Abstract
Cumulative exposure to solar ultraviolet (SUV) irradiation is regarded as the major etiologic factor in the development of skin cancer. The activation of the mitogen-activated protein kinase (MAPK) cascades occurs rapidly and is vital in the regulation of SUV-induced cellular responses. The T-LAK cell-originated protein kinase (TOPK), an upstream activator of MAPKs, is heavily involved in inflammation, DNA damage, and tumor development. However, the chemopreventive and therapeutic effects of specific TOPK inhibitors in SUV-induced skin cancer have not yet been elucidated. In the current study, ADA-07, a novel TOPK inhibitor, was synthesized and characterized. Pull-down assay results, ATP competition and in vitro kinase assay data revealed that ADA-07 interacted with TOPK at the ATP-binding pocket and inhibited its kinase activity. Western blot analysis showed that ADA-07 suppressed SUV-induced phosphorylation of ERK1/2, p38, and JNKs, and subsequently inhibited AP-1 activity. Importantly, topical treatment with ADA-07 dramatically attenuated tumor incidence, multiplicity, and volume in SKH-1 hairless mice exposed to chronic SUV. Our findings suggest that ADA-07 is a promising chemopreventive or potential therapeutic agent against SUV-induced skin carcinogenesis that acts by specifically targeting TOPK.
Keywords: ADA-07, SUV, skin cancer, TOPK
Introduction
Skin cancer is one of the most frequently diagnosed malignancies in the United States, and its incidence has been increasing at an astonishing rate over the past few decades, exceeding the number of all other human cancers combined (1). Non-melanoma skin cancer (NMSC) includes basal cell carcinomas (BCC) and squamous cell carcinomas (SCC) and is the most common type of skin cancer with substantial associated morbidity and mortality (2). BCCs comprise approximately 80% of skin cancers with SCC as the second most common skin cancer. SCCs are more aggressive and have a higher potential for metastasis compared to BCCs (3). Solar ultraviolet (SUV) irradiation is well documented as a prominent environmental carcinogen responsible for various physiological and biological effects, including immune suppression, cellular aging, and DNA damage (4, 5). Furthermore, strong epidemiological and molecular evidence indicates that cumulative exposure to SUV irradiation is the major etiologic factor in the development of NMSC (6-8). The SUV spectrum can be divided into 3 subtypes according to wavelength and include UVA (320-400 nm), UVB (280-320 nm) and UVC (200-280 nm) (9). Previous studies indicated that although UVC is filtered out by stratospheric ozone, UVA and UVB each has strong carcinogenic effects on the skin, which can lead to DNA damage, erythema, sunburn, immunosuppression, and, eventually, skin cancer (5, 10, 11). Therefore, targeting SUV-induced signaling could be an effective strategy for developing agents for effective chemoprevention and chemotherapy against skin carcinogenesis.
Activation of intracellular signaling pathways in response to SUV irradiation plays a crucial role in SUV-induced skin cancer. The mitogen-activated protein kinases (MAPKs) are serine/threonine protein kinases that are strongly activated by SUV irradiation, and are essential in the regulation of fundamental cellular processes, such as proliferation, differentiation, and apoptosis (12). The MAPK cascades comprise the extracellular signal-regulated kinases (ERKs), p38 MAPKs, and c-Jun NH2-terminal kinases (JNKs). The activation of these pathways occurs rapidly and is vital in the regulation of SUV-induced cellular responses that can lead to skin carcinogenesis (12). T-LAK cell-originated protein kinase (TOPK) is a member of the MEK3/6-related MAPKK family and is highly expressed in many cancers (13). TOPK is an upstream activator of ERKs, p38 MAPKs and JNKs and has been identified as an oncogenic protein that is involved in various cellular functions, such as DNA damage, neoplastic transformation and inflammation (14-16). Moreover, an accumulation of data provides evidence that the inhibition of TOPK might be useful in cancer chemoprevention and treatment (17, 18). However, very few effective TOPK inhibitors have been discovered. Here, we report that a novel compound, ADA-07 (5-((1s, 3s)-adamantan-1-yl)-3-(hydroxyimino) indolin-2-one), is a potent TOPK inhibitor that effectively suppresses SUV-induced activation of MAPKs signal transduction resulting in reduced SUV-induced skin carcinogenesis. These results suggest that ADA-07 might have beneficial effects in the prevention and treatment of SUV-induced skin carcinogenesis by directly targeting TOPK.
Materials and Methods
Chemicals and reagents
ADA-07 was synthesized in-house and MS analysis was performed (Supplementary Figure 1A and B). Cell culture media were all obtained from Invitrogen (Grand Island, NY). Fetal bovine serum (FBS) was from Gemini Bio-Products (West Sacramento, CA). Tris, NaCl, and SDS for molecular biology and buffer preparation were purchased from Sigma-Aldrich (St. Louis, MO). The active TOPK and MEK1 human recombinant proteins for the kinase assays were from SignalChem (Richmond, BC, Canada) and Millipore (Billerica, MA), respectively. The antibodies against phosphorylated TOPK (Thr9), ERK1/2 (Thr202/204), p38, JNKs, c-Jun and total TOPK, ERK1/2, p38, JNKs, p-c-Jun and PCNA were from Cell Signaling Biotechnology (Danvers, MA). Antibodies to detect β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The CellTiter 96 Aqueous One Solution Cell Proliferation Assay Kit and the luciferase assay substrate were purchased from Promega (Madison, WI). CNBr-activated Sepharose™ 4B beads were purchased from GE Healthcare Bio-Sciences (Uppsala, Sweden).
Cell culture
The human skin keratinocytes (HaCaT cells, obtained in 2011), normal human dermal fibroblasts (NHDF, obtained in 2009), JB6 P+ mouse epidermal cells (obtained in 1999), human epidermoid carcinoma A431 cells (obtained in 2000) and the HEK293T cell line (obtained in 2013) were purchased from American Type Culture Collection (ATCC; Manassas, VA). The human squamous cell carcinoma SCC12 cell line (obtained in 2008) was purchased from Thermo Fisher Scientific (Waltham, MA). All the cell lines were cytogenetically tested and authenticated before freezing. Each vial was thawed and maintained for a maximum of 10 passages. JB6 P+ mouse epidermal skin cells were cultured in Eagle's Minimum Essential Medium (MEM) with 5% FBS and 1% antibiotics. The HaCaT, NHDF, HEK293T and A431 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS and 1% antibiotics. The SCC12 cell line was cultured in Dulbecco's modified Eagle's medium/Ham's F-12 50/50 Mix (DMEM: F-12K 50/50) medium supplemented with 10% FBS and 1% antibiotics. All cell culture conditions were performed following ATCC's instructions.
MTS assay
Cells (1 × 104 cells/well) were seeded into 96-well plates for determining cytotoxicity. After an overnight incubation, cells were treated with different concentrations of ADA-07 and incubated for 24 or 48 h. Then 20 μL of the CellTiter 96 Aqueous One Solution (Promega Corporation, Madison, WI) were added to each well and cells were incubated for an additional 1 h at 37°C. Absorbance was measured at an optical density of 492 and 690 nm using the Thermo Multiskan plate-reader (Thermo Fisher Scientific, Waltham, MA).
Anchorage-independent cell growth assay
Cells (8 × 103/well) were seeded into 6-well plates with 0.3% Basal Medium Eagle agar containing 10% FBS with epidermal growth factor (EGF, 10 ng/ml) and different concentrations of ADA-07 and then cultured for 1 to 2 weeks. Colonies were scored under a microscope using the Image-Pro PLUS (v6.) computer software program (Media Cybernetics. Rockville, MD).
Crystal violet staining assay
Cell proliferation was determined by a crystal violet staining assay. Cells (3 × 104/well) were seeded into 24-well plates. After an overnight incubation, cells were treated with different concentrations of ADA-07 and incubated for several days. Then, each well was washed 3 times with phosphate buffered saline (PBS) and stained with 0.2% (w/v) crystal violet in 2% (v/v) ethanol. After 10 min, cells were washed 3 times with distilled water, and the remaining dye was dissolved in 0.5% (w/v) sodium dodecyl sulfate in 50% (v/v) ethanol. Absorbance was measured at an optical density of 540 nm using the Thermo Multiskan plate-reader (Thermo Fisher Scientific).
SUV irradiation system
The SUV irradiation system (UVA-340 lamps) was purchased from Q-Lab Corporation (Cleveland, OH) and used to stimulate cells in this study. The UVA-340 lamps provide the best possible simulation of sunlight in the critical short wavelength region from 365 nm down to the solar cutoff of 295 nm with a peak emission of 340 nm (19). Using this system, cells were exposed once at a dose of 60 kJ UVA/m2 and 2.9 kJ UVB/m2.
Western blot analysis
HaCaT or JB6 P+ cells (1 × 106) were cultured in 10-cm dishes for 24 h and then the medium was replaced with 0.1% FBS medium for 24 h. Cells were then treated with different concentrations of ADA-07 for 4 h followed by exposure to SUV (60 kJ UVA/m2 and 2.9kJ UVB/m2). After SUV, cells were incubated for 15 min at 37°C in a 5% CO2 humidified incubator. SCC12 and A431 cells (1.5 × 106) were cultured in 10-cm dishes overnight and then starved for 24 h. Cells were then treated with different concentrations of ADA-07 for an additional 24 h. Protein concentration in cell lysates was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA). Equal amounts of proteins were resolved by SDS-PAGE and then transferred onto polyvinylidene difluoride (PVDF) membranes (EMD Millipore Corp., Billerica, MA) and membranes were blocked with 5% nonfat milk for 1 h at room temperature. Blots were probed with appropriate primary antibodies (1:1000) overnight at 4°C followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody (1:5000) for hybridization. Protein bands were visualized with a chemiluminescent reagent (GE Healthcare Biosciences).
Lentiviral Infection
Lentivirus plasmids shTOPK (#1, TRCN0000001807; 5′-CCGGGAATATGGCAAGAGGGTTAAACTCGAGTTTAACCCTCTTGCCATATTCTTTTT -3′, #2 TRCN0000001806; 5′-CCGGCACCAAGCAAATTATCAGAAACTCGAGTTTCTGATAATTTGCTTGGTGTTTTT -3′) were purchased from GE Healthcare Dharmacon (OpenBioSystem). pLKO.1-puro Non-Target shRNA Control Plasmid DNA was purchased from Sigma-Aldrich Co. LLC (St. Louis, MO). To generate knockdown TOPK cells, the lentiviral expression vector of TOPK or shRNA control plasmid DNA was transfected into HEK293T cells together with pMD2.0G and psPAX, which were purchased from Thermo Scientific (Huntsville, AL). Cells were transfected using iMFectin poly DNA transfection reagent (GenDEPOT) according to the manufacturer's instructions. Viral supernatant fractions were collected at 48 h after transfection and filtered through a 0.45 μm syringe filter followed by infection into the appropriate cells together with 10 μg/mL polybrene (Millipore). At 16 h after infection, the medium was replaced with fresh complete growth medium containing the appropriate concentration of puromycin. At 3 to 4 days after infection, the selected cells were used for experiments.
Luciferase reporter assay
Confluent monolayers of JB6 P+ cells stably transfected with an AP-1 luciferase reporter plasmid were trypsinized and viable cells (4 × 104) suspended in 1 ml of 5% FBS-MEM were added to each well of a 24-well plate. After a 24 h incubation at 37°C in a 5% CO2 humidified incubator, cells were starved in 0.1% serum medium for another 24 h and then treated with different concentrations of ADA-07 for 1 h. Cells were then exposed to SUV (60 kJ UVA/m2 and 2.9kJ UVB/m2) and harvested after a 3 h incubation. Finally, the cells were disrupted with 100 μl of lysis buffer (0.1 M potassium phosphate pH 7.8, 1% Triton X-100, 1 mM dithiothreitol (DTT), and 2 mM EDTA) and luciferase activity was measured using a luminometer (Luminoskan Ascent, Thermo Electro, Waltham, MA).
Stable control or TOPK knockdown cells were co-transfected with 100 ng of the AP-1 luciferase reporter plasmid and 50 ng of an internal control β-galactosidase plasmid. Cells were transfected using iMFectin poly DNA transfection reagent (GenDEPOT) according to the manufacturer's instructions. After 12 h of transfection, cells were incubated with different concentrations of ADA-07 for another 24 h. Luciferase and β-galactosidase activities were measured using the Luminoskan Ascent and Multiskan MCC (Labsystems), respectively. The luciferase activity was normalized to β-galactosidase activity.
Pull-down assays
ADA-07 (2.5 mg) was coupled to CNBr-activated Sepharose 4B (GE Healthcare Biosciences, Pittsburgh, PA) matrix-beads (0.5 g) in 0.5 M NaCl and 40% DMSO (pH 8.3) overnight at 4°C, according to the manufacturer's instructions. Active TOPK, MEK or HaCaT cell lysates (500 μg) were mixed with ADA-07-conjugated Sepharose 4B beads or with Sepharose 4B beads alone as a control in reaction buffer (50 mM Tris-HCl pH 7.5, 5 mM EDTA, 150 mM NaCl, 1 mM dithiothreitol [DTT], 0.01% NP-40, 2 μg/mL bovine serum albumin, 0.02 mM phenylmethylsulfonyl fluoride [PMSF], and 1 × protease inhibitor cocktail). After gentle rocking at 4°C overnight, the beads were washed 5 times with buffer (50 mM Tris-HCl pH 7.5, 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% NP-40, and 0.02 mM PMSF). Binding was examined by Western blotting. For the ATP competition assay, active TOPK (200 ng) was incubated with different concentrations of ATP (0, 10, or 100 μM) in reaction buffer at 4°C overnight. ADA-07-conjugated Sepharose 4B beads or Sepharose 4B beads alone were added and incubated at 4°C overnight, followed by 5 washes with buffer. Then, binding was examined by Western blotting.
In vitro kinase assay
The in vitro kinase assay was conducted according to the instructions provided by Millipore (Billerica, MA). Briefly, reactions were performed in the presence of 10 μCi [γ−32P] ATP with active TOPK (200 ng) or MEK1 (200 ng), ADA-07 (0.5, 1, 3, or 5 μM) or HI-032 (10 μM, in-house synthesis) or PD098059 (10 μM, Sigma) in 40 μL of reaction buffer (40 mM MOPS/NaOH pH 7.0, 1 mM EDTA, 10 mM MnCl2, and 0.8 M ammonium sulphate) at 30°C for 30 min. HI-032 or PD098059, a well-known TOPK or MEK1 inhibitor, was used as a positive control. Reactions were stopped by adding 10 μl protein loading buffer and the mixture was separated by SDS-PAGE. The relative amounts of incorporated radioactivity were assessed by autoradiography.
Molecular modeling
The computer modeling of ADA-07 with TOPK was performed using the Schrödinger Suite 2015 software programs (20). The TOPK crystal structures were prepared under the standard procedure of the Protein Preparation Wizard in Schrödinger Suite 2015. Hydrogen atoms were added consistent with a pH of 7 and all water molecules were removed. The ATP binding site-based receptor grid was generated for docking. ADA-07 was prepared using the LigPrep program (Schrödinger) and the lowest energy conformations for docking were determined by using default parameters under the extra precision (XP) mode and the program Glide. The protein-ligand docking analysis was conducted using the induced fit docking program of Schrödinger, which can provide the ligand binding flexibility with binding pocket residues.
Mouse skin tumorigenesis study
Female SKH-1 hairless mice were purchased from Charles River and were acclimated for 2 weeks before the study and had free access to food and water. All animals were maintained according to the guidelines approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC). The animals were housed in climate-controlled quarters with a 12-h light/dark cycle. The skin carcinogenesis experiments were conducted using 6-8 wk old mice with a mean body weight of 25 g. Skin carcinogenesis was induced by a solar simulated ultraviolet irradiation system. The SUV irradiation source (Q-Lab Corporation, Westlake, OH) emitted at wavelengths of 295 to 365 nm and the peak emission was 340 nm.
The two mouse models included an early-stage prevention model and a late-stage prevention model. These models were designed for detecting either the respective chemopreventive effect or the potential therapeutic value of ADA-07 against SUV-induced skin damage and carcinogenesis. For the early-stage prevention study, SKH-1 mice were divided into 7 groups and an oil-in-water emulsion cream was applied topically as the vehicle with or without ADA-07. The three control groups included 1) mice (n = 3) not treated with vehicle or SUV; 2) mice (n = 3) treated with vehicle but no SUV; and 3) mice (n = 3) treated with 1 mg ADA-07 in vehicle but not exposed to SUV. Experimental groups included 1) mice (n = 12) treated with SUV only; 2) mice (n = 12) treated with vehicle followed by SUV 1 h later; 3) mice (n = 12 each group) treated with topical application of 0.1 or 1 mg of ADA-07 in vehicle 1 h before SUV irradiation. The topical applications and SUV treatment were applied 3 times a week for a total of 15 weeks. At 15 weeks, SUV irradiation was discontinued and topical applications continued with tumor growth monitoring for an additional 13 weeks until week 28 at which time mice were euthanized and tissues harvested.
For the late-stage prevention model, the control and experimental groups were identical to those for the early prevention model. However, the experimental groups of mice were exposed to SUV irradiation 3 times a week for 15 weeks without application of vehicle or ADA-07 in vehicle. At the end of 15 weeks, SUV exposure was stopped and application of vehicle with or without ADA-07 was begun and continued for 13 weeks until week 28 at which time the study was discontinued and mice euthanized and tissues harvested.
For both studies, the SUV irradiation was progressively increased by 10% each week. At week 1, mice were irradiated with SUV at a dose of 36 kJ/m2 UVA and 1.8 kJ/m2 UVB. At week 6, the dose of SUV reached 60 kJ/m2 UVA and 2.9 kJ/m2 UVB and this dose was maintained from week 6 to week 15. The respective doses of vehicle or ADA-07 were applied topically to the dorsal area. Mice were weighed and tumors were measured once a week until week 28 or when tumor load reached 1 cm3 total volume. At that time mice were euthanized and one-half of each sample was immediately fixed in 10% formalin and processed for hematoxylin and eosin (H&E) staining and immunohistochemistry. The other one-half of the sample was frozen and used for Western blot analysis.
Immunohistochemistry staining
Skin tissues were embedded in paraffin and subjected to immunohistochemistry. Tissues were de-paraffinized and hydrated and then permeabilized with 0.5% Triton X-100/1 × PBS for 10 min. Tissues were hybridized with PCNA (1:4000) as the primary antibody and biotinylated anti-mouse IgG as the secondary antibody. Slides were stained using the Vectastain Elite ABC Kit (Vector Laboratories, Inc., Burlingame, CA) to detect protein targets according to the manufacturer's instructions. After developing with 3, 3′-diaminobenzidine, the sections were counterstained with hematoxylin.
Statistical analysis
All quantitative data are expressed as mean values ± standard deviation (S.D.) of at least 3 independent experiments. Significant differences were determined by a Student t test or one-way ANOVA. A probability value of p < 0.05 was used as the criterion for statistical significance.
Results
ADA-07 inhibits proliferation of NMSC cells
Because of the steady increase in skin cancer incidence, chemoprevention is a practical approach to the control of SUV-induced skin cancer that has received much public attention (21). An accumulation of evidence has indicated that TOPK might be an attractive target for chemopreventive and therapeutic agents and it might contribute to p38 activation and JNKs phosphorylation during the SUV-induced DNA damage response (22, 23). Thus, identifying a novel compound that can prevent SUV-induced skin cancer by directly targeting TOPK is important. ADA-07 was synthesized as a potential TOPK inhibitor (Figure 1A). To determine whether ADA-07 exerted any cytotoxic effects against normal skin cells, JB6 P+ mouse epidermal skin cells and normal human dermal fibroblasts (NHDF) were treated with different concentrations of ADA-07 for 24 or 48 h. The results showed that ADA-07 had no cytotoxicity at concentrations less than 10 μM (Supplementary Figure 1C, D). We examined TOPK expression in JB6, NHDF, A431 and SCC12 cells (Supplementary Figure 2). The results showed that TOPK is expressed at higher levels in skin carcinoma cells (A431 and SCC12) compared to normal skin cell lines (JB6 and NHDF). This suggests that ADA-07 might specifically target skin carcinoma cells based on the higher expression of TOPK.
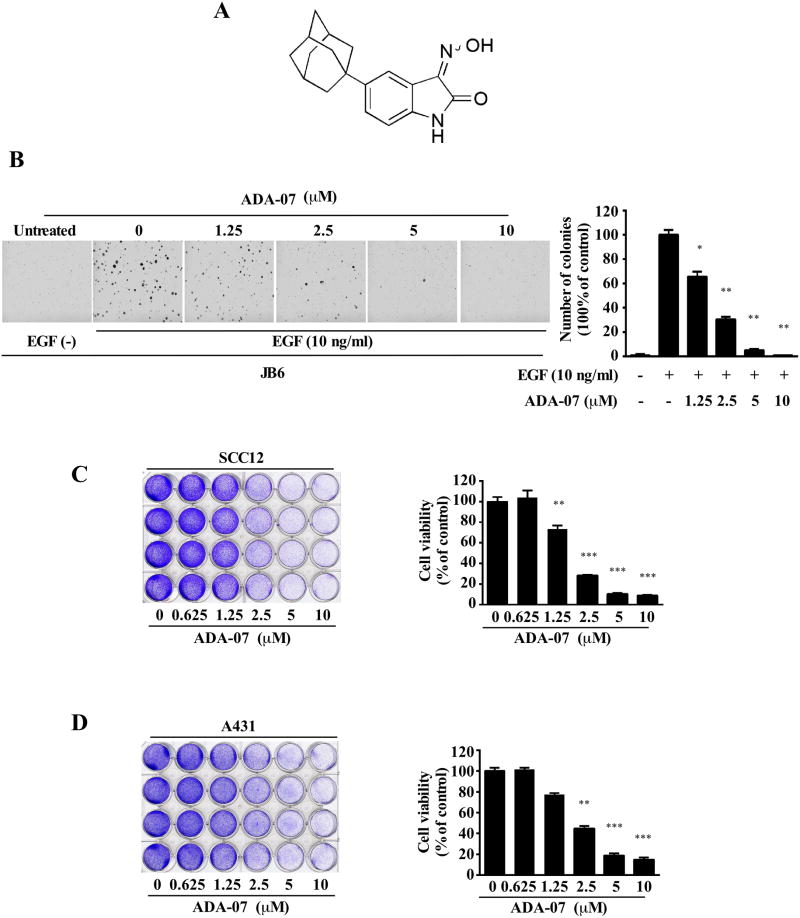
Figure 1. ADA-07 inhibits proliferation of NMSC cell lines.
A, chemical structure of ADA-07. B, ADA-07 blocks EGF-induced neoplastic transformation of JB6 P+ cells. Cells were exposed to EGF (10 ng/ml) and treated with increasing concentrations of ADA-07. Representative photographs are shown and data are presented as mean values ± S.D. from triplicate experiments. ADA-07 suppresses cell proliferation of SCC12 (C) or A431 (D) cell lines. Cells were treated with different concentrations of ADA-07 for several days and cell proliferation was determined by a crystal violet staining assay. The graph shows data from multiple experiments expressed as mean values ± S.D. The asterisk (*) indicates a significant (*, p < 0.05; **, p < 0.001; ***, p < 0.0001) decrease in cell proliferation with ADA-07 treatment.
In order to confirm whether ADA-07 absorbs at UVA or UVB wavelengths, we examined its ability to absorb SUV light between 250 and 320 nm. The result indicated that ADA-07 mainly absorbs light in the wavelength range of 250-275 nm and the peak absorption of ADA-07 is 259 nm, which indicated that ADA-07 did not show significant absorbance of UVA or UVB (Supplementary Figure 3). In addition, an anchorage-independent growth assay was performed to assess the effect of ADA-07 on cell transformation. Data indicated that EGF-induced colony formation of JB6 P+ cells was attenuated after treatment with different concentrations of ADA-07 (Figure 1B). Similarly, cell proliferation was determined by a crystal violet staining assay. Results showed that ADA-07 strongly decreased SCC12 and A431 cell proliferation in a dose-dependent manner (Figure 1 C, D). Overall, these results indicated that ADA-07 exhibited strong anti-tumor efficacy against NMSC cell growth and deserves further investigation.
ADA-07 directly suppresses TOPK kinase activity
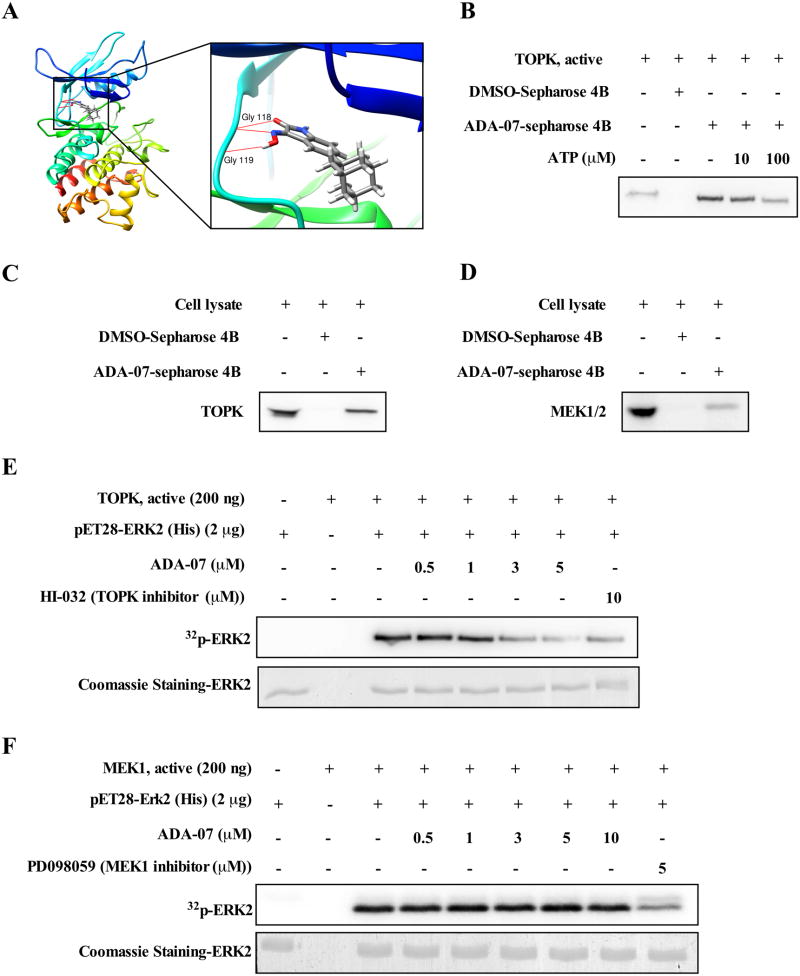
To better understand the function of ADA-07 in skin carcinogenesis, homology modeling and subsequent molecular docking were conducted to determine whether ADA-07 binds to TOPK. The binding model indicated that ADA-07 formed interactions within the ATP-binding pocket of TOPK and two potential hydrogen bonds were formed with the hinge residues Gly118 and Gly119 of TOPK and ADA-07 (Figure 2A, Supplementary Figure 4). In addition, an ATP competition assay showed that the binding ability of ADA-07 with TOPK was altered in the presence of ATP (Figure 2B). Further, an in vitro binding assay was performed using ADA-07-conjugated beads and HaCaT cell lysates. Results showed that both TOPK and MEK1/2 were detected in the ADA-07-conjugated beads group but not in the beads only group (Figure 2C, D). Next, we conducted an in vitro TOPK or MEK1 kinase activity assay with increasing concentrations of ADA-07. The results indicated that ADA-07 effectively inhibited TOPK kinase activity but not MEK1 activity (Figure 2E, F). Overall, the data suggested that ADA-07 directly binds to TOPK and suppresses TOPK kinase activity.
Figure 2. TOPK is a specific target of ADA-07.
A, computational docking model of ADA-07 with TOPK. B, ADA-07 binds with TOPK in an ATP-competitive manner. Active TOPK (200 ng) was incubated with different concentrations of ATP (0, 10 or 100 μM) and then mixed with ADA-07-conjugated Sepharose 4B beads or Sepharose 4B beads. Proteins were pulled down and analyzed by Western blot. Data are representative of 3 independent experiments that gave similar results. ADA-07 binds to either TOPK (C) or MEK1/2 (D) ex vivo. Lysates from HaCaT cells (500 μg) were incubated with ADA-07-conjugated Sepharose 4B beads or Sepharose 4B beads alone and the pulled-down proteins were analyzed by Western blot. Data are representative of 3 independent experiments that gave similar results. ADA-07 inhibits TOPK kinase (E) activity but not MEK1 kinase (F) activity in vitro. Active TOPK (200 ng) was mixed with ADA-07 (0, 0.5, 1, 3, or 5 μM) or HI-032 (TOPK inhibitor, 10 μM) and then incubated with a [γ-32P] ATP mixture. Similarly, MEK1 (200 ng) was mixed with ADA-07 (0, 0.5, 1, 3, 5, or 10 μM) or PD098059 (MEK1 inhibitor, 5 μM) and then incubated with a [γ-32P] ATP mixture. For E and F, the results are visualized by autoradiography and Coomassie blue staining serves as a loading control. Data are representative of 3 independent experiments that gave similar results.
ADA-07 attenuates SUV-induced TOPK downstream signaling
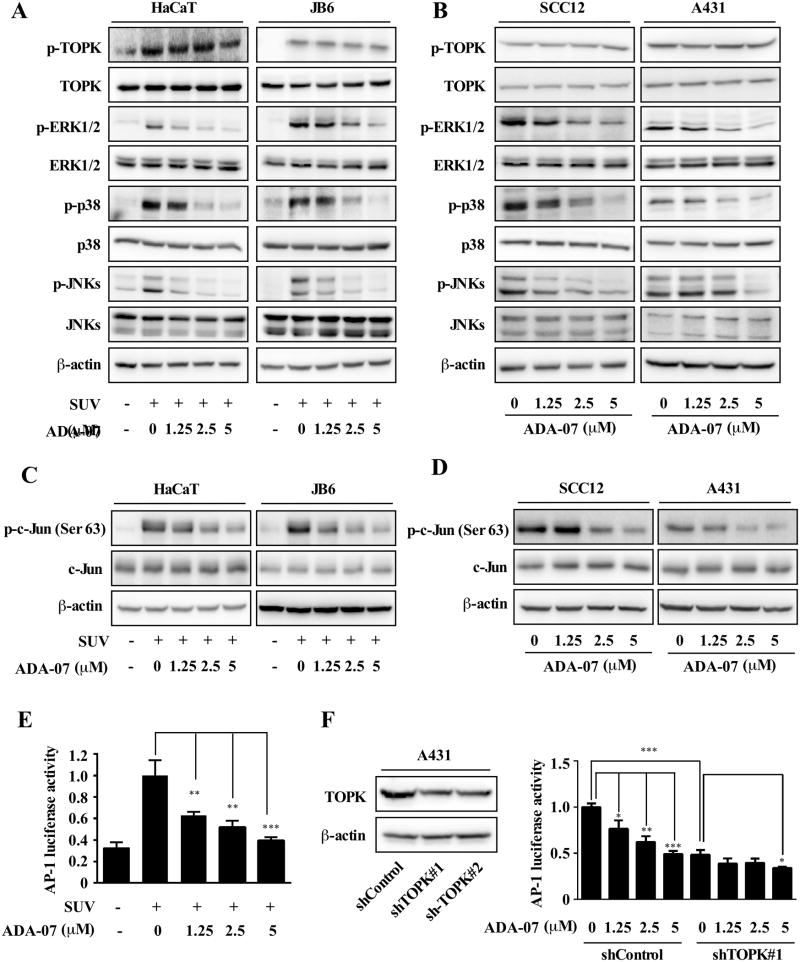
MAPK signaling cascades are well-known targets in SUV exposure and are involved in the regulation of SUV-induced cellular responses (12, 16, 24). Particularly, the activation of the ERKs pathway is relevant to UVA, whereas activation of JNKs and p38 MAPKs is directly triggered by UVB or UVC irradiation (25, 26). Additionally, evidence indicated that hyperactivation of TOPK results in uncontrolled cell proliferation in many human cancers including skin cancer (13, 16, 27). Therefore we examined the effect of ADA-07 on the SUV-induced TOPK signaling pathway in HaCaT and JB6 P+ cells. At the same time we also examined the TOPK signaling pathway in SCC12 and A431 skin cancer cells after treatment with ADA-07. Results showed that the level of phosphorylation of ERK1/2, p38, and JNKs in HaCaT and JB6 P+ cells exposed to SUV (60 kJ UVA/m2 and 2.9 kJ UVB/m2) was markedly suppressed after treatment with different concentrations of ADA-07 (Figure 3A). Similarly, the level of phosphorylation of ERK1/2, p38, and JNKs was blocked by ADA-07 in SCC12 and A431 cells dose-dependently (Figure 3B). Although no significant changes were observed in the phosphorylated and total TOPK protein levels, our results showed that ADA-07 directly binds to TOPK and suppresses TOPK kinase activity, and then blocks the phosphorylation of ERK1/2, p38, and JNKs, which are the downstream of TOPK. Previous reports revealed that AP-1 could be activated by SUV irradiation through the MAPK cascades (28, 29); and TOPK was involved in the UVB-induced JNK1-c-Jun-dependent signaling pathway leading to AP-1 activation (22). Therefore, we examined the protein levels of phosphorylation and total c-Jun by Western blot analysis. The results indicated that the phosphorylated c-Jun protein levels in HaCaT and JB6 P+ cells exposed to SUV were significantly suppressed after treatment with different concentrations of ADA-07 (Figure 3C). Similarly, the protein levels of phosphorylated c-Jun were blocked dose-dependently by ADA-07 in SCC12 and A431 cells (Figure 3D). To examine the effect of ADA-07 on SUV-induced transactivation of AP-1, we exposed JB6 P+ cells stably transfected with an AP-1 luciferase reporter plasmid to ADA-07 and SUV. The data showed that ADA-07 inhibited SUV-induced transactivation of AP-1 (Figure 3E) dose-dependently. Moreover, we also measured AP-1 activity using a combination of ADA-07 and knockdown of TOPK. The data indicated that AP-1 activity was suppressed after knockdown of TOPK in A431 cell line. More specifically, in the control group, the AP-1 activity was inhibited by ADA-07 in a dose-dependent manner. However, in the TOPK knockdown group, the AP-1 activity was only slightly affected by ADA-07 at the highest concentration (5 μM), which confirmed that ADA-07 suppresses AP-1 activity by directly targeting TOPK (Figure 3F). These results provide evidence showing that ADA-07 treatment suppresses SUV-induced TOPK downstream signaling and AP-1 activity in NMSC cells. Based on these results, we hypothesized that ADA-07 might decrease SUV-induced carcinogenesis in vivo.
Figure 3. ADA-07 attenuates SUV-induced TOPK downstream signaling.
A, ADA-07 inhibits SUV-induced phosphorylation of ERK1/2, p38, and JNKs in HaCaT and JB6 P+ cell lines. The cells were cultured, treated for 4 h with different concentrations of ADA-07 and then exposed to SUV (60 kJ UVA/m2 and 2.9 kJ UVB/m2), followed by an additional 15-minute incubation. Cells were harvested and the levels of phosphorylated and total proteins were determined by Western blot analysis with specific antibodies as indicated. B, ADA-07 inhibits phosphorylation of ERK1/2, p38, and JNKs in SCC12 and A431 cell lines. The cells were cultured and treated with different concentrations of ADA-07 for 24 h. Cells were harvested and the levels of phosphorylated and total proteins were determined by Western blot analysis with specific antibodies as indicated. C, ADA-07 suppresses the phosphorylation c-Jun protein levels in HaCaT and JB6 P+ cells exposed to SUV (60 kJ UVA/m2 and 2.9 kJ UVB/m2). D, ADA-07 attenuates phosphorylation c-Jun protein levels in SCC12 and A431 cells dose-dependently. E, ADA-07 inhibits SUV-induced AP-1 activity. For the luciferase assay, JB6 P+ cells stably transfected with an AP-1 luciferase reporter plasmid were cultured. After starvation for 24 h, cells were treated for 1 h with ADA-07 (0, 1.25, 2.5, or 5 μM). Cells were then exposed to SUV (60 kJ UVA/m2 and 2.9 kJ UVB/m2) and harvested 3 h later. Luciferase activity was measured and AP-1 activity is expressed relative to control cells without SUV treatment. F, ADA-07 represses AP-1 activation in A431 cells and knockdown of TOPK also suppresses AP-1 activation. Knockdown of TOPK in A431 cell line (left). Stable control or TOPK knockdown cells were co-transfected with 100 ng of the AP-1 luciferase reporter plasmid and 50 ng of internal control β-galactosidase plasmid. Cells were transfected using iMFectin poly DNA transfection reagent (GenDEPOT) according to the manufacturer's instructions. After 12 h of transfection, cells were incubated with the different concentrations of ADA-07 for another 24 h. Luciferase and β-galactosidase activities were measured using reagents included in the reporter assay system (right). Data are shown as mean values ± S.D obtained from triplicate experiments. Significant differences were evaluated using one-way ANOVA and the asterisks (*) indicate a significant effect (*, p < 0.05; **, p < 0.001; ***, p < 0.0001).
ADA-07 suppresses SUV-induced skin carcinogenesis in SKH-1 hairless mice
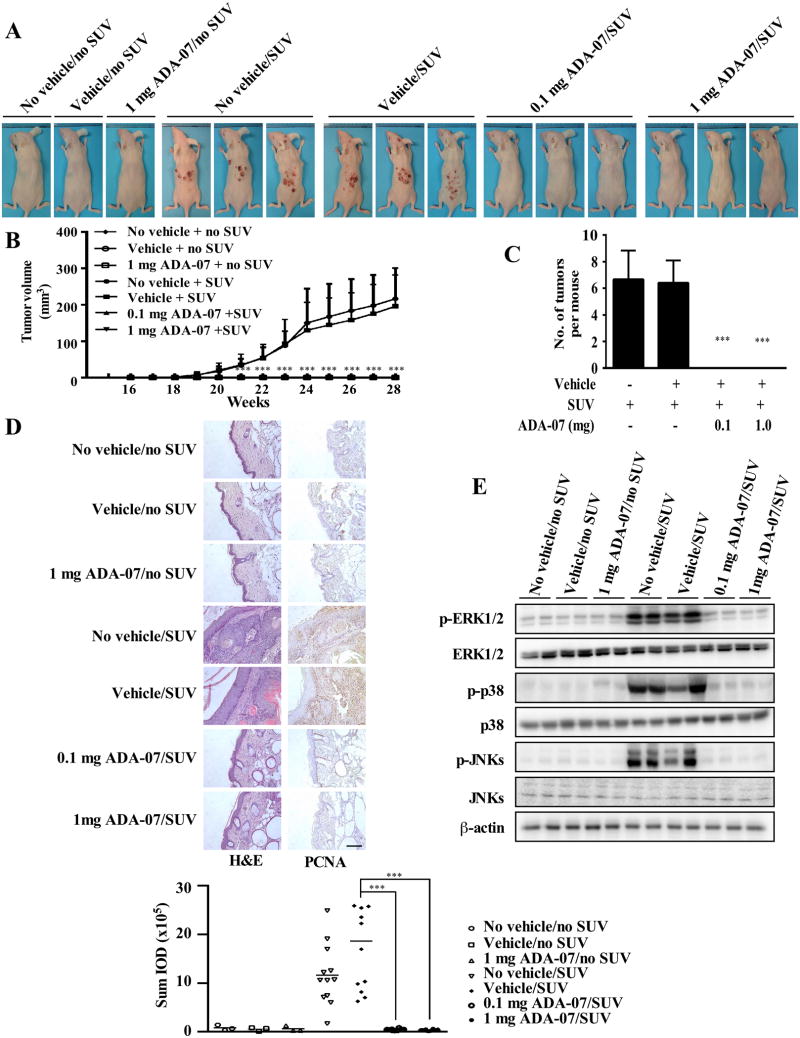
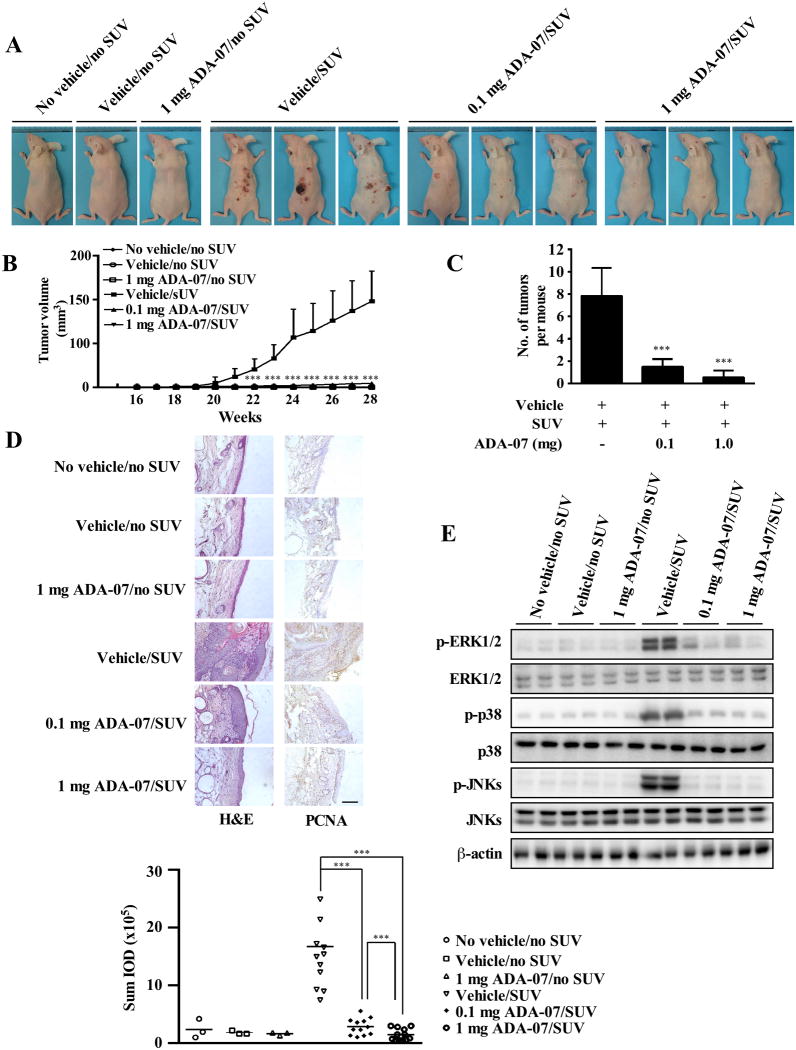
SUV irradiation, comprising both UVA and UVB, more closely resembles the natural environment and exerts a variety of effects on cells and tissues. To study the chemopreventive effect and potential therapeutic value of ADA-07 in vivo, we used two different SUV-induced mouse skin tumorigenesis models. Specifically, we set up animal studies that included an early-stage prevention model (Figure 4 and Supplementary Figure 5) and a late-stage prevention model (Figure 5 and Supplementary Figure 6). Topical application of ADA-07 on mouse dorsal skin had no effect on body weight (Supplementary Figure 5A and 6A) but resulted in a total inhibition of SUV-induced papilloma formation in the early-stage prevention group (Figure 4A, B, C, and Supplementary Figure 5B), and a substantial suppression of SUV-induced tumor incidence in the late-stage prevention group (Figure 5A and Supplementary Figure 6B). Compared with the vehicle-treated group, although some papillomas were observed in the late-stage prevention study at 28 weeks after SUV exposure, results indicated that topical treatment with 0.1 or 1 mg ADA-07 significantly reduced tumor volume and multiplicity (Figure 5B, C). Skin cancer development is well-known to be a multistep process, and SCCs frequently progress from actinic keratosis (AK), a sun-induced keratinocyte intraepithelial neoplasia (KIN or in situ cSCC) (30). Thus, in order to evaluate the histopathological differences between each group, skin and tumor samples were processed for H&E staining at the end of the study (28 weeks). In the early-stage prevention study, results revealed that chronic SUV irradiation induced malignant SCCs in SUV-treated and vehicle and SUV-treated groups, respectively. However, treatment with 0.1 or 1 mg ADA-07 decreased SUV-induced epidermal thickness, which represents typical changes in skin histological inflammation (Figure 4D, left panels). Similarly, both AKs and SCCs were induced by SUV exposure in the late-stage prevention model. Notably, ADA-07 dramatically decreased epidermal thickness and inhibited the formation of SCCs (Figure 5D, left panels). Additionally, immunohistochemical data showed that PCNA, which is a well-known marker for evaluating cell proliferation, was significantly increased in the SUV- or vehicle and SUV-treated groups. In contrast, the expression of PCNA was decreased in the ADA-07-treated groups, compared with the SUV- or vehicle and SUV-treated groups in both prevention models (Figure 4D, 5D, right panels). Moreover, Western blot analysis of mouse skin showed that phosphorylation of ERK1/2, p38, and JNKs induced by SUV was dramatically suppressed in the ADA-07-treated groups (Figure 4E, 5E). Overall, these results clearly indicated that ADA-07 exerts a strong preventive effect and also has potential therapeutic value against SUV-induced mouse skin carcinogenesis through its inhibition of TOPK activation.
Figure 4. ADA-07 suppresses SUV-induced skin carcinogenesis in an SKH-1 hairless mouse early-stage prevention model.
SKH-1 hairless mice were treated as described in Materials and Methods. The respective doses of vehicle with or without ADA-07 were applied topically to the dorsal area 1 h before SUV exposure. Mice were exposed to SUV 3 times a week for 15 weeks, and tumor incidence and multiplicity were recorded weekly until the end of the experiment at week 28. A, external appearance of tumors. B, ADA-07 suppresses SUV-induced tumor volume. Tumor volume was calculated according to the following formula: tumor volume (mm3) = length × width × height × 0.52. C, ADA-07 suppresses the average number of SUV-induced tumors at week 28. For B and C, data are represented as mean values ± S.D. and significant differences were determined by one-way ANOVA. The asterisk (*) indicates a significant decrease compared to the SUV only or the vehicle and SUV-treated groups (***, p < 0.001). D, ADA-07 inhibits SUV-induced skin carcinogenesis in mouse skin epidermal tissue. Dorsal trunk skin samples were harvested and stained with H&E (left panels) or with an antibody to detect PCNA (right panels). Representative staining shows the pathologic changes in the epidermis from each of the groups (scale bar = 100 μm). Expression of PCNA was quantified using Image-Pro Plus software and stained cells were counted from 5 separate areas on each slide (lower panel). An average of 3 samples was calculated per group. Data are expressed as mean percent of control ± S.D. and the asterisks indicate a significant difference in PCNA expression (***, p < 0.001). E, ADA-07 inhibits SUV-induced phosphorylation of ERK1/2, p38, and JNKs in mouse skin. The expression levels of phosphorylated and total proteins were analyzed by Western blot.
Figure 5. ADA-07 suppresses SUV-induced skin carcinogenesis in an SKH-1 hairless mouse late-stage prevention model.
SKH-1 hairless mice were treated as described in Materials and Methods. Mice were exposed to SUV for 15 weeks and then treatment with vehicle with or without ADA-07 was applied topically to the dorsal area for an additional 13 weeks. Tumor incidence and multiplicity were recorded weekly until the end of the experiment at week 28. A, external appearance of tumors. B, ADA-07 suppresses SUV-induced tumor volume. Tumor volume was calculated according to the following formula: tumor volume (mm3) = length × width × height × 0.52. C, ADA-07 decreases the average number of SUV-induced tumors at week 28. For B and C, data are represented as mean values ± S.D. and differences were determined by one-way ANOVA. The asterisk (*) indicates a significant decrease compared to the group exposed to SUV only or the group treated with vehicle followed by SUV (***, p < 0.001). D, ADA-07 inhibits SUV-induced skin carcinogenesis in mouse skin epidermal tissue. Dorsal trunk skin samples were harvested and stained with H&E (left panels) or with an antibody to detect PCNA (right panels). Representative staining shows the pathologic changes in the epidermis from each of the groups (scale bar = 100 μm). Expression of PCNA was quantified using Image-Pro Plus software and stained cells were counted from 5 separate areas on each slide (lower panel). An average of 3 samples was calculated per group. Data are expressed as mean percent of control ± S.D. and the asterisks indicate a significant difference in PCNA expression (***, p < 0.001). E, ADA-07 inhibits SUV-induced phosphorylation of ERK1/2, p38, and JNKs in mouse skin. The expression levels of phosphorylated and total proteins were analyzed by Western blot.
Discussion
Solar ultraviolet (SUV) irradiation represents the most important environmental risk factor that is involved in the development of skin cancer (31, 32). Studies demonstrated that accumulated SUV irradiation exposure leads to an increased risk of both BCC and SCC and nearly 90% of NMSC is estimated to be caused by chronic exposure to SUV (33, 34). Particularly, an early sign of SUV-induced skin cancer is the development of AKs, which have been identified as precursors to SCC. Epidemiological evidence demonstrated that approximately 0.025-16% of AKs progress into invasive SCCs within one year, whereas approximately 26% of AKs spontaneously regress within one year (30, 35). Thus, SCC is preventable and often curable if detected early. An accumulation of evidence indicated that specific proteins are involved in SUV-induced skin carcinogenesis. Specifically, approximately 88% of human skin cancers contain p53 mutations (36) and activating RAS mutations have been found in UV-induced mouse skin cancers (37). The importance of EGFR, RSK2, ERKs and COX-2 in UV-induced skin carcinogenesis has also been reported in the past few years (38-41). Interestingly, TOPK, a newly identified oncogene, has been studied in a wide range of human cancers (13, 15). Although previous studies indicated that TOPK is highly expressed in melanoma and clinical samples of solar dermatitis (16, 42), only one potential TOPK inhibitor against SUV-induced skin inflammation has been discovered. In the current study, we identified a novel TOPK inhibitor referred to as ADA-07 (Figure 1A) and showed its potential chemopreventive or therapeutic effects against SUV-induced skin carcinogenesis.
As indicated earlier, MAPK signaling pathways are activated during UV-induced carcinogenesis. Disturbance of the MAPK cascades by UV occurs rapidly and can lead to cellular malfunction, altered gene expression or loss of cell cycle control, which further contributes to the formation of cancer cells (4). The p38 and JNKs proteins, which are subgroups of the MAPK family, are highly expressed in solar dermatitis (42), and both play significant roles in SUV-induced inflammation and apoptosis (43-47). ERK1/2 are well-characterized MAP kinases. They are activated in keratinocytes following SUV irradiation and are involved in regulating cell proliferation, differentiation, apoptosis and tumorigenesis (48-50). TOPK is an upstream kinase of p38 and JNKs and can phosphorylate ERK1/2 (13). Abnormal levels of TOPK can signal downstream, triggering the activation of the ERK1/2, p38 or JNKs pathways. In the present study, we demonstrated that ERKs signaling, as well as p38 MAPKs and JNKs signaling, was strongly enhanced by SUV irradiation (Figure 3A). However, our findings indicated that ADA-07 not only dramatically suppressed SUV-induced activation of ERK1/2, p38 or JNKs in HaCaT and JB6 P+ cells, but also decreased MAPK signaling in skin cancer cells (Figure 3A, B). Evidently, the activator protein-1 (AP-1) transcription factor is crucial for skin cancer development (51) and the activity of AP-1 is increased by SUV exposure through the activation of the MAPK signaling pathways (12). Moreover, TOPK was involved in the UVB-induced JNK1-c-Jun-dependent signaling pathway leading to AP-1 activation (22). Our data clearly showed that SUV-induced transactivation of AP-1 was inhibited by ADA-07 dose-dependently (Figure 3E). Similarly, the AP-1 activity was significantly suppressed by ADA-07 in a dose-dependent manner in A431 cells, whereas after knockdown of TOPK only showed a slight decrease of AP-1 activity at the highest concentration of ADA-07 (Figure 3F). More directly, results of an in vitro kinase assay showed that ADA-07 effectively inhibited TOPK kinase activity, but not MEK1 activity (Figure 2E, F). Collectively, our findings further suggested that the chemopreventive or therapeutic effects of ADA-07 against SUV-induced skin carcinogenesis might be due to the specific inhibition of TOPK.
Evidence indicated that only 3 TOPK inhibitors, HI-TOPK-032, OTS514 and Cefradine have been discovered (17, 18, 42). However, the chemopreventive or therapeutic effects of these compounds against SUV-induced skin carcinogenesis have not yet been studied. Importantly, our in vivo results showed that ADA-07 was an effective chemopreventive agent and a potential therapeutic agent against SUV-induced skin tumorigenesis. The SKH-1 hairless mouse is widely considered to be the most suitable model for studies of SUV-induced carcinogenesis, and also is very useful for the study of topical compounds that alter SUV-induced skin cancer development (38, 52). Generally, skin tumors are induced in mice by chronic exposure to SUV, and tumors progress from foci of epithelial hyperplasia to premalignant papillomas and ultimately into a malignant SCC (52). In this study, our system of SUV irradiation mimics natural sunlight that includes both UVA and UVB spectrums. Results indicated that SCCs were successfully induced by 15 weeks of SUV exposure (3 times a week) in our SKH-1 hairless mouse models. Importantly, the topical application of ADA-07 completely suppressed the incidence of SUV-induced skin cancer development in an early-stage prevention study (Figure 4, Supplementary Figure 5). In addition, we showed that SKH-1 mice exposed to SUV and treated with topical ADA-07 displayed a significantly decreased tumor incidence, multiplicity, volume and malignancy rate compared to vehicle-treated mice in the late-stage prevention study (Figure 5, Supplementary Figure 6). These data support the idea that cumulative length of SUV exposure is a risk factor for SUV-induced NMSC and highlights the fact that ADA-07 might ultimately decrease skin carcinogenesis through its direct inhibition of TOPK.
Overall, this study suggests that TOPK is an important mediator of SUV-induced skin carcinogenesis. The novel compound, ADA-07, inhibited TOPK by blocking its kinase activity thereby suppressing ERK1/2, p38, JNKs and AP-1 activation. The chemopreventive effect and potential therapeutic value of ADA-07 was confirmed in the SKH-1 hairless mouse models. Therefore, ADA-07 is a promising chemopreventive and potential therapeutic agent that might be applied to human skin malignancies.
Supplementary Material
Acknowledgments
The authors thank Todd Schuster for supporting experiments, Tara Adams for supporting animal experiments and Nicki Brickman for assistance in submitting our manuscript (The Hormel Institute, University of Minnesota).
Funding: This work was funded by The Hormel Foundation and National Institutes of Health grants CA027502 (Z. Dong), CA166011 (Z. Dong), CA187027 (Z. Dong) and CA196639 (Z. Dong).
Glossary
Abbreviation
- AK
actinic keratosis
- AP-1
activator protein-1
- BCC
basal cell carcinomas
- EGF
epidermal growth factor
- ERKs
extracellular signal-regulated kinases
- JNKs
c-Jun NH2-terminal kinases
- MAPK
mitogen-activated protein kinase
- NHDF
normal human dermal fibroblasts
- NMSC
Non-melanoma skin cancer
- SCC
squamous cell carcinomas
- SUV
solar ultraviolet
- TOPK
T-LAK cell-originated protein kinase
References
- 1.Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Archives of dermatology. 2010;146:279–82. doi: 10.1001/archdermatol.2010.4. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA dermatology. 2015;151:1081–6. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 3.Samarasinghe V, Madan V, Lear JT. Management of high-risk squamous cell carcinoma of the skin. Expert review of anticancer therapy. 2011;11:763–9. doi: 10.1586/era.11.36. [DOI] [PubMed] [Google Scholar]
- 4.de Gruijl FR, van Kranen HJ, Mullenders LH. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. Journal of photochemistry and photobiology B, Biology. 2001;63:19–27. doi: 10.1016/s1011-1344(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Camarillo C, Ocampo EA, Casamichana ML, Perez-Plasencia C, Alvarez-Sanchez E, Marchat LA. Protein kinases and transcription factors activation in response to UV-radiation of skin: implications for carcinogenesis. International journal of molecular sciences. 2012;13:142–72. doi: 10.3390/ijms13010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns EM, Tober KL, Riggenbach JA, Kusewitt DF, Young GS, Oberyszyn TM. Extended UVB Exposures Alter Tumorigenesis and Treatment Efficacy in a Murine Model of Cutaneous Squamous Cell Carcinoma. Journal of skin cancer. 2013;2013:246848. doi: 10.1155/2013/246848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marks R. An overview of skin cancers. Incidence and causation. Cancer. 1995;75:607–12. doi: 10.1002/1097-0142(19950115)75:2+<607::aid-cncr2820751402>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Sarasin A. The molecular pathways of ultraviolet-induced carcinogenesis. Mutation research. 1999;428:5–10. doi: 10.1016/s1383-5742(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 9.Jung SK, Lee KW, Byun S, Kang NJ, Lim SH, Heo YS, et al. Myricetin suppresses UVB-induced skin cancer by targeting Fyn. Cancer Res. 2008;68:6021–9. doi: 10.1158/0008-5472.CAN-08-0899. [DOI] [PubMed] [Google Scholar]
- 10.Cooper SJ, Bowden GT. Ultraviolet B regulation of transcription factor families: roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Current cancer drug targets. 2007;7:325–34. doi: 10.2174/156800907780809714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods in enzymology. 2000;319:359–66. doi: 10.1016/s0076-6879(00)19035-4. [DOI] [PubMed] [Google Scholar]
- 12.Bode AM, Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Science's STKE : signal transduction knowledge environment. 2003;2003:Re2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 13.Zhu F, Zykova TA, Kang BS, Wang Z, Ebeling MC, Abe Y, et al. Bidirectional signals transduced by TOPK-ERK interaction increase tumorigenesis of HCT116 colorectal cancer cells. Gastroenterology. 2007;133:219–31. doi: 10.1053/j.gastro.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 14.Ayllon V, O'Connor R. PBK/TOPK promotes tumour cell proliferation through p38 MAPK activity and regulation of the DNA damage response. Oncogene. 2007;26:3451–61. doi: 10.1038/sj.onc.1210142. [DOI] [PubMed] [Google Scholar]
- 15.Hu F, Gartenhaus RB, Eichberg D, Liu Z, Fang HB, Rapoport AP. PBK/TOPK interacts with the DBD domain of tumor suppressor p53 and modulates expression of transcriptional targets including p21. Oncogene. 2010;29:5464–74. doi: 10.1038/onc.2010.275. [DOI] [PubMed] [Google Scholar]
- 16.Zykova TA, Zhu F, Vakorina TI, Zhang J, Higgins LA, Urusova DV, et al. T-LAK cell-originated protein kinase (TOPK) phosphorylation of Prx1 at Ser-32 prevents UVB-induced apoptosis in RPMI7951 melanoma cells through the regulation of Prx1 peroxidase activity. The Journal of biological chemistry. 2010;285:29138–46. doi: 10.1074/jbc.M110.135905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DJ, Li Y, Reddy K, Lee MH, Kim MO, Cho YY, et al. Novel TOPK inhibitor HI-TOPK-032 effectively suppresses colon cancer growth. Cancer research. 2012;72:3060–8. doi: 10.1158/0008-5472.CAN-11-3851. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo Y, Park JH, Miyamoto T, Yamamoto S, Hisada S, Alachkar H, et al. TOPK inhibitor induces complete tumor regression in xenograft models of human cancer through inhibition of cytokinesis. Science translational medicine. 2014;6:259ra145. doi: 10.1126/scitranslmed.3010277. [DOI] [PubMed] [Google Scholar]
- 19.Nakano H, Gasparro FP, Uitto J. UVA-340 as energy source, mimicking natural sunlight, activates the transcription factor AP-1 in cultured fibroblasts: evidence for involvement of protein kinase-C. Photochemistry and photobiology. 2001;74:274–82. doi: 10.1562/0031-8655(2001)074<0274:uaesmn>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Schrödinger. Schrödinger Suite 2015. Schrödinger, LLC; New York, NY: 2015. [Google Scholar]
- 21.Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. Journal of the American Academy of Dermatology. 2006;54:933–46. doi: 10.1016/j.jaad.2005.08.062. quiz 47-50. [DOI] [PubMed] [Google Scholar]
- 22.Oh SM, Zhu F, Cho YY, Lee KW, Kang BS, Kim HG, et al. T-lymphokine-activated killer cell-originated protein kinase functions as a positive regulator of c-Jun-NH2-kinase 1 signaling and H-Ras-induced cell transformation. Cancer research. 2007;67:5186–94. doi: 10.1158/0008-5472.CAN-06-4506. [DOI] [PubMed] [Google Scholar]
- 23.Liu K, Yu D, Cho YY, Bode AM, Ma W, Yao K, et al. Sunlight UV-induced skin cancer relies upon activation of the p38alpha signaling pathway. Cancer research. 2013;73:2181–8. doi: 10.1158/0008-5472.CAN-12-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim Y, He YY. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes & Diseases. 2014;1:188–98. doi: 10.1016/j.gendis.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiological reviews. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 26.Englaro W, Derijard B, Ortonne JP, Ballotti R. Solar ultraviolet light activates extracellular signal-regulated kinases and the ternary complex factor in human normal keratinocytes. Oncogene. 1998;16:661–4. doi: 10.1038/sj.onc.1201536. [DOI] [PubMed] [Google Scholar]
- 27.Park JH, Nishidate T, Nakamura Y, Katagiri T. Critical roles of T-LAK cell-originated protein kinase in cytokinesis. Cancer science. 2010;101:403–11. doi: 10.1111/j.1349-7006.2009.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Ouyang W, Li J, Wei L, Ma Q, Zhang Z, et al. Loss of tumor suppressor p53 decreases PTEN expression and enhances signaling pathways leading to activation of activator protein 1 and nuclear factor kappaB induced by UV radiation. Cancer research. 2005;65:6601–11. doi: 10.1158/0008-5472.CAN-04-4184. [DOI] [PubMed] [Google Scholar]
- 29.Huang C, Ma WY, Dong Z. The extracellular-signal-regulated protein kinases (Erks) are required for UV-induced AP-1 activation in JB6 cells. Oncogene. 1999;18:2828–35. doi: 10.1038/sj.onc.1202639. [DOI] [PubMed] [Google Scholar]
- 30.Glogau RG. The risk of progression to invasive disease. Journal of the American Academy of Dermatology. 2000;42:23–4. doi: 10.1067/mjd.2000.103339. [DOI] [PubMed] [Google Scholar]
- 31.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nature reviews Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 32.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer--the role of sunlight. Advances in experimental medicine and biology. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 33.Seebode C, Lehmann J, Emmert S. Photocarcinogenesis and Skin Cancer Prevention Strategies. Anticancer research. 2016;36:1371–8. [PubMed] [Google Scholar]
- 34.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–6. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet (London, England) 1988;1:795–7. doi: 10.1016/s0140-6736(88)91658-3. [DOI] [PubMed] [Google Scholar]
- 36.Kanjilal S, Strom SS, Clayman GL, Weber RS, el-Naggar AK, Kapur V, et al. p53 mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer research. 1995;55:3604–9. [PubMed] [Google Scholar]
- 37.Pierceall WE, Kripke ML, Ananthaswamy HN. N-ras mutation in ultraviolet radiation-induced murine skin cancers. Cancer research. 1992;52:3946–51. [PubMed] [Google Scholar]
- 38.Oi N, Chen H, Ok Kim M, Lubet RA, Bode AM, Dong Z. Taxifolin suppresses UV-induced skin carcinogenesis by targeting EGFR and PI3K. Cancer prevention research (Philadelphia, Pa) 2012;5:1103–14. doi: 10.1158/1940-6207.CAPR-11-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao K, Chen H, Liu K, Langfald A, Yang G, Zhang Y, et al. Kaempferol targets RSK2 and MSK1 to suppress UV radiation-induced skin cancer. Cancer prevention research (Philadelphia, Pa) 2014;7:958–67. doi: 10.1158/1940-6207.CAPR-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G, Fu Y, Malakhova M, Kurinov I, Zhu F, Yao K, et al. Caffeic acid directly targets ERK1/2 to attenuate solar UV-induced skin carcinogenesis. Cancer prevention research (Philadelphia, Pa) 2014;7:1056–66. doi: 10.1158/1940-6207.CAPR-14-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubet RA, et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Molecular carcinogenesis. 1999;25:231–40. [PubMed] [Google Scholar]
- 42.Fan X, Duan Q, Ke C, Zhang G, Xiao J, Wu D, et al. Cefradine blocks solar-ultraviolet induced skin inflammation through direct inhibition of T-LAK cell-originated protein kinase. Oncotarget. 2016;7:24633–45. doi: 10.18632/oncotarget.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chouinard N, Valerie K, Rouabhia M, Huot J. UVB-mediated activation of p38 mitogen-activated protein kinase enhances resistance of normal human keratinocytes to apoptosis by stabilizing cytoplasmic p53. The Biochemical journal. 2002;365:133–45. doi: 10.1042/BJ20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hildesheim J, Awwad RT, Fornace AJ., Jr p38 Mitogen-activated protein kinase inhibitor protects the epidermis against the acute damaging effects of ultraviolet irradiation by blocking apoptosis and inflammatory responses. The Journal of investigative dermatology. 2004;122:497–502. doi: 10.1111/j.1523-1747.2004.22229.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. The Journal of biological chemistry. 1996;271:31929–36. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 46.Hochedlinger K, Wagner EF, Sabapathy K. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene. 2002;21:2441–5. doi: 10.1038/sj.onc.1205348. [DOI] [PubMed] [Google Scholar]
- 47.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science (New York, NY) 2000;288:870–4. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 48.He YY, Huang JL, Chignell CF. Delayed and sustained activation of extracellular signal-regulated kinase in human keratinocytes by UVA: implications in carcinogenesis. The Journal of biological chemistry. 2004;279:53867–74. doi: 10.1074/jbc.M405781200. [DOI] [PubMed] [Google Scholar]
- 49.Kim HH, Shin CM, Park CH, Kim KH, Cho KH, Eun HC, et al. Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts. Journal of lipid research. 2005;46:1712–20. doi: 10.1194/jlr.M500105-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Archives of dermatological research. 2010;302:5–17. doi: 10.1007/s00403-009-0994-y. [DOI] [PubMed] [Google Scholar]
- 51.Eckert RL, Adhikary G, Young CA, Jans R, Crish JF, Xu W, et al. AP1 transcription factors in epidermal differentiation and skin cancer. Journal of skin cancer. 2013;2013:537028. doi: 10.1155/2013/537028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, Kusewitt DF. The hairless mouse in skin research. Journal of dermatological science. 2009;53:10–8. doi: 10.1016/j.jdermsci.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.