Abstract
Gastrointestinal stromal tumor (GIST) is the most common type of sarcoma and usually harbors either a KIT or PDGFRA mutation. However, the molecular basis for tumor malignancy is not well defined. While the Wnt/β-catenin signaling pathway is important in a variety of cancers, its role in GIST is uncertain. Through analysis of nearly 150 human GIST specimens, we found that some human GISTs expressed β-catenin and contained active, dephosphorylated nuclear β-catenin. Furthermore, advanced human GISTs expressed reduced levels of the Wnt antagonist DKK4. Accordingly, in human GIST T1 cells, Wnt stimulation increased β-catenin–mediated transcriptional activity in a reporter assay as well as transcription of the downstream target genes axin2 and cyclin D1. In contrast, DKK4 overexpression in GIST T1 cells reduced Wnt/β-catenin signaling. Additionally, we showed that nuclear β-catenin stability was partially regulated by the E3 ligase COP1, as demonstrated with co-immunoprecipitation and COP1 knockdown. Three molecular inhibitors of the Wnt/β-catenin pathway demonstrated anti-tumor efficacy in various GIST models, both in vitro and in vivo. Notably, the tankyrase inhibitor G007-LK alone had substantial activity against tumors of genetically engineered KitV558Δ/+ mice, and the effect was increased by the addition of the Kit inhibitor imatinib mesylate. Collectively, our findings demonstrate that Wnt/β-catenin signaling is a novel therapeutic target for selected untreated or imatinib-resistant GISTs.
Keywords: Wnt/β-catenin signaling, gastrointestinal stromal tumor, targeted therapy
INTRODUCTION
Gastrointestinal stromal tumor (GIST) is the most common subtype of human sarcoma and typically arises from the stomach or small intestine (1). Most GISTs have an activating mutation in either KIT or PDGFRA (2, 3). Targeting the KIT or PDGFRA oncogenic proteins with the selective tyrosine kinase inhibitor imatinib mesylate has markedly increased survival in advanced GIST (4). Despite its efficacy, though, imatinib is rarely curative. The median time to resistance to imatinib is 2 years, most often due to secondary KIT mutations (5).
The molecular basis for aggressive tumor biology in GIST remains poorly defined. For instance, the criteria for assessing the risk of recurrence following the resection of a localized, primary GIST are based on the pathologic factors of tumor mitotic activity, size, and location, while tumor KIT mutation status is not an independent predictor of recurrence, implying that other signaling processes may be involved (6). Multiple regulatory proteins or molecular alterations have been associated with aggressive phenotypes in GIST (7). For example, expression of miR-196a was associated with poor survival and malignant progression in GIST (8) and amplification of cell cycle genes correlated with increased malignant potential in GIST (9). Deletions of 9p targeting CDKN2A are common in GIST and loss of p16 has been demonstrated in high-risk GIST (10, 11). In addition, the CINSARC gene signature is linked to expression of p16 and RB1 with increased risk of metastatic disease (12). A subpopulation of GISTs with wild-type KIT and PDGFRA instead has a BRAF mutation (13) or succinate dehydrogenase (SDH) deficiency (14). Very recently, loss of myc-associated protein (MAX) was shown to be an early genomic event of GIST progression (15). A greater understanding of the mechanisms of GIST tumor malignancy is necessary to identify additional therapeutic targets given the limitations of current tyrosine kinase inhibitors once resistance develops.
Wnt pathway components are well known to contribute to tumor progression and metastasis in a variety of cancers (16–18). Wnt signaling includes canonical and noncanonical pathways. Canonical Wnt signaling is initiated by Wnt ligands binding to Frizzled (FZD)/low-density lipoprotein receptor-related protein (LRP) receptor complexes, which inactivate the GSK3β-Axin-APC destruction complex leading to signaling via the β-catenin/T cell factor (TCF) pathway (19). The dickkopf-related protein (DKK) family comprises secreted antagonists of Wnt signaling that bind and block the Wnt receptors LRP5/6. Several DKKs have been reported to be downregulated in various cancers and restoration of their expression can inhibit cell proliferation and tumor growth (17, 20). Wnt/β-catenin signaling plays an important role in tumor initiation and progression through regulation of the cell cycle, stem cell self-renewal, and epithelial-to-mesenchymal transition (19, 21). Aberrant accumulation of nuclear β-catenin is the hallmark of Wnt activation. Stabilized β-catenin associated with its co-transcriptional factor TCF/lymphoid enhancer factor (TCF/LEF) in the nucleus triggers upregulation of proto-oncogenes such as MYC and cyclin D1. Multiple pathways have been reported to regulate the nuclear translocation and stability of β-catenin (22, 23). The role of Wnt/β-catenin signaling in GIST has not been defined.
In this study, we showed that Wnt/β-catenin components were overexpressed in a subset of human GISTs. Consistently, we found aberrant accumulation of nuclear β-catenin in patient-derived xenografts (PDXs) and murine imatinib-resistant models. DKK4 mRNA expression was reduced in most metastases relative to primary, untreated tumors and overexpression of DKK4 inhibited the Wnt/β-catenin signaling in vitro. Furthermore, COP1 (photomorphogenesis protein 1), a tumor suppressor and E3 ligase, was also involved in the regulation of nuclear β-catenin stability and its transcriptional activity in GIST cells. Lastly, we showed that Wnt signaling was required for tumor survival in multiple preclinical GIST models, both in imatinib-naïve and imatinib-resistant GISTs. Therefore, our findings highlight the significant role of Wnt/β-catenin activation in GIST and provide the rationale for targeting Wnt/β-catenin signaling as a potential therapeutic strategy for GIST.
MATERIALS AND METHOD
Human tissue microarray and tumor samples
IRB approval was obtained for all human tissue studies. A human GIST tissue paraffin microarray was constructed containing more than 100 archival specimens from patients who underwent resection at Memorial Sloan Kettering Cancer Center between 1984 and 2004. We also obtained 46 more recent GIST specimens. Mitotic rate (number of mitoses per 50 high power fields) and mutation status for each specimen were recorded. Single cell suspensions were generated from fresh tumors after mincing and digestion with 10 mg/ml collagenase type II (Worthington Biochemicals) plus 50 μg/ml DNAse I (Roche Diagnostics) in Ca2+ and Mg2+-free HBSS (Invitrogen) at 37°C for 30 minutes. The tumor suspensions were filtered through a 100 μm cell strainer and mixed with an equal volume of 100% fetal bovine serum (FBS) to quench the collagenase. In some cases, KIT+ cells were purified using human CD117 microbeads (Miltenyi Biotec) to a purity that exceeded 90% by flow cytometry.
Patient derived-xenografts (PDXs)
Human HG130 was derived from an imatinib-resistant liver metastasis that contained KIT exon 13 V654A and KIT exon 11 W557R mutations. Human HG324 was derived from an imatinib-resistant peritoneal GIST metastasis containing a KRAS G12V and SDHB mutation. Bulk tumor cells were freshly isolated from tumors using collagenase as above and injected into the flanks of NSG mice.
GIST cell lines and treatments
The human GIST882 cell line (KIT exon 13 mutant) was kindly provided by Dr. Jonathan Fletcher. Human GIST T1 and murine S2 GIST cells (both KIT exon 11 mutant) have been described previously (24, 25). We established the HG129 cell line from a primary, untreated gastric GIST harboring a KIT exon 11 mutation in 2012 (26). Human HG209 was derived from an imatinib- and sunitinib-resistant peritoneal metastasis, and has a KIT exon 11 YIDPTQL 570–576 deletion and a KIT exon 17 point mutation (C. 2446 C>G p. D816H) in 2013. Cell lines were cultured in RPMI 1640 supplemented with 10% FBS and 1% penicillin/streptomycin. PKF118-310, XAV939, and G007-LK were purchased from Calbiochem. Imatinib was purchased from LC Laboratories. MG132 and cycloheximide were from Sigma-Aldrich. A Dojindo assay (Cell Counting Kit 8, Dojindo) was carried out at 72h to measure the cell viability after incubation of 1×104 cells in 96-well plates with PKF118-310, XAV939, G007-LK, imatinib, or media plus 0.5% DMSO.
Real-time PCR
Total RNA was extracted from human GIST tissues or cells, reverse transcribed, and amplified with PCR TaqMan probes for human DKK4 (Hs00205290_ml), FZD6 (Hs00171574_ml), FZD7 (Hs00275833_sl), LRP6 (Hs00233945_ml), CCND1 (Hs00765553_ml), CTNNB1 (Hs00355049_ml), Axin2 (Hs00610344_ml), TCF4 (Hs00162613_ml), and GAPDH (Applied Biosystems). Quantitative PCR was performed using a ViiATM7 real-time PCR system (Applied Biosystems). Data were calculated by the 2−ΔΔCt method as described in the manufacturer’s instructions and were expressed as fold increase over the indicated controls.
Gene knockdown and overexpression
For transient COP1 knockdown or β-catenin knockdown, GIST T1 cells were transfected with 30 nM of On-Target plus Smartpool siRNA for human COP1 (E-007949-00), On-Target plus Smartpool siRNA for human β-catenin (L-003482-00), or a non-target control siRNA (D-001810-10-05; Thermo Scientific) using Lipofectamine RNAiMAX (Invitrogen) for 48h. To generate overexpressing cells, GIST T1 were transfected with empty control vector (pCMV6-mock, PS100001), human β-catenin (pCMV6-β-catenin, RC208947), DKK4 (pCMV6-DKK4, RC221217), or COP1 plasmid (pCMV6-COP1, RC210492, all from Origene) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
Microarray analysis
RNA was isolated from human KIT+ cells using the RNAeasy Plus Mini Kit (Qiagen). Gene expression microarray analysis was performed by the Genomics Core Laboratory of Sloan Kettering Institute using Human Genome U133A 2.0 microarrays (Affymetrix). Microarray data were analyzed using Partek Genomics Suite version 6.5. After log transformation and quantile normalization, ANOVA was performed to compare multiple groups. Statistically significant genes with a False Discovery Rate <0.05 were selected and analyzed using KEGG Pathway Analysis software (Ingenuity Systems).
Immunohistochemistry
Formalin-fixed and paraffin-embedded human tumors, xenograft tumors, and tumors from KitV558Δ/+ mice were sectioned at 5 μm thickness. Antigen retrieval was achieved with citrate buffer. Immunohistochemistry was performed using the following antibodies and the corresponding isotype control IgG: anti-human β-catenin IgG (Clone β-catenin 1, DAKO), Ki-67 (Vector Labs) and β-catenin (6B3, Cell Signaling Technology).
Western blot
Western blot of whole protein lysates or nuclear proteins from frozen tumor tissues or cells was performed as previously described (24). Antibodies for cleaved caspase 3 (Asp175) (5A1E), cleaved PARP (Asp214) (D64E10), cyclin D1 (92G2), β-catenin (6B3), active β-catenin (D13A1), TCF4 (C48H11), axin 1 (C95H11), tubulin (DM1A) and GAPDH (D16H11) were purchased from Cell Signaling Technology. Other antibodies included lamin B1 (ab16048, Abcam), COP1 (ab56400, Abcam), tankyrase1/2 (H-350, Santa Cruz), and active β-catenin (Clone 8E7, Millipore). ImageJ software was used to measure the relative density for signaling expression.
Luciferase reporter assay
GIST T1 cells were plated in a 12-well plate (1×105 cells/well) and transfected with 0.5 μg of a TCF/LEF reporter plasmid or a negative reporter plasmid (CCS-018L, Qiagen) using Lipofectamine 2000 (Invitrogen) combined with or without an expression vector for β-catenin (pCMV6-β-catenin) or mock control. After 48h of transfection, cells were treated with recombinant 150 ng/ml human Wnt3a (R&D systems) for 4h. Cell lysates were analyzed using the Dual-Glo luciferase Assay System (Promega). Firefly luciferase activity was normalized with an internal control (Renilla luciferase activity) for transfection efficiency. Relative TCF/LEF reporter activity was expressed as fold change over the indicated control.
Cycloheximide (CHX) chase assays and proteasome inhibition assay
GIST T1 cells were transiently transfected with control siRNA or COP1 Smartpool siRNA for 48h. Cells were collected after the addition of 200 μg/ml CHX at the indicated time points (0, 1, 2, 4, 6h) with or without 10 μM MG132. Cell lysates from nuclear extracts were used for signaling. Quantification was achieved by ImageJ software. The final β-catenin turnover rate at each time point is the percentage of β-catenin/lamin B1 expressed relative to the t = 0 hour control siRNA.
Mouse in vivo studies
For PKF118-310 studies in a xenograft model, human GIST T1 cells or HG129 cells (1×106) were injected s.c. into the flanks of NSG mice. Murine S2 cells were injected into the flanks of C57BL/6J mice (Jackson Laboratory). After the tumors reached 100 mm3, PKF118-310 (0.8 mg/kg) or vehicle 0.01% DMSO in PBS was injected intratumorally every other day for 12 days. Because PKF118-310 is toxic when administered systemically, we also investigated the in vivo efficacy of G007-LK, another Wnt pathway inhibitor, on tumor growth. The age and sex-matched KitV558Δ/+ mice were treated with imatinib (600 mg/L in drinking water), G007-LK (28.5 mg/kg in MCT solution given by daily intraperitoneal (i.p.) injection) (27), or imatinib plus G007-LK. Control mice received regular drinking water and daily i.p. injection of MCT solution (0.5% methyl cellulose plus 0.2% Tween 80 in sterile water). Mice were sacrificed after 2 weeks of treatment. All mouse studies were approved by the Institutional Animal Care and Use Committee.
Statistical analysis
Data are expressed as mean ± SEM or median. Differences were detected by unpaired 2-tailed Student’s t tests unless otherwise indicated using Prism 6.0 software (Graph Pad Software). p<0.05 was considered significant.
RESULTS
Wnt/β-catenin signaling is activated in a subset of human GISTs
To identify genes and pathways that might drive GIST tumor malignancy, we compared the transcriptome of freshly isolated KIT+ tumor cells from 4 metastatic, resistant tumors to 8 primary, untreated tumors. There were 462 statistically significant genes with a False Discovery Rate of <0.05 and a fold change >2.0. KEGG pathway analysis revealed differences in cell cycle, DNA replication, and the Wnt signaling pathway (Supplemental Table S1). Specifically, metastatic, resistant tumors (Met/Res) had lower expression of the Wnt inhibitor DKK4 (18.8 fold change) and higher expression of the Frizzled 5 ligand Wnt5A compared to primary, untreated tumors (Prim/UT) (8.6 fold change) (Supplemental Table S2). Lack of DKK4 has been shown to promote canonical Wnt signaling and tumor invasion in hepatocellular carcinoma (20). We validated the DKK4 findings in KIT+ tumor cells (Supplemental Fig. 1A) and in 46 bulk human GIST tumors by quantitative PCR (Fig. 1A left). Meanwhile, we found that β-catenin mRNA (CTNNB1) was highly expressed in all GISTs, with a similar magnitude as GAPDH mRNA, and there was no difference between primary, untreated and metastatic, resistant tumors (Fig. 1A right). In addition, we found that CTNNB1 was expressed to a greater extent in freshly isolated KIT+ tumor cells from 3 patients compared to KIT− (i.e., non-tumor) cells (Supplemental Fig. 1B), further supporting the potential importance of β-catenin in GIST tumorigenesis.
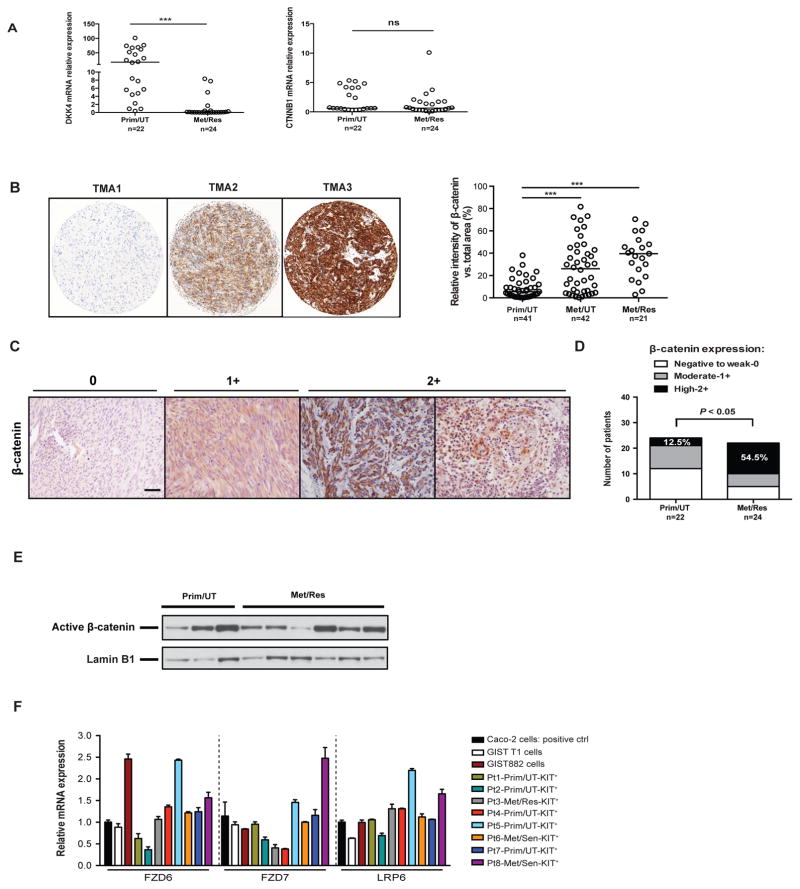
Figure 1. Wnt/β-catenin signaling is activated in a subset of human GISTs.
(A) Real-time PCR showing relative mRNA expression of DKK4 and β-catenin (CTNNB1) in 46 bulk human GIST tumors. Lines represent the medians. Prim/UT– untreated primary GIST (n = 22), Met/Res – resistant metastatic GIST (n = 24). Student’s t test; ***P < 0.001. (B) Representative (left) and quantitative (right) staining of β-catenin in a human GIST tissue microarray of surgical specimens from the time period 1982–2004. The percentage of relative intensity of β-catenin versus total area was calculated using Metamorph software. Medians are indicated. Student’s t test; ***P < 0.001. Prim/UT– primary, untreated GIST; Met/UT– metastatic, untreated GIST; Met/Res – metastatic, resistant GIST. (C) Representative staining of β-catenin in 46 additional human GIST specimens. Scale bar, 20 μm. (D) The corresponding β-catenin intensity was scored as negative to weak (0), moderate (1+), or high (2+). The fraction of high β-catenin intensity score is significantly higher in metastatic, resistant GIST (54.5%) than in primary, untreated GIST (12.5%). (E) Western blot analysis of nuclear active β-catenin isolated from high β-catenin-expressing human GISTs. (F) Relative mRNA expression of FDZ6, FDZ7, and LRP6 in human GIST cell lines and freshly isolated KIT+ tumor cells from 8 human specimens as detected by real-time PCR, compared to Caco-2 colorectal cancer cells. Prim/UT– untreated primary GIST, Met/Res – resistant metastatic GIST, Met/Sen – sensitive metastatic GIST.
To evaluate the potential role of Wnt/β-catenin signaling in GIST biology, we first examined β-catenin staining in a human GIST tissue paraffin microarray comprising more than 100 archival specimens, many of which were from patients who underwent surgery prior to the advent of tyrosine kinase inhibitors. We found high β-catenin staining density in many metastatic tumors, regardless of whether they were untreated or imatinib-resistant (Fig. 1B). In addition, we also analyzed the intensity of β-catenin staining in whole tissue sections of 46 additional human GIST specimens (Fig. 1C). There was low or moderate β-catenin staining in many of the primary, untreated tumors. In contrast, a greater percentage of metastatic, imatinib-resistant specimens had high β-catenin staining (Fig. 1D). To determine whether β-catenin was present in the nucleus, we isolated nuclear protein extracts from frozen human GISTs that had high β-catenin staining by immunohistochemistry. We found that active β-catenin, which was specifically detected by dephosphorylated Ser37 and Thr41 (28), was present in the nuclear extracts of selected primary, untreated and metastatic, imatinib-resistant tumors (Fig. 1E). Furthermore, we performed quantitative PCR for several Wnt co-receptor and ligand genes in human GIST cell lines as well as freshly isolated KIT+ cells from 8 selected human GISTs, including primary, untreated and metastatic, imatinib-resistant or sensitive tumors. The expression of multiple co-receptor genes of Wnt (FZD6, FZD7, and LRP6) was variable, but the majority of specimens had similar or higher expression than the Caco-2 colorectal adenocarcinoma cell line, which has a high level of Wnt activation (Fig. 1F).
Wnt/β-catenin signaling is present in GIST murine models
To further assess the role of the Wnt/β-catenin signaling in GIST, we generated patient derived xenografts (PDXs) from several patients with metastatic, imatinib-resistant GISTs. Because we have not been able to generate any primary, untreated PDXs thus far, we used GIST T1 xenografts as a comparison because they also expressed highly β-catenin and grow aggressively in vivo. While β-catenin tended to localize mostly to the membrane and cytoplasm of GIST T1 tumors, it was extensively expressed in the nuclei of the PDX tumors (Fig. 2A). The amount of active nuclear β-catenin was also higher in the imatinib-resistant PDX models compared to GIST T1 xenografts (Fig. 2B). TCF4 protein was also higher in the PDX models. Upregulation of the Wnt target genes cyclin D1 and downregulation of DKK4 were also seen in the PDX models compared to GIST T1 xenografts by quantitative PCR (Fig. 2C), suggesting that activation of the canonical Wnt pathway could contribute to tumor malignancy in the PDX models.
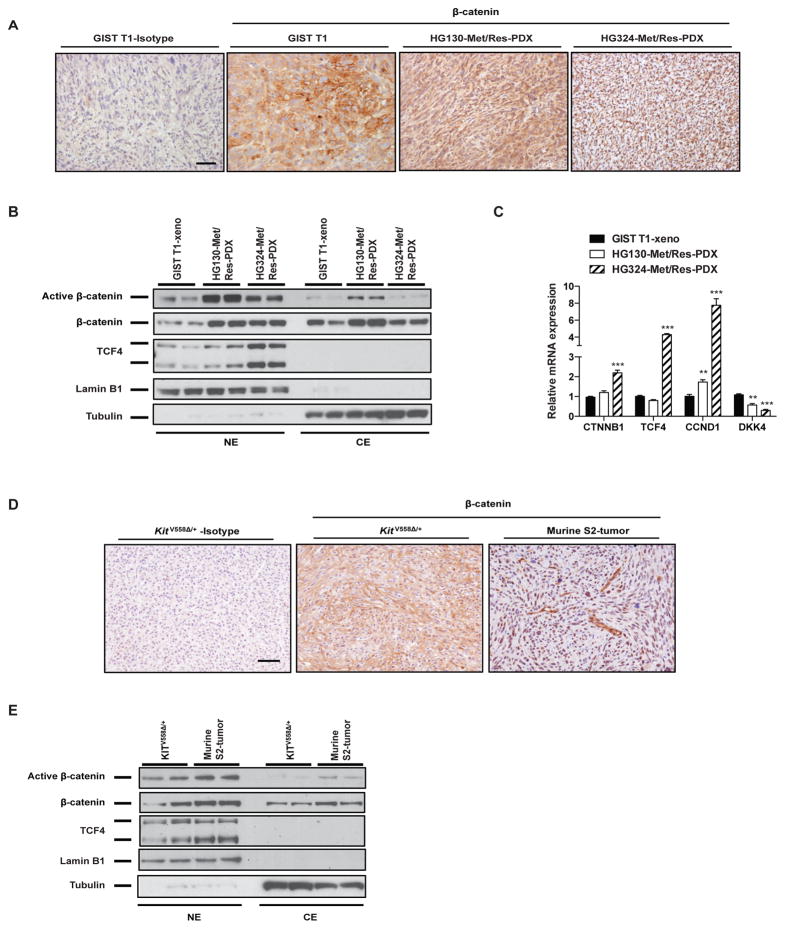
Figure 2. Wnt signaling is present in GIST murine models.
(A) Representative staining of β-catenin in GIST T1 xenograft and 2 imatinib-resistant PDX models. Scale bar, 20 μm. (B) Immunoblots of nuclear (NE) and cytoplasmic extracts (CE) from GIST T1 xenografts and PDXs. (C) Real-time PCR showing relative mRNA expression of indicated Wnt genes in PDXs compared to GIST T1 xenografts. Bars indicate means ± SEM. Student’s t test; **P < 0.01, ***P < 0.001. (D) Representative staining of β-catenin in tumors from KitV558Δ/+ mice and murine GIST S2 orthotopic liver tumors. Scale bar, 20 μm. (E) Immunoblots of nuclear (NE) and cytoplasmic extracts (CE) from KitV558Δ/+ tumors or S2 tumors.
We showed similar findings in the murine imatinib-resistant GIST cell line S2, which we previously derived from a KitV558Δ/+ mouse that develops GIST (24). We established an orthotopic liver model of GIST metastasis (Supplemental Fig. 2A). Compared to the original KitV558Δ/+ tumor, the S2 liver tumors had lower Kit expression, yet high DOG1 expression (Supplemental Fig. 2B), and similar levels of downstream Kit signaling, such as phospho-STAT3, ERK, and S6 (Supplemental Fig. 2C). There was high nuclear β-catenin staining in murine S2 tumors. In contrast, the KitV558Δ/+ mouse tumor had mostly membranous β-catenin staining by immunohistochemistry (Fig. 2D). By using nuclear and cytoplasmic protein extraction, we detected greater β-catenin nuclear translocation and dephosphorylation in S2 tumors compared to KitV558Δ/+ mouse tumors (Fig. 2E). Taken together, these data suggested that there was active Wnt/β-catenin signaling in multiple models of murine and human GIST, as we had observed in the human GIST specimens.
Wnt3a and DKK4 regulate Wnt signaling in GIST cell lines
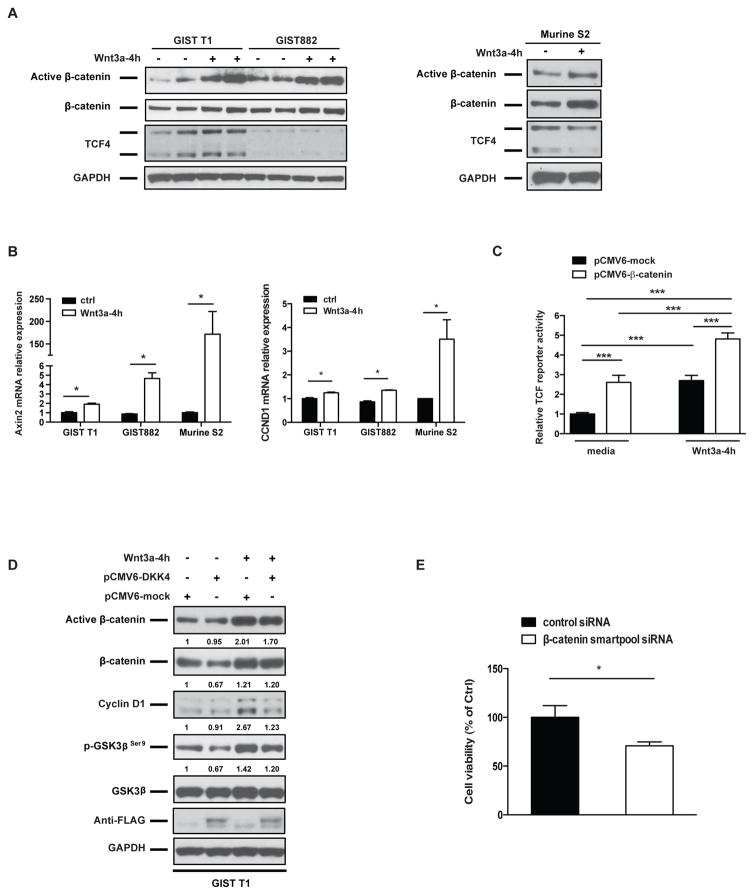
Wnt ligands stimulate canonical Wnt signaling by inactivating the GSK3β-Axin-APC destruction complex, thereby activating the β-catenin/TCF pathway. To test the biological effect of Wnt activation in GIST cells, we stimulated human GIST cell lines and the murine S2 line in vitro with recombinant Wnt3a. We found that active β-catenin was significantly increased (Fig. 3A). The Wnt responsive genes axin2 and cyclin D1 were also induced by Wnt3a stimulation (Fig. 3B). To further assess the effect of Wnt3a on β-catenin transcriptional activity, we transfected GIST T1 cells, which have higher TCF4 levels compared to GIST882 cells (Fig. 3A), with a luciferase reporter linked to a TCF/LEF promoter. In addition, we also transfected GIST T1 cells with a mock or a β-catenin expression plasmid. We found that TCF/LEF reporter activity was increased by β-catenin overexpression. The effect was augmented in the presence of Wnt3a (Fig. 3C). Since DKK4 mRNA was higher in most primary, untreated GISTs compared to most metastatic, resistant GISTs (Fig. 1A), we overexpressed DKK4 in GIST T1 cells to determine its effect on β-catenin. DKK4 overexpression was confirmed by detection of the vector construct with a FLAG antibody. We found that DKK4 overexpression reduced β-catenin protein levels by 33% (Fig. 3D). Consistent with reduced β-catenin, GSK3β phosphorylated at Serine 9, which inhibits formation of the destruction complex (19), was also reduced. Ectopic expression of DKK4 reduced active β-catenin and cyclin D1 levels in the presence of Wnt3a stimulation. Together, these in vitro data demonstrated that Wnt ligands induced β-catenin signaling while DKK4 antagonized it in GIST cells. Lastly, to identify the functional significance of β-catenin expression in GIST cells, we transfected the GIST T1 cells with control siRNA or β-catenin smartpool siRNA. Knockdown of β-catenin also decreased cell viability at 72h (Figure 3E).
Figure 3. Wnt3a and DKK4 regulate Wnt signaling in GIST cell lines.
(A) Immunoblots of GIST T1, GIST882, and murine S2 whole cell lysates after treatment with either 150 ng/ml Wnt3a or control PBS for 4h. (B) Real-time PCR of Axin 2 and CCND1 (cyclin D1) mRNA expression after treatment with either 150 ng/ml Wnt3a or control PBS for 4h. Bars indicate means ± SEM. Student’s t test; *P < 0.05. (C) TCF/LEF reporter activity in GIST T1 cells in the presence of Wnt3a and/or β-catenin overexpression. GIST T1 cells were co-transfected with a negative reporter or TCF/LEF reporter and a mock or β-catenin expression plasmid. 48h after transfection, cells were treated with either 150 ng/ml of Wnt3a or control PBS for 4h. Relative luciferase signaling (indicating TCF/LEF activity) was determined after normalizing to renilla luciferase (indicating transfection efficiency), and expressed as relative fold changes compared to indicated control. All bars, means ± SEM. Student’s t test; *P < 0.001. (D) Immunoblots of whole-cell lysates of GIST T1 cells that had been transfected with mock or DKK4 expression plasmid and 48h later treated with either 150 ng/ml of Wnt3a or PBS for 4h. Western blot bands were quantified by Image J Software and normalized to GAPDH, and then expressed as the fold-change compared to pCMV6-mock control. (E) Cell viability assay (Dojindo) of human GIST T1 cells following 72h of transfection of control siRNA or b-catenin smartpoolsiRNA. Bars, mean ± SEM. Student’s t test; *P < 0.05.
Nuclear β-catenin stability is partially regulated by COP1 in GIST cells
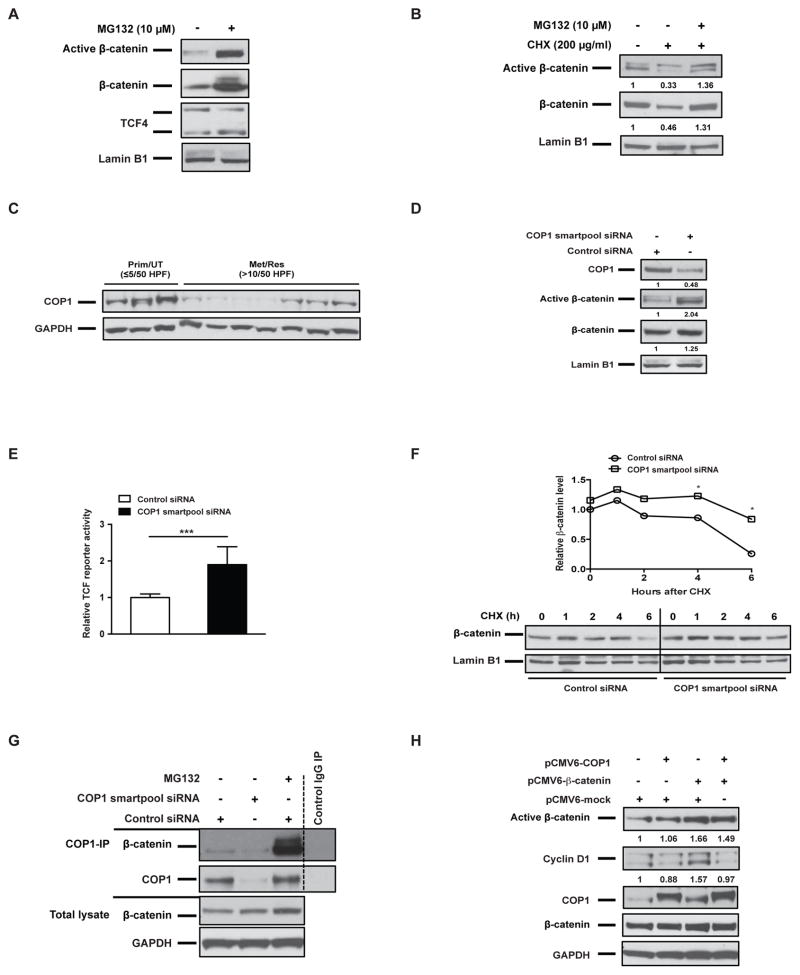
β-catenin is unstable due to ubiquitin-mediated proteasomal degradation (19). To address the role of proteasomal degradation of nuclear β-catenin in GIST, we treated GIST T1 cells for 6h with the proteasome inhibitor MG132. Upon proteasome inhibition, nuclear dephosphorylated and total β-catenin both stabilized, as expected (Fig. 4A). Incubation of GIST T1 cells with the protein synthesis inhibitor cycloheximide (CHX) for 6h decreased both active and total β-catenin in the nuclei. However, the addition of MG132 restored the accumulation of nuclear active and total β-catenin in GIST T1 cells, indicating that nuclear β-catenin was undergoing proteasomal degradation (Fig. 4B). Several E3 ubiquitin ligases have been shown to promote nuclear β-catenin degradation, such as c-cbl, TRIM33, and COP1 (22, 23, 29). In human GIST specimens, TRIM33 was almost undetectable by western blot (Supplemental Fig. 3), while COP1 was highly expressed in primary, untreated GISTs, but reduced in many metastatic, resistant GISTs (Fig. 4C). Furthermore, COP1 was highly expressed in GIST T1 cells, especially in the nucleus (Fig. 4D), whereas c-cbl and TRIM33 expression was not detectable. Notably, COP1 functions as an E3 ligase for ETV1 and ETV4 (30, 31). ETV1 is a lineage transcription factor in GIST (32) and we previously explored a critical role of ETV4 in GIST (33). COP1 knockdown with Smartpool siRNA in GIST T1 cells stabilized active nuclear and total β-catenin levels (Fig. 4D). Consistently, COP1 knockdown significantly increased TCF/LEF reporter activity compared to control siRNA, indicating that COP1 loss facilitated β-catenin–mediated transcription (Fig. 4E). A CHX-chase assay further confirmed that COP1 knockdown significantly increased the half-life of nuclear β-catenin (Fig. 4F). To assess whether COP1 binds to β-catenin in GIST T1 cells, we performed COP1 immunoprecipitation (Fig. 4G). There was a direct interaction between endogenous COP1 and β-catenin. Furthermore, MG132 increased the amount of β-catenin that was co-immunoprecipitated with COP1. The binding specificity was confirmed by COP1 knockdown and control IgG immunoprecipitation. Lastly, GIST T1 cells were transfected with ectopic COP1. Overexpressing COP1 alone did not decrease the endogenous β-catenin level in GIST T1 cells, suggesting that there was sufficient endogenous β-catenin turnover. However, in the setting of increased β-catenin transcriptional activation induced by ectopic β-catenin, COP1 overexpression inhibited active β-catenin and cyclin D1 (Fig. 4H). Overall, these data suggested that β-catenin stability and transcriptional activity are partially regulated by COP1 in GIST.
Figure 4. Nuclear β-catenin stability is partially regulated by COP1 in GIST cells.
Immunoblots of nuclear extracts of GIST T1 cells treated with (A) the proteasome inhibitor MG132 10 μM for 6h or pretreated with (B) the protein biosynthesis inhibitor cycloheximide (CHX) for 6h. (C) Immunoblots of COP1 expression in whole-cell lysates of human GIST specimens. (D) Immunoblots of nuclear extracts of GIST T1 cells transfected with control siRNA or COP1 Smartpool siRNA for 48h. (E) TCF/LEF reporter activity as detected by luciferase of GIST T1 cells transfected with a negative reporter or a TCF/LEF reporter, and control siRNA or COP1 Smartpool siRNA for 48h, Bars, mean ± SEM. Student’s t test; ***P < 0.001. (F) Representative CHX-chase assay (of 2 performed) to determine the stability (half-life) of nuclear β-catenin in GIST T1 cells 48h after transfection with control siRNA or COP1 Smartpool siRNA. Cells were collected after the addition of 200 μg/ml CHX at indicated time points (0, 1, 2, 4, 6h). Relative nuclear β-catenin levels were determined by normalizing to the loading control (lamin B1) and then normalizing to the t = 0h control siRNA. Immunoblots of nuclear extracts are shown. (G) GIST T1 cells were transfected with control siRNA or COP1 Smartpool siRNA for 48h and then treated with MG132 10 μM for 2h prior to harvest. Whole-cell lysates were immunoprecipitated by either anti-COP1 or control IgG, Western blot was performed as indicated. Whole-cell lysates were used as input. (H) Immunoblots of whole-cell lysates of GIST T1 cells transfected with the indicated constructs for 48h. Western blot bands were quantified by Image J Software and normalized to GAPDH, and then expressed as the fold-change compared to control.
Wnt inhibition induces GIST cell death
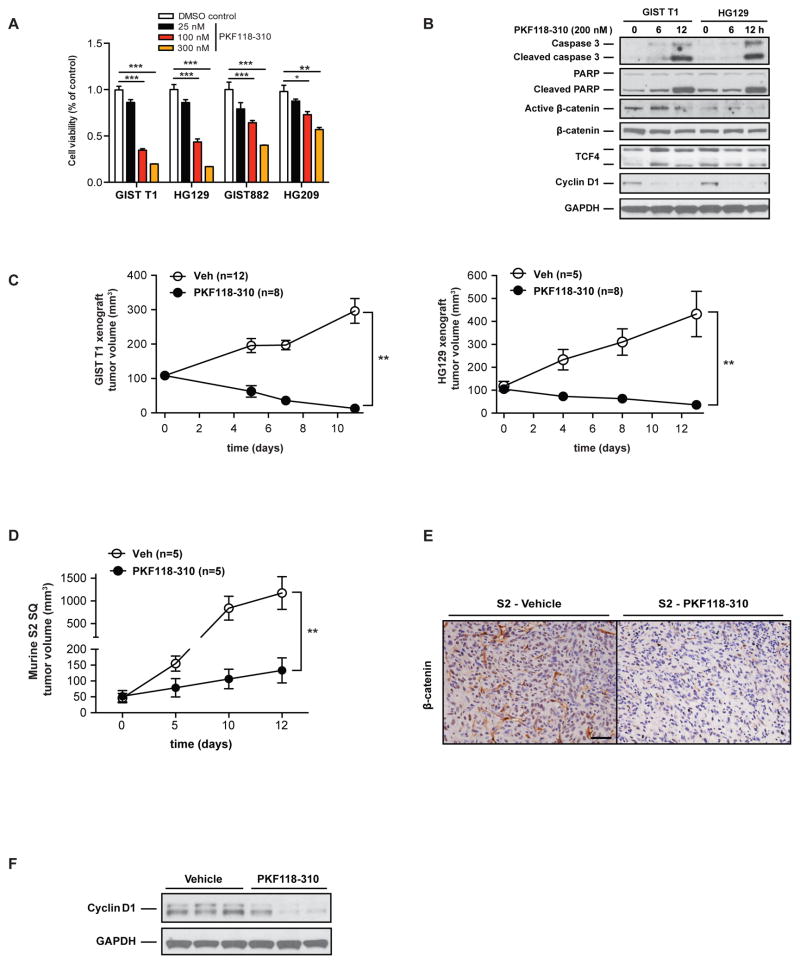
To investigate the importance of Wnt signaling in GIST cell survival, we tested the effect of the Wnt inhibitor PKF118-310, a specific inhibitor that disrupts the β-catenin/TCF complex (34). PKF118-310 significantly inhibited the viability of imatinib-sensitive (T1, HG129, and GIST882) and imatinib-resistant (HG209) cells at low IC50 values (Fig. 5A), but did not affect the in vitro viability of a murine hepatocellular carcinoma cell line or B16 melanoma (Supplemental Fig. 4). The mechanism depended on apoptosis as shown by activation of caspase 3 and PARP (Fig. 5B). The Wnt/β-catenin target gene cyclin D1 was markedly reduced, while total β-catenin and TCF4 levels were relatively unaffected. To determine if Wnt inhibition affected tumor growth in vivo, we treated established flank tumor xenografts of GIST T1 cells and HG129 cells with intratumoral injection of PKF118-310 and found significant tumor regression (Fig. 5C). We confirmed these findings in subcutaneous tumors of the imatinib-resistant murine S2 GIST cell line as PKF118-310 treatment reduced tumor growth (Fig. 5D), tumor β-catenin staining (Fig. 5E), and cyclin D1 expression (Fig. 5F). Thus, targeting β-catenin/TCF was sufficient to inhibit GIST growth in vitro and in vivo in imatinib-sensitive and resistant GIST models.
Figure 5. Wnt inhibition induces GIST cell death.
(A) Cell viability assay (Dojindo) of human GIST cell lines following 72h of PKF118-310 or vehicle (DMSO control) treatment. Bars indicate means ± SEM. Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001. (B) Immunoblots of GIST T1 and HG129 cells treated with 200 nM PKF118-310 or vehicle (DMSO control) at the indicated time points. (C) GIST T1 or HG129 cells were injected into the flanks of NSG mice or (D) murine S2 cells were injected into the flanks of C5BL/6J mice and when the tumors were 100 mm3, intratumoral injections of PKF118-310 (0.8 mg/kg) or vehicle 0.01% DMSO in PBS were administered every other day for 6 doses. Graph shows Mean ± SEM. Student’s t test; **P < 0.01. (E) Representative immunostaining for β-catenin in PKF118-310 or vehicle-treated murine S2 tumors. Scale bar, 20 μm. (F) Immunoblots of whole-cell lysates from vehicle or PKF118-310 treated S2 tumors.
Wnt inhibition enhances the efficacy of imatinib in vitro and in vivo
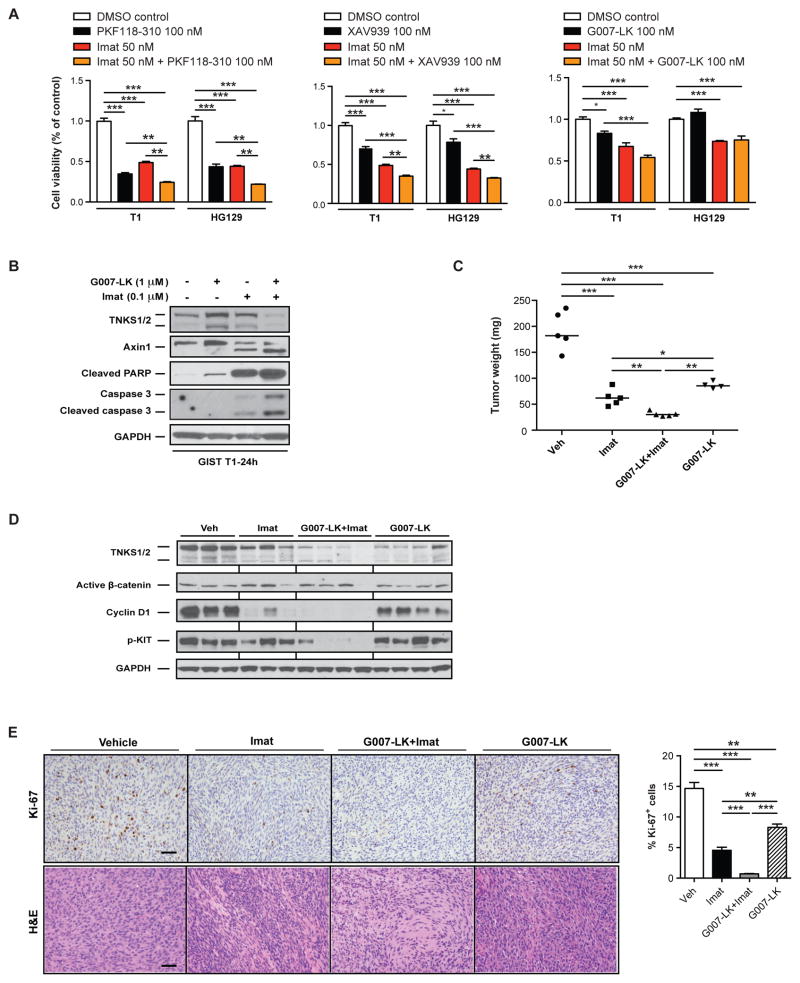
We next considered whether inhibition of Wnt/β-catenin would enhance the anti-tumoral effect of imatinib in GIST. Imatinib treatment increased the transcription of CTNNB1 (β-catenin) and Axin2 mRNA in KitV558Δ/+ tumors (Supplemental Fig. 5A). Although imatinib reduced active β-catenin protein levels at 1 week of treatment, its expression was restored after an additional week of therapy (Supplemental Fig. 5B), making it an attractive target in addition to KIT. Therefore, we tested whether Wnt inhibition increased the sensitivity of GIST cells to imatinib. Indeed, treatment with PKF118-310 and imatinib further reduced cell viability compared to imatinib alone in the imatinib-sensitive cell lines GIST T1 and HG129 (Fig. 6A left). We also studied the effect of XAV939 and GK007-LK, which antagonize Wnt signaling by inhibiting tankyrase1/2 (TNKS1/2) and stabilizing axins through the prevention of PARsylation and ubiquitination (27, 35). Similarly, combination therapy with imatinib reduced cell viability in most cases (Fig. 6A middle and right). We confirmed that tankyrase inhibition increased Axin1 protein levels and found that dual inhibition of Wnt and KIT signaling led to more apoptosis compared to single drug treatment, as measured by cleaved caspase 3 and PARP (Fig. 6B). Since G007-LK can be administered systemically (unlike PKF118-310) and was effective in treating both xenografts and genetically engineered models of colorectal cancer (27), we investigated its efficacy in vivo and synergism with imatinib in genetically engineered KitV558Δ/+ mice. G007-LK alone had substantial anti-tumor activity, which was further increased by combination with imatinib as measured by tumor weight (Fig. 6C). Combination therapy further reduced TNKS1/2, cyclin D1, and phospho-KIT at 2 weeks (Fig. 6D), decreased cellular proliferation as measured by Ki-67 staining, and increased tumor destruction by H&E (Fig. 6E). Taken together, antagonizing Wnt signaling augmented the anti-tumoral effect of imatinib in vitro and in vivo.
Figure 6. Wnt inhibition enhances the efficacy of imatinib in vitro and in vivo.
(A) Cell viability assay of human GIST cell lines at 72h after the indicated treatment. (B) Immunoblots of whole-cell lysates of GIST T1 cells 24h after the indicated treatment. (C) Tumor weight in KitV558Δ/+ mice treated for 2 weeks with vehicle (Veh), imatinib (Imat), G007-LK plus imatinib, or G007-LK alone. n=4–5 mice per group. Medians are indicated. (D) Immunoblots of whole-cell lysates from vehicle or treated KitV558Δ/+ tumors. (E) Representative H&E, Ki-67 staining and quantification in KitV558Δ/+ tumors after 2 weeks of the indicated treatment. Scale bar, 50 μm. Bars indicate means ± SEM. Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
Although aberrant activation of the Wnt/β-catenin pathway has been linked to tumor progression in a variety of cancers(16, 18, 36), its role in GIST is unclear. We identified the Wnt/β-catenin pathway on KEGG pathway analysis comparing mRNA expression of freshly isolated KIT+ tumor cells from primary, untreated human GISTs with low mitotic rates to metastatic, resistant tumors with high mitotic rates. We observed that most primary, untreated human GISTs had low staining, while many metastatic tumors (untreated or imatinib-resistant) had high levels of β-catenin. Notably, elevated expression of cytoplasmic β-catenin alone has been linked to tumor progression and poor outcome in some human cancers (37). Previously, it was reported that GISTs lacked nuclear β-catenin by immunohistochemistry, distinguishing it from mesenteric fibromatosis (38), although different techniques may explain the discrepancy with our data. Moreover, in a broad survey of 45 tumors and 23 cell lines, active β-catenin was found to be common in sarcoma and was present in the single human GIST specimen that was tested (39). It remains to be shown whether use of different fixatives or time to tissue fixation affects the quality of β-catenin staining by immunohistochemistry.
Wnt/β-catenin signaling can be activated in a variety of ways in cancer. In 140 human GISTs analyzed for mutations in over 300 commonly mutated genes in cancer, we have not identified a β-catenin mutation, and only 1 patient had an APC mutation (unpublished data). Instead, we found that the Wnt pathway antagonist DKK4 was substantially lower in metastatic, resistant GISTs compared to primary, untreated GISTs. We also detected other critical components of Wnt/β-catenin signaling in human and murine GISTs, including frizzled receptors, TCF4 and LRP6.
To prove functional Wnt/β-catenin signaling, we performed a number of experiments in human GIST T1 cells. Wnt3a increased TCF/LEF reporter activity. Meanwhile, ectopic expression of DKK4 partially reduced total β-catenin protein levels and inhibitory phospho-GSK3β. In the presence of Wnt3a, DKK4 also reduced active β-catenin and cyclin D1. Epigenetic silencing of Wnt antagonism and aberrant DNA methylation of Wnt pathway genes have been linked to poor prognosis in some cancers (40–42). Several studies have shown that concurrent hypermethylation of multiple Wnt/β-catenin inhibitors leads to gene silencing and contributes to aberrant Wnt pathway activation (43). Further investigation is needed to delineate the mechanism of DKK4 inactivation in GIST.
Although activation of Wnt/β-catenin signaling can occur through a variety of mechanisms, nuclear β-catenin stabilization is essential for its oncogenic function. We showed that proteasome inhibition stabilized nuclear dephosphorylated and total β-catenin and increased TCF/LEF reporter activity, demonstrating that the ubiquitin-proteasome pathway contributes to the regulation of Wnt/β-catenin signaling. Recently, several E3 ligases have been shown to control transcriptionally active nuclear β-catenin (22, 44). Among them, TRIMM33 was almost undetectable in most human GIST specimens and cell lines. COP1 was of particular interest, since it was expressed variably in human GIST specimens and cell lines. Moreover, our unpublished data also showed that in GIST cells, COP1 knockdown with siRNA significantly increased stability of c-jun. C-jun has been reported to stabilize β-catenin via its association with TCF4 and regulate gene transcription stimulated by the canonical Wnt signaling pathway (45). Accordingly, our in vitro data demonstrated that COP1 knockdown increased nuclear β-catenin half-life and stability, and transcriptional activity in a TCF-reporter assay. Meanwhile, based on COP1 immunoprecipitation, endogenous COP1 interacted with β-catenin in GIST T1 cells. Similarly, we also detected interaction between endogenous β-catenin and COP1 in some human GIST specimens, including primary, untreated and metastatic, resistant GISTs (Supplemental Fig. 3). Furthermore, ectopic expression of COP1 reduced active β-catenin and cyclin D1 expression induced by β-catenin overexpression. The coordinated action of COP1 and other E3 ligases, such as β-TrCP, has been reported to negatively regulate β-catenin (29). While our data demonstrated that COP1 loss contributed to upregulation of β-catenin in GIST, other mechanisms are likely involved and may involve genomic or epigenetic changes.
Since the Wnt pathway regulates multiple cellular processes linked to tumor progression, several small molecule inhibitors or blocking antibodies have been developed for potential therapeutic use (46, 47). Promising data using these agents in different tumor types and animal models have been reported (27, 47). Indeed, we showed that PKF118-310, a selective inhibitor targeting the canonical Wnt/β-catenin signaling cascade, was effective in both imatinib-sensitive and resistant GIST cells in vitro and tumors in vivo. Alternatively, 2 different tankyrase inhibitors that antagonize Wnt/β-catenin signaling were effective against GIST cell lines in vitro. Strikingly, the tankyrase inhibitor G007-LK had almost as much anti-tumor efficacy as imatinib in KitV558Δ/+ mice. Thus, inhibition of Wnt/β-catenin signaling may be useful in patients with GIST that has become refractory to imatinib or other tyrosine kinase inhibitors. Furthermore, we found that 2 weeks of imatinib therapy increased β-catenin mRNA in KitV558Δ/+ tumors and restored active β-catenin levels, suggesting that Wnt/β-catenin signaling is a compensatory mechanism for KIT inhibition. Consistent with these findings, the combination of imatinib and G007-LK was even more effective than either alone in KitV558Δ/+ mice.
Although we have demonstrated Wnt/β-catenin activation in human GIST, these findings occurred only in a subset of GIST patients, and more investigation is needed to correlate β-catenin expression with clinicopathologic features. In addition, the efficacy of our in vivo treatments was assessed at short-term intervals, so the toxicity and efficacy of longer treatment durations need to be defined. It is possible that resistance to Wnt inhibition may develop over time. Our data support a clinical trial of Wnt/β-catenin inhibition in GIST. Multiple agents targeting the Wnt/β-catenin pathway are already being tested in patients with cancer (48), which should indicate their safety and effectiveness.
In summary, our findings show that activation of the canonical Wnt pathway and accumulation of nuclear active β-catenin were present in a subset of human GISTs that were treatment naïve, responsive to imatinib, or resistant to imatinib. The mechanism involved reduction of DKK4 and enhanced the nuclear β-catenin stability by COP1 loss. Inhibiting Wnt/β-catenin signaling alone or in combination with imatinib demonstrated anti-tumor efficacy in multiple cells and pre-clinical models in GIST. Our data provide the rationale for targeting Wnt/β-catenin signaling as a new strategy in the treatment of GIST.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by NIH grants R01 CA102613 and T32 CA09501, Betsy Levine-Brown and Marc Brown, David and Monica Gorin, and the Stephanie and Fred Shuman through the Windmill Lane Foundation (RP DeMatteo); GIST Cancer Research Fund (RP DeMatteo and CR Antonescu); F32 CA162721 and the Claude E. Welch Fellowship from the Massachusetts General Hospital (TS Kim); and P50 CA140146-01 (CR Antonescu).
We are grateful to the Tissue Procurement Service for assistance in the acquisition of human tumor specimens; Laboratory of Comparative Pathology; Research Animal Resource Center; and Molecular Cytology Core Facility. We thank Russell Holmes for logistical and administrative support.
References
- 1.Ducimetiere F, Lurkin A, Ranchere-Vince D, Decouvelaere AV, Peoc’h M, Istier L, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6:e20294. doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 3.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 4.Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247–58. doi: 10.1146/annurev-med-043010-091813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–90. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 6.Corless CL, Ballman KV, Antonescu CR, Kolesnikova V, Maki RG, Pisters PW, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary gi stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563–70. doi: 10.1200/JCO.2013.51.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865–78. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 8.Niinuma T, Suzuki H, Nojima M, Nosho K, Yamamoto H, Takamaru H, et al. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012;72:1126–36. doi: 10.1158/0008-5472.CAN-11-1803. [DOI] [PubMed] [Google Scholar]
- 9.Hur K, Lee HJ, Woo JH, Kim JH, Yang HK. Gene expression profiling of human gastrointestinal stromal tumors according to its malignant potential. Dig Dis Sci. 2010;55:2561–7. doi: 10.1007/s10620-009-1061-4. [DOI] [PubMed] [Google Scholar]
- 10.Haller F, Gunawan B, von Heydebreck A, Schwager S, Schulten HJ, Wolf-Salgo J, et al. Prognostic role of E2F1 and members of the CDKN2A network in gastrointestinal stromal tumors. Clin Cancer Res. 2005;11:6589–97. doi: 10.1158/1078-0432.CCR-05-0329. [DOI] [PubMed] [Google Scholar]
- 11.Schmieder M, Wolf S, Danner B, Stoehr S, Juchems MS, Wuerl P, et al. p16 expression differentiates high-risk gastrointestinal stromal tumor and predicts poor outcome. Neoplasia. 2008;10:1154–62. doi: 10.1593/neo.08646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagarde P, Perot G, Kauffmann A, Brulard C, Dapremont V, Hostein I, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18:826–38. doi: 10.1158/1078-0432.CCR-11-1610. [DOI] [PubMed] [Google Scholar]
- 13.Agaram NP, Wong GC, Guo T, Maki RG, Singer S, Dematteo RP, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47:853–9. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janeway KA, Kim SY, Lodish M, Nose V, Rustin P, Gaal J, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A. 2011;108:314–8. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaefer IM, Wang Y, Liang CW, Bahri N, Quattrone A, Doyle L, et al. MAX inactivation is an early event in GIST development that regulates p16 and cell proliferation. Nat Commun. 2017;8:14674. doi: 10.1038/ncomms14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson AL, Rahrmann EP, Moriarity BS, Choi K, Conboy CB, Greeley AD, et al. Canonical Wnt/beta-catenin signaling drives human schwann cell transformation, progression, and tumor Maintenance. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-13-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kypta RM, Waxman J. Wnt/beta-catenin signalling in prostate cancer. Nat Rev Urol. 2012;9:418–28. doi: 10.1038/nrurol.2012.116. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Morris JPt, Yan W, Schofield HK, Gurney A, Simeone DM, et al. Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer Res. 2013;73:4909–22. doi: 10.1158/0008-5472.CAN-12-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatima S, Lee NP, Tsang FH, Kolligs FT, Ng IO, Poon RT, et al. Dickkopf 4 (DKK4) acts on Wnt/beta-catenin pathway by influencing beta-catenin in hepatocellular carcinoma. Oncogene. 2012;31:4233–44. doi: 10.1038/onc.2011.580. [DOI] [PubMed] [Google Scholar]
- 21.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 22.Xue J, Chen Y, Wu Y, Wang Z, Zhou A, Zhang S, et al. Tumour suppressor TRIM33 targets nuclear beta-catenin degradation. Nat Commun. 2015;6:6156. doi: 10.1038/ncomms7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chitalia V, Shivanna S, Martorell J, Meyer R, Edelman E, Rahimi N. c-Cbl, a ubiquitin E3 ligase that targets active beta-catenin: a novel layer of Wnt signaling regulation. J Biol Chem. 2013;288:23505–17. doi: 10.1074/jbc.M113.473801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavnar MJ, Zeng S, Kim TS, Sorenson EC, Ocuin LM, Balachandran VP, et al. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J Exp Med. 2013;210:2873–86. doi: 10.1084/jem.20130875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen NA, Zeng S, Seifert AM, Kim TS, Sorenson EC, Greer JB, et al. Pharmacological inhibition of KIT activates MET signaling in gastrointestinal stromal tumors. Cancer Res. 2015;75:2061–70. doi: 10.1158/0008-5472.CAN-14-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim TS, Cavnar MJ, Cohen NA, Sorenson EC, Greer JB, Seifert AM, et al. Increased KIT inhibition enhances therapeutic efficacy in gastrointestinal stromal tumor. Clin Cancer Res. 2014;20:2350–62. doi: 10.1158/1078-0432.CCR-13-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau T, Chan E, Callow M, Waaler J, Boggs J, Blake RA, et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013;73:3132–44. doi: 10.1158/0008-5472.CAN-12-4562. [DOI] [PubMed] [Google Scholar]
- 28.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–5. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 29.Xu S, Tong M, Huang J, Zhang Y, Qiao Y, Weng W, et al. TRIB2 inhibits Wnt/beta-Catenin/TCF4 signaling through its associated ubiquitin E3 ligases, beta-TrCP, COP1 and Smurf1, in liver cancer cells. FEBS Lett. 2014;588:4334–41. doi: 10.1016/j.febslet.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 30.Vitari AC, Leong KG, Newton K, Yee C, O’Rourke K, Liu J, et al. COP1 is a tumour suppressor that causes degradation of ETS transcription factors. Nature. 2011;474:403–6. doi: 10.1038/nature10005. [DOI] [PubMed] [Google Scholar]
- 31.Baert JL, Monte D, Verreman K, Degerny C, Coutte L, de Launoit Y. The E3 ubiquitin ligase complex component COP1 regulates PEA3 group member stability and transcriptional activity. Oncogene. 2010;29:1810–20. doi: 10.1038/onc.2009.471. [DOI] [PubMed] [Google Scholar]
- 32.Chi P, Chen Y, Zhang L, Guo X, Wongvipat J, Shamu T, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010;467:849–53. doi: 10.1038/nature09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 35.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 36.Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125:1269–85. doi: 10.1172/JCI76452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Knowles E, Zardawi SJ, McNeil CM, Millar EK, Crea P, Musgrove EA, et al. Cytoplasmic localization of beta-catenin is a marker of poor outcome in breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2010;19:301–9. doi: 10.1158/1055-9965.EPI-09-0741. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery E, Torbenson MS, Kaushal M, Fisher C, Abraham SC. Beta-catenin immunohistochemistry separates mesenteric fibromatosis from gastrointestinal stromal tumor and sclerosing mesenteritis. Am J Surg Pathol. 2002;26:1296–301. doi: 10.1097/00000478-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Vijayakumar S, Liu G, Rus IA, Yao S, Chen Y, Akiri G, et al. High-frequency canonical Wnt activation in multiple sarcoma subtypes drives proliferation through a TCF/beta-catenin target gene, CDC25A. Cancer Cell. 2011;19:601–12. doi: 10.1016/j.ccr.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schade B, Lesurf R, Sanguin-Gendreau V, Bui T, Deblois G, O’Toole SA, et al. beta-Catenin signaling is a critical event in ErbB2-mediated mammary tumor progression. Cancer Res. 2013;73:4474–87. doi: 10.1158/0008-5472.CAN-12-3925. [DOI] [PubMed] [Google Scholar]
- 41.Xu C, Xie D, Yu SC, Yang XJ, He LR, Yang J, et al. beta-Catenin/POU5F1/SOX2 transcription factor complex mediates IGF-I receptor signaling and predicts poor prognosis in lung adenocarcinoma. Cancer Res. 2013;73:3181–9. doi: 10.1158/0008-5472.CAN-12-4403. [DOI] [PubMed] [Google Scholar]
- 42.Galamb O, Kalmar A, Peterfia B, Csabai I, Bodor A, Ribli D, et al. Aberrant DNA methylation of WNT pathway genes in the development and progression of CIMP-negative colorectal cancer. Epigenetics. 2016;11:588–602. doi: 10.1080/15592294.2016.1190894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moskalev EA, Luckert K, Vorobjev IA, Mastitsky SE, Gladkikh AA, Stephan A, et al. Concurrent epigenetic silencing of wnt/beta-catenin pathway inhibitor genes in B cell chronic lymphocytic leukaemia. BMC Cancer. 2012;12:213. doi: 10.1186/1471-2407-12-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shivanna S, Harrold I, Shashar M, Meyer R, Kiang C, Francis J, et al. The c-Cbl Ubiquitin Ligase Regulates Nuclear beta-Catenin and Angiogenesis by Its Tyrosine Phosphorylation Mediated through the Wnt Signaling Pathway. J Biol Chem. 2015;290:12537–46. doi: 10.1074/jbc.M114.616623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP, Li L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J Cell Biol. 2008;180:1087–100. doi: 10.1083/jcb.200710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 47.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109:11717–22. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Hao J. Development of anticancer agents targeting the Wnt/beta-catenin signaling. Am J Cancer Res. 2015;5:2344–60. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.