Abstract
Objectives
Sinus hypoplasia is a hallmark characteristic in cystic fibrosis (CF). Chronic rhinosinusitis (CRS) is nearly universal from a young age, impaired sinus development could be secondary to loss of the cystic fibrosis transmembrane conductance regulator (CFTR) or consequences of chronic infection during maturation. The objective of this study is to assess sinus development relative to overall growth in a novel CF animal model.
Methods
Sinus development was evaluated in CFTR-/- and CFTR+/+ rats at 3 stages of development – newborns, 3 weeks, and 16 weeks. MicroCT scanning, cultures, and histology were performed. Three-dimensional sinus and skull volumes were quantified.
Results
At birth, sinus volumes were decreased in CFTR-/- rats compared to wild-type (in mm3 +/- SEM; 11.3 +/- 0.85 vs. 14.5 +/- 0.73; p<0.05) despite similar weights (in g +/- SEM; 8.4+/-0.46 vs. 8.3+/-0.51; p=0.86). CF rat weights declined by 16 weeks (378.4 +/- 10.6 vs. 447.4 +/- 15.9; p<0.05), sinus volume increased similar to wild-type rats (201.1 +/- 3.77 vs. 203.4 +/- 7.13; p=0.8). The ratio of sinus volume to body weight indicates hypoplasia is present at birth (1.37 +/- 0.12 vs. 1.78 +/- 0.11; p<0.05) and increases compared to CFTR+/+ animals by 16 weeks (0.53 +/- 0.02 vs. 0.46 +/- 0.02; p<0.05). Rats didn't develop histological evidence of chronic infection.
Conclusion
CF rat sinuses are smaller at birth, but develop volumes similar to wild-type rats with maturation. This suggests that loss of CFTR may confer sinus hypoplasia at birth, but normal development ensues without chronic sinus infection.
Keywords: cystic fibrosis, CFTR, sinusitis, chronic sinusitis, chronic rhinosinusitis, CF rat, animal models, sinus development, sinus hypoplasia, sinusitis
Introduction
Cystic fibrosis (CF) is the most prevalent genetic disease affecting the Caucasian population, with an annual incidence of 1 in 2,500-3,5000 newborns born with the lethal disease.1,2 In CF, absent or aberrant cystic fibrosis transmembrane conductance regulator (CFTR) confers lifelong complications of both the upper and lower airways.3 Chronic rhinosinusitis (CRS) typically begins at an early age with significant nasal polyposis, which can be one of the first presenting signs for some individuals. There are marked differences in CF sinus anatomy compared to the normal population, such as sinus hypoplasia or aplasia and demineralization of the uncinate process. 4-7 Indeed, pansinus hypoplasia is nearly universal and represents a classic hallmark of CF. Because chronic infection generally arises prior to complete pneumatization of the sinuses, it unknown whether defective CFTR directly interferes with normal embryogenesis and development or whether hypoplasia is secondary to onset of infection.2,8,9
Since first linking CF to loss of CFTR almost 30 years ago, researchers have developed a number of animal models in efforts to reproduce human disease.10,11 Traditionally, transgenic CF murine models have been widely utilized in both pharmaceutical testing and studies of CF pathogenesis. However, their small size and inability to develop spontaneous sinus disease preclude the accurate evaluation of CFTR impact on sinus development.12,13 Recently, CF ferret14 and pig15 models have been developed, both of which exhibit robust CF respiratory phenotypes. Importantly, the CF pig has provided new insight regarding CFTR influence on sinus development, particularly because the pig possesses large sinuses and develops spontaneous sinusitis, which is similar to what is observed in humans.16,17 In one study, Chang et al. found that sinus hypoplasia was present prior to the onset of infection and that sinus development was delayed well into adulthood.4 Despite their semblance to the human CF phenotype, both pigs and ferrets are associated with a number of limitations that restrict their widespread use. For example, they are costly, require specialized animal husbandry, and experience increased gestation and time to sexual maturation.18
Recently, the development of the CFTR-/- rat model (Rattus norvegicus; SD-CFTRtm1sage), generated by Sigma Advanced Genetic Engineering Labs in collaboration with investigators at the University of Alabama Birmingham (UAB), has permitted a number of advantages. For example, the rat develops a substantial amount of submucosal glands19 and significantly larger sinuses in comparison to mice, which facilitates evaluation of sinus volumes and pneumatization patterns.20 Moreover, rats display a brief gestational period followed by rapid sexual maturation, which is favorable in the setting of longitudinal and breeding studies.18
The goal of this study is to evaluate sinus development in comparison to overall growth at longitudinal time points in the novel CF rat model.
Methods
Acquisition of Rat Models
Institutional Animal Care and Use Committee (IACUC) approval was obtained prior to the initiation of the study. Male CFTR+/+ and CFTR-/- rats were bred in the CF Animal Core facility at UAB and generated by SAGE Labs, Inc. using zinc-finger endonuclease based gene disruption on a Sprague-Dawley rat background as previously described.18 CFTR-/- rats have a 16 base-pair deletion of exon 3, which leads to loss of CFTR expression.18 Special diets and laxatives were administered to CFTR-/- rat models to allow longitudinal evaluation of CF-related pathology for a period of months rather than weeks post-weaning. This curtailed prior issues regarding reduced body weight and decreased survival in CF rats, which prohibited such longitudinal studies.
MicroCT
Sinus development was evaluated in CFTR-/- and CFTR+/+ rats at three different stages of development: newborn (defined 2 days old), 3 weeks (weaning), and 16 weeks. Three-dimensional (3D) sinus and skull volumes were quantified for assessment. Borders of rat nasal sinuses were defined by the following criteria: anterior starting point is defined by the beginning of the maxillary sinuses; posterior is marked by the termination of the ethmoid sinuses; superior is considered to be everything superior to the base of the septum. However, it should be noted that maxillary sinuses inferior to the base of the septum were also included. Following these border demarcations, volumetric analysis included all of the maxillary sinuses and ethmoid sinuses, and the nasal lumen posterior to the base of the septum. Excised rat skulls (fresh, unfixed) were scanned using the Scanco μCT40 desktop cone-beam micro-CT scanner and μCT Tomography software v5.44 (Scanco Medical AG, Brüttisellen, Switzerland). All skulls were wrapped in a dry kimwipe for scans.
Newborn rat skulls were placed vertically in a 20mm diameter scanning holder and scanned at the following settings: 20μm resolution, 500 projections/180°, 70kVp, 114μA with an integration time of 200ms. Three-week-old rat skulls were placed vertically in a 30mm diameter scanning holder and scanned at the following settings: 30μm resolution, 250 projections/180°, 70kVp, 114μA with an integration time of 200ms. Sixteen-week-old rat skulls were placed vertically in a 36mm diameter scanning holder and scanned at the following settings: 36μm resolution, 250 projections/180°, 70kVp, 114μA with an integration time of 200ms. All scans were automatically reconstructed into 2-D slices using the μCT Evaluation Program (v6.5-2, Scanco Medical). 3D images were obtained from the 3D evaluation software (μCT Ray v3.8, Scanco Medical). In all instances, sinus cavities were outlined and volume was thresholded using grayscale values of -1000 to 50, in order to distinguish them from bone and tissue.
Histology of Rat Nasal Tissue
CFTR+/+ and CFTR-/- rats were euthanized at three separate stages of development—newborns, 3 weeks, and 16 weeks—and sinonasal cavities assessed for any evidence of inflammation and infection on histological examination. Rat nasal septum samples were fixed in 10% formalin. Blocks were then decalcified, grossly sectioned, embedded in paraffin and subsequently sectioned and mounted on glass sides. Finally, slides were stained with hematoxylin and eosin for identification of inflammatory cells and exudate as well as alcian blue periodic acid Schiff (AB-PAS) for identification of mucosubstances. All slides were assessed in a blinded fashion for the presence of inflammatory exudate, lymphoid aggregates, distended submucosal glands, and goblet cell hyperplasia.
Statistical Analysis
Statistical analyses were performed in Microsoft Excel using Student's t-test for comparing differences in sinus volumes and body weight between CFTR+/+ and CFTR-/- rats. A p-value<0.05 was considered statistically significant. All reported values are reported as the mean +/- standard error of the mean [SEM].
Results
CFTR+/+ and CFTR-/- rats were bred and genotyped by the UAB CF Animal Models Core as previously described.18
The CFTR -/- rat exhibits pansinus hypoplasia and delayed sinus development in the neonatal period (Figure 1A)
Figure 1.

Interleaved scatter plots demonstrating sinus volume (A) and body weight (B) as a function of time in CFTR+/+ and CFTR-/- rats. Error bars represent SEM. Significant findings are bracketed with an asterisk.
After birth, 2-day old rats were sacked and sinus volumes evaluated with microCT scanning (Figure 4). We found that the CFTR-/- rats (n=7) had significantly decreased sinus volume (in mm3) when compared to wild type (n=6) (11.3 +/- 0.85 vs. 14.5 +/- 0.73, respectively; p<0.05). At 3 weeks, however, sinus volumes were not significantly different between CFTR+/+ (n=5) and CFTR-/- (n=6) rats (58.0 +/- 3.3 vs. 59.3+/-3.5, respectively; p=0.8) (Figure 5). Similarly, 16-week old sacked CFTR+/+ (n=5) and CFTR-/- (n=5) rats again demonstrated similar sinus volumes (201.1 +/- 3.8 vs. 203.4 +/- 7.1; p=0.78) (Figure 6).
Figure 4.

3D replication of CFTR+/+ (superior) and CFTR-/- (inferior) newborn rat sinuses in the coronal (A), transverse (B), and sagittal (C) planes.
Figure 5.

3D replication of CFTR+/+ (superior) and CFTR-/- (inferior) 3-week old rat sinuses in the coronal (A), transverse (B), and sagittal (C) planes.
Figure 6.

3D replication of CFTR+/+ (superior) and CFTR-/- (inferior) 16-week old rat sinuses in the coronal (A), transverse (B), and sagittal (C) planes.
Body weight declines in the CFTR -/- rat over time (Figure 1B)
Body weight was evaluated in both CFTR+/+ (n=6) and CFTR-/- (n=7) rats. Both groups exhibited similar weights (g) at birth (8.4 +/- 0.46 [CFTR-/-] vs. 8.3+/-0.51 [CFTR+/+]; p=0.86). At age 3 weeks, body weight of CFTR-/- rats (n=6) began to decline compared to that of CFTR+/+ (n=5) (43.1 +/- 1.73 vs. 49.4 +/- 3.0, respectively; p=0.09). By age 16 weeks, CFTR-/- rats (n=5) demonstrated significantly reduced body weight as compared to wild type (378.4 +/- 10.6 vs. 447.4 +/- 15.9; p<0.05).
The ratio of sinus volume/weight decreases over time in both CFTR +/+ vs. CFTR -/- rats (Figure 2)
Figure 2.

Interleaved scatter plot demonstrating ratio of sinus volume to body weight as a function of time in CFTR+/+ and CFTR-/- rats. Error bars represent SEM. Significant findings are bracketed with an asterisk.
At birth, newborn CFTR-/- rats demonstrated a significantly reduced sinus volume-to-weight ratio when compared to wild type due to significant sinus hypoplasia (1.37+/-0.12 vs. 1.78+/-0.03; p-value <0.05). By age 3 weeks, normalization of sinus hypoplasia results in comparable ratios between both groups (1.34+/-0.06 [CFTR-/-] vs. 1.22+/-0.09 [CFTR+/+]; p=0.27). At age 16 weeks, however, CFTR-/- rats exhibit a significantly increased sinus volume-to-weight ratio due to a rapidly declining body weight (0.53 +/- 0.02 vs. 0.46 +/- 0.02; p<0.05), which suggests that sinus development is maintained despite significant nutritional deficiency in CFTR-/- rat.
CF sinus epithelia demonstrated no evidence of infection (Figure 3)
Figure 3.
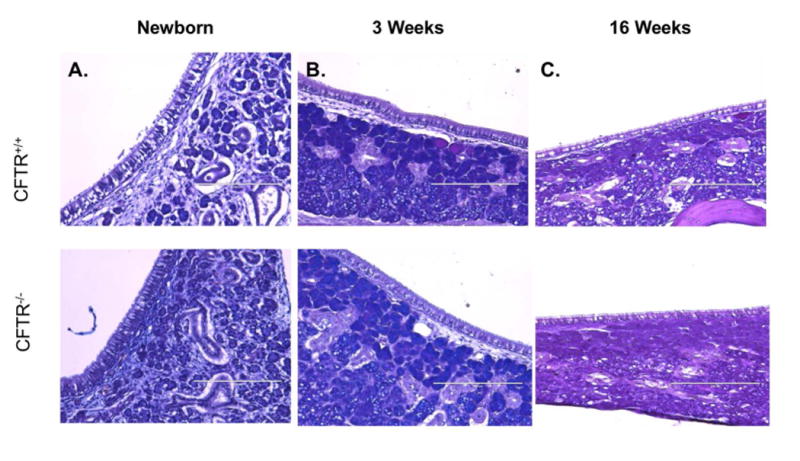
AB-PAS staining of CFTR+/+ (superior) and CFTR-/- (inferior) rat maxillary sinus epithelia at newborn (A), 3- (B) and 16-weeks (C).
CFTR+/+ and CFTR-/- rat maxillary sinonasal epithelia were examined for any obvious evidence of infection, including inflammatory exudate and lymphoid aggregates. Both genotypes exhibited non-distended mucus within submucosal glands. Goblet cells were similar between groups with no observable hyperplasia or trapped mucus in the CFTR-/- group. Overall, there were no differences between wild type and knockout rats.
Discussion
Use of animal models has been crucial to furthering our understanding of CF pathogenesis. Although murine models have dominated previous studies, researchers have since developed a number of animal models in an attempt to better emulate the widespread multi-organ pathology exhibited by humans.
In the present study, we aimed to assess sinus development as it relates to nutritional growth in CF rats. Our findings suggest that CFTR plays an important role during in utero sinus development, which was evident from the significantly hypoplastic sinuses present in newborn CF rats. Interestingly, we found that sinus development in the CF rat rapidly attained normal size by weaning age, continuing to enlarge through the age of four months. This is in stark contrast to that demonstrated by porcine models, which exhibit persistent sinus hypoplasia with development of chronic sinus infection.4 Unlike pigs, however, CF rats did not develop any evidence of infection despite exhibiting neonatal sinus hypoplasia. This suggests that, in the absence of infection, sinus development progresses as expected, if not at an increased rate, to become comparable to that demonstrated by adult wild type rats. Furthermore, these findings support previous reports by highlighting the importance of CFTR in the regulation of sinus development.4 While the pathogenesis of CRS in CF continues to remain uncertain, we can conclude that in the CFTR-/- rat model, neonatal sinus hypoplasia does not represent the inciting event for development of sinus inflammation and infection. Of note, the majority of rat epithelia is olfactory in nature and this may play a role in why they lack upper respiratory disease. However, this olfactory epithelium is not present within the maxillary sinus, which in our study, showed normalization of growth in the absence of infection. Therefore, findings here suggest that infection is a key component for persistent sinus hypoplasia.8
Development of CF sinus disease is unquestionably multi-factorial, but we can conclude that sinus hypoplasia begins in utero with CFTR dysfunction in the CF rat and pig and likely reflects what is seen in humans. The sinuses are fluid-filled in utero and the sinus development could perhaps be affected by the aberrant ionic composition of the fluid from CFTR deficiency. It is interesting to note that sinus volumes “catch up” to wild type with time despite declining body weight over time.21 While widespread nutritional deficiency and low body weight are classic findings in CF, they do not appear to have a significant effect on the development of rat sinuses outside of the newborn period.
Although rats do not appear to develop evidence of sinus infection, it does not preclude their use for future studies regarding sinus disease. Early studies indicate respiratory infection in CF rats can be initiated with introduction of Pseudomonas aeruginosa. If infection can be induced, then the impact on sinus development can be measured. Future studies challenging the sinonasal cavities of the CF rat with Pseudomonas aeruginosa are planned.
Conclusion
CF rats have sinus hypoplasia at birth, which is similar to both CF pigs and human CF sinusitis. However, they do not form spontaneous infections as seen in the CF pig and human CF sinus disease. In the absence of infection, the rat continued to exhibit progressive growth of the sinonasal cavities. This indicates persistent sinus hypoplasia may require both absent or dysfunctional CFTR and persistent chronic infection and inflammation during pneumatization.
Acknowledgments
Funding Support: This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006-01) and National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-04, CF Research Center Pilot Award) to B.A.W. and National Institutes of Health (T32CA091078) to K.E.T.
Footnotes
Please address all requests for reprints to this author.
Level of Evidence: NA.
Disclosures: All authors have read and approved the manuscript. Dr. Bradford A. Woodworth is a consultant for Cook Medical, Olympus, and Smith and Nephew. An oral presentation of this work was presented at the 2017 American Rhinologic Society's Spring Meeting on April 27, 2017 in San Diego, California. Manuscript ID#: 1930
References
- 1.Grosse SD, Boyle CA, Botkin JR, et al. Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. MMWR Recomm Rep. 2004;53(RR-13):1–36. [PubMed] [Google Scholar]
- 2.Chang EH. New insights into the pathogenesis of cystic fibrosis sinusitis. Int Forum Allergy Rhinol. 2014;4(2):132–137. doi: 10.1002/alr.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy. 2013;27(5):387–395. doi: 10.2500/ajra.2013.27.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang EH, Pezzulo AA, Meyerholz DK, et al. Sinus hypoplasia precedes sinus infection in a porcine model of cystic fibrosis. Laryngoscope. 2012;122(9):1898–1905. doi: 10.1002/lary.23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le C, McCrary H, Chang E. Cystic Fibrosis Sinusitis. In: Woodworth BPDR, DD, editor. Rhinosinusitis with Nasal Polyposis. Vol. 792016. pp. 29–37. [Google Scholar]
- 6.Woodworth B, Ahn C, Flume P, Schlosser R. The delta F508 mutation in cystic fibrosis and imapct on sinus development. Am J Rhinol. 2007;21:122–127. doi: 10.2500/ajr.2007.21.2905. [DOI] [PubMed] [Google Scholar]
- 7.Eggesbo H, Sovik S, Dolvik S, Eiklid K, Kolmannskog F. CT characterization of developmental variations of the paranasal sinuses in cystic fibrosis. Acta Radiol. 2001;42:482–493. doi: 10.1080/028418501127347214. [DOI] [PubMed] [Google Scholar]
- 8.King V. Upper respiratory disease, sinusitis, and polyposis. Clinical Review of Allergy. 1991;9:143–157. [PubMed] [Google Scholar]
- 9.Kim HJ, Friedman EM, Suley M, Duncan NO, McCluggage C. Paranasal Sinus Development in Chronic Sinusitis, Cystic Fibrosis, Normal Comparison Population: A Computer Tomography Correlation Study. American Journal of Rhinology. 1997;11(4):275–281. doi: 10.2500/105065897781446676. [DOI] [PubMed] [Google Scholar]
- 10.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 11.Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol. 2007;36(1):1–7. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho-Oliveira I, Scholte BJ, Penque D. What have we learned from mouse models for cystic fibrosis? Expert Rev Mol Diagn. 2007;7(4):407–417. doi: 10.1586/14737159.7.4.407. [DOI] [PubMed] [Google Scholar]
- 13.Woodworth B, Antunes M, Bhargave G, Palmer J, Cohen N. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: a comparative study. Am J Rhinol. 2007;21:533–537. doi: 10.2500/ajr.2007.21.3068. [DOI] [PubMed] [Google Scholar]
- 14.Sun X, Sui H, Fisher JT, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120(9):3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers CS, Hao Y, Rokhlina T, et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008;118(4):1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavelle GM, White MM, Browne N, McElvaney NG, Reeves EP. Animal Models of Cystic Fibrosis Pathology: Phenotypic Parallels and Divergences. Biomed Res Int. 2016;2016:5258727. doi: 10.1155/2016/5258727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean N, Ranganath N, Jones B, et al. Porcine nasal epithelial cultures for studies of cystic fibrosis sinusitis. Int Forum Allergy Rhinol. 2014;4:565–570. doi: 10.1002/alr.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuggle KL, Birket SE, Cui X, et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One. 2014;9(3):e91253. doi: 10.1371/journal.pone.0091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smolich JJ, Stratford BF, Maloney JE, Ritchie BC. New features in the development of the submucosal gland of the respiratory tract. J Anat. 1978;127(Pt 2):223–238. [PMC free article] [PubMed] [Google Scholar]
- 20.Tipirneni K, Cho DY, Skinner D, et al. Characterization of primary rat nasal epithelial cultures in CFTR knockout rats as a model for CF sinus disease. Laryngoscope. 2017 doi: 10.1002/lary.26720. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai HC, Kosorok M, Sondel S, et al. Growth status in children with cystic fibrosis based on National Cystic Fibrosis Patient Registry data: Evaluation of various criteria used to identify malnutrition. The Journal of Pediatrics. 1998;132(3):478–485. doi: 10.1016/s0022-3476(98)70024-1. [DOI] [PubMed] [Google Scholar]


