilet J, Brot-Laroche E, Rivero F, et al. 2012. A new role for the architecture of microvillar actin bundles in apical retention of membrane proteins. Mol Biol Cell 23: 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GP, de Monvel JB, Petit C. 2011. How the genetics of deafness illuminates auditory physiology. Annu Rev Physiol 73: 311–334. [DOI] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. 2005. Disruption of Bardet–Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 37: 1135–1140. [DOI] [PubMed] [Google Scholar]
- Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. 2008. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015. [DOI] [PubMed] [Google Scholar]
- Sadeqzadeh E, de Bock CE, O’Donnell MR, Timofeeva A, Burns GF, Thorne RF. 2014. FAT1 cadherin is multiply phosphorylated on its ectodomain but phosphorylation is not catalysed by the four-jointed homologue. FEBS Lett 588: 3511–3517. [DOI] [PubMed] [Google Scholar]
- Sandoz D, Chailley B, Boisvieux-Ulrich E, Lemullois M, Laine MC, Bautista-Harris G. 1988. Organization and functions of cytoskeleton in metazoan ciliated cells. Biol Cell 63: 183–193. [DOI] [PubMed] [Google Scholar]
- Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, et al. 2011. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 145: 513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome I, Curto M, McClatchey AI. 2004. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell 6: 855–864. [DOI] [PubMed] [Google Scholar]
- Schluter MA, Margolis B. 2012. Apicobasal polarity in the kidney. Exp Cell Res 318: 1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbagh M, Borg JP. 2014. Insight into planar cell polarity. Exp Cell Res 328: 284–295. [DOI] [PubMed] [Google Scholar]
- Sfakianos J, Togawa A, Maday S, Hull M, Pypaert M, Cantley L, Toomre D, Mellman I. 2007. Par3 functions in the biogenesis of the primary cilium in polarized epithelial cells. J Cell Biol 179: 1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Komatsu K, Hirao M, Toyooka Y, Koyama H, Tissir F, Goffinet AM, Uemura T, Fujimori T. 2014. Celsr1 is required for the generation of polarity at multiple levels of the mouse oviduct. Development 141: 4558–4568. [DOI] [PubMed] [Google Scholar]
- Shibayama T, Carboni JM, Mooseker MS. 1987. Assembly of the intestinal brush border: Appearance and redistribution of microvillar core proteins in developing chick enterocytes. J Cell Biol 105: 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe CW, Lu X. 2011. Kif3a regulates planar polarization of auditory hair cells through both ciliary and non-ciliary mechanisms. Development 138: 3441–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S. 1962. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol 15: 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin SP. 1968. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci 3: 207–230. [DOI] [PubMed] [Google Scholar]
- Speder P, Adam G, Noselli S. 2006. Type ID unconventional myosin controls left–right asymmetry in Drosophila. Nature 440: 803–807. [DOI] [PubMed] [Google Scholar]
- Spektor A, Tsang WY, Khoo D, Dynlacht BD. 2007. Cep97 and CP110 suppress a cilia assembly program. Cell 130: 678–690. [DOI] [PubMed] [Google Scholar]
- Strutt D, Warrington SJ. 2008. Planar polarity genes in the Drosophila wing regulate the localisation of the FH3-domain protein Multiple Wing Hairs to control the site of hair production. Development 135: 3103–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ohno S. 2006. The PAR-aPKC system: Lessons in polarity. J Cell Sci 119: 979–987. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S. 2002. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci 115: 3565–3573. [DOI] [PubMed] [Google Scholar]
- Takemura R, Masaki T, Hirokawa N. 1988. Developmental organization of the intestinal brush-border cytoskeleton. Cell Motil Cytoskeleton 9: 299–311. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S. 1994. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol 125: 1371–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarchini B, Jolicoeur C, Cayouette M. 2013. A molecular blueprint at the apical surface establishes planar asymmetry in cochlear hair cells. Dev Cell 27: 88–102. [DOI] [PubMed] [Google Scholar]
- Tasouri E, Tucker KL. 2011. Primary cilia and organogenesis: Is Hedgehog the only sculptor? Cell Tissue Res 345: 21–40. [DOI] [PubMed] [Google Scholar]
- ten Klooster JP, Jansen M, Yuan J, Oorschot V, Begthel H, Di Giacomo V, Colland F, de Koning J, Maurice MM, Hornbeck P, et al. 2009. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev Cell 16: 551–562. [DOI] [PubMed] [Google Scholar]
- Tepass U. 2012. The apical polarity protein network in Drosophila epithelial cells: Regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol 28: 655–685. [DOI] [PubMed] [Google Scholar]
- Tissir F, Qu Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, Fujimori T, Labeau J, Tyteca D, Courtoy P, et al. 2010. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci 13: 700–707. [DOI] [PubMed] [Google Scholar]
- Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. 2005. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell 16: 2443–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde KJ, Dhekne HS, Swertz MA, Sirigu S, Ropars V, Vinke PC, Rengaw T, van den Akker PC, Rings EH, Houdusse A, et al. 2013. An overview and online registry of microvillus inclusion disease patients and their MYO5B mutations. Hum Mutat 34: 1597–1605. [DOI] [PubMed] [Google Scholar]
- Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD. 2012. Microtubules enable the planar cell polarity of airway cilia. Curr Biol 22: 2203–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald FA, Oriolo AS, Mashukova A, Fregien NL, Langshaw AH, Salas PJ. 2008. Atypical protein kinase C (ι) activates ezrin in the apical domain of intestinal epithelial cells. J Cell Sci 121: 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Deretic D. 2014. Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res 38: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, et al. 2005. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet 37: 980–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. 2006. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci 26: 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Morita Y, Mazelova J, Deretic D. 2012. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J 31: 4057–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansleeben C, Feitsma H, Montcouquiol M, Kroon C, Cuppen E, Meijlink F. 2010. Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec24b. Development 137: 1067–1073. [DOI] [PubMed] [Google Scholar]
- Ward HH, Brown-Glaberman U, Wang J, Morita Y, Alper SL, Bedrick EJ, Gattone VH 2nd, Deretic D, Wandinger-Ness A. 2011. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol Biol Cell 22: 3289–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Beales PL. 2011. Ciliopathies: An expanding disease spectrum. Pediatr Nephrol 26: 1039–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Morton DG, Bestman J, Kemphues KJ. 2000. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development 127: 1467–1475. [DOI] [PubMed] [Google Scholar]
- Wei Q, Zhang Y, Li Y, Zhang Q, Ling K, Hu J. 2012. The BBSome controls IFT assembly and turnaround in cilia. Nat Cell Biol 14: 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al. 2011. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci 108: 2759–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum U, Liu X, Schmitt A, Udovichenko IP, Williams DS. 1998. Myosin VIIa as a common component of cilia and microvilli. Cell Motil Cytoskeleton 40: 261–271. [DOI] [PubMed] [Google Scholar]
- Wong LL, Adler PN. 1993. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J Cell Biol 123: 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Huen D, Morely T, Johnson G, Gubb D, Roote J, Adler PN. 2008. The multiple-wing-hairs gene encodes a novel GBD-FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics 180: 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga T, Hoff S, Schell C, Helmstadter M, Kretz O, Kuechlin S, Yakulov TA, Engel C, Muller B, Bensch R, et al. 2015. The polarity protein Inturned links NPHP4 to Daam1 to control the subapical actin network in multiciliated cells. J Cell Biol 211: 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaria S, Mao Y, Kuta A, Ferreira de Sousa C, Gaufo GO, McNeill H, Hindges R, Guthrie S, Irvine KD, Francis-West PH. 2014. Regulation of neuronal migration by Dchs1-Fat4 planar cell polarity. Curr Biol 24: 1620–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilber Y, Babayeva S, Seo JH, Liu JJ, Mootin S, Torban E. 2013. The PCP effector Fuzzy controls cilial assembly and signaling by recruiting Rab8 and Dishevelled to the primary cilium. Mol Biol Cell 24: 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Guo W, Lipschutz JH. 2009. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell 20: 2522–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Fogelgren B, Lipschutz JH. 2011. The small GTPase Cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells. J Biol Chem 286: 22469–22477. [DOI] [PMC free article] [PubMed] [Google Scholar]