Abstract
Fatigue affects most cancer patients and has numerous potential causes, including cancer itself and cancer treatment. Cancer-related fatigue (CRF) is not relieved by rest, can decrease quality of life, and has no FDA-approved therapy. Thyrotropin-releasing hormone (TRH) has been proposed as a potential novel treatment for CRF, but its efficacy against CRF remains largely untested. Thus, we tested the TRH analog, taltirelin (TAL), in mouse models of CRF. To model fatigue, we used a mouse model of chemotherapy, a mouse model of radiation therapy, and mice bearing colon 26 carcinoma tumors. We used the treadmill fatigue test to assess fatigue-like behavior after treatment with TAL. Additionally, we used wild-type and TRH receptor knockout mice to determine which TRH receptor was necessary for the actions of TAL. Tumor-bearing mice displayed muscle wasting and all models caused fatigue-like behavior, with mice running a shorter distance in the treadmill fatigue test than controls. TAL reversed fatigue-like behavior in all three models and the mouse TRH1 receptor was necessary for the effects of TAL. These data suggest that TAL may be useful in alleviating fatigue in all cancer patients and provide further support for evaluating TAL as a potential therapy for CRF in humans.
Keywords: treadmill fatigue test, treadmill running, cancer-related fatigue, radiation-induced fatigue, colon 26 carcinoma, muscle wasting, mouse fatigue model
Graphical Abstract

1. Introduction
Cancer-related fatigue (CRF) is a significant problem. It is prevalent among cancer patients, is not relieved by rest, can significantly reduce quality of life, and may be caused by any combination of the cancer itself, or its treatments (for review, see refs 1, 2). Consequently, CRF can result in decreased patient compliance and overall worse clinical outcomes. To treat fatigue, non-pharmacological (e.g., physical exercise and cognitive behavioral therapy) and pharmacological interventions (e.g., methylphenidate [MPH] or modafinil) have been tested. Whereas non-pharmacological interventions may effectively alleviate CRF in many patients, the current drug treatment options may only be effective in specific subsets of patients [3–6]. Indeed, a recent meta-analysis examining the efficacy of clinical trials of non-pharmacological and drug interventions for CRF found that, while the non-pharmacological interventions were often efficacious, drug treatments were not [7]. Non-pharmacological interventions, however, are not feasible or effective effective treatment options, CRF remains undertreated and poorly understood. Given the unmet need, new, effective CRF treatments are needed.
Thyrotropin-releasing hormone (TRH), a tripeptide neurohormone/neuromodulator, showed some promise as a potential novel treatment for CRF [8]. This study was limited, however, by its small sample size of eight participants and by its use of TRH, which degrades quickly in the blood [9] and has low oral bioavailability and poor blood-brain barrier penetration, thus requiring parenteral administration of large doses. In contrast, taltirelin (TAL), an analog of TRH, has greater stability than TRH in vivo [10] and a comparable in vitro efficacy [11]. Additionally, it is orally bioavailable and efficacious in rodents [12, 13]. As such, TAL provides a stronger candidate for exploring the potential for TRH receptor agonism as a treatment for CRF.
Only one human TRH receptor has been identified. The mouse has two subtypes, the TRH1 and TRH2 receptor. The mouse TRH1 receptor is orthologous to the human TRH receptor and their amino acid sequences share 94.6% identity [14]. Moreover, the TRH1 receptor serves the same endocrine role as the human TRH receptor (i.e., stimulating release of thyroid-stimulating hormone, or TSH) and is highly expressed in the anterior pituitary of the rat [15, 16]. The TRH2 receptor, in contrast, only shares 56.8% amino acid sequence identity with the human TRH receptor [14] has no known endocrine role, and, in rats, is most highly expressed in the thalamus, brainstem, and spinal cord [15]. Given the subtype differences, it may be important to know which TRH receptor may mediate the effect(s) in the mouse before any observations from mice treated with TRH agonists can be definitively translated to other animals or humans.
Numerous mouse models have been developed and used to recapitulate and better understand CRF produced by various causes, including chemotherapy [17–19], radiation [20], and cancer and cancer cachexia [21–23]. Given the many causes of CRF and the possibility that the underlying mechanisms of CRF may vary depending on the cause, it is important to determine if TAL has anti-fatigue effects in multiple models of CRF. Thus, the current study used three mouse models of CRF. The first was a model of chemotherapy-induced fatigue, adapted from a published model [19]. The second was a mouse model of peripheral irradiation that causes fatigue-like behavior by mimicking the peripheral radiation exposure of radiation therapy [20]. The third was a well-established and widely-studied model of cancer cachexia [24–26] in which mice develop tumors that cause fatigue-like behavior [21], muscle wasting, and weight loss [25, 27]. Once fatigue was induced, we tested if TAL alleviated fatigue-like behavior in these models. Additionally, we used TRH receptor knockout mice to determine whether the actions of TAL were specifically mediated by a TRH receptor and to identify the responsible receptor(s).
2. Materials and methods
2.1. Animals
Adult male or female C57BL/6NCr mice (7–8 weeks old at the start of experiments) and female Balb/cAnNCr mice (9 weeks old at the start of experiments) were obtained from Charles River Laboratories (Frederick, MD). Adult male and female wild-type (WT) and mice lacking the TRH1 (R1KO), TRH2 (R2KO), or both TRH1 and TRH2 (R1R2KO) receptor (10–11 weeks old at the start of experiments) were used for the indicated TFT and serum hormone analysis. The TRH receptor knockout and WT mice were bred in our colony for these studies and have been previously characterized [11, 28, 29]. Female C57BL/6 mice were used for chemotherapy-induced fatigue experiments, which used 5-FU to induce fatigue, as this strain and sex was used to establish the TFT [30] and in other studies using 5-FU [19, 31]. Male C57BL/6 mice were used for the radiation-induced fatigue experiment as this strain and sex was used to establish this model [20]. Balb/c mice were used for the colon 26 carcinoma (C26) model as cells used to produce tumors were originally derived from this strain [24]. Male C57BL/6 mice and the Balb/c mice were individually housed for the duration of the studies. Mice were kept on a 12h:12h light cycle (lights off at 6PM). Food and water were provided ad libitum. All experiments were performed with prior approval of the NIDDK or NHLBI Institutional Animal Care and Use Committees.
2.2. Drugs
TAL was purchased from Tocris (Minneapolis, MN) and Santa Cruz Biotechnology (Dallas, TX), MPH was generously provided by Dr. Jonathan Katz of the National Institute of Drug Abuse, 5-fluorouracil (5-FU) was purchased from Fresenius Kabi (Lake Zurich, IL), and TRH was purchased from Sigma-Aldrich (St. Louis, MO). Ketamine was purchased from Lloyd Laboratories (Shenandoah, IA) and xylazine was purchased from Putney (Portland, ME).
For i.p. injection or oral gavage administration, drugs were prepared for a volume of 6 or 10 μL/g mouse body weight, respectively. Each day of treatment, 5-FU was diluted with PBS to the necessary concentration. MPH was prepared fresh daily in PBS. TAL was dissolved in PBS and aliquots were stored at −20°C. TAL aliquots were thawed and brought to room temperature prior to injection. TRH was prepared, stored, and thawed in the same manner as TAL.
2.3. Drug Treatment
To study alleviation of fatigue, we administered one of three interventions: TAL, MPH, or PBS. TAL was injected i.p. at 1 mg/kg based on previous studies [11, 32]. To adjust for lower oral bioavailability, a higher dose (10 mg/kg) was used to test the oral efficacy of TAL. MPH was injected i.p. at 6 mg/kg based upon pilot studies. In pilot studies testing intervention dosing regimens, we found b.i.d. dosing (between 9 and 11 AM and 3 and 5 PM) the day prior to the test, followed by a single dose 30 min prior to testing was effective. Thus, we used this regimen.
2.4. Treadmill Fatigue Test (TFT)
The TFT was performed per our published method [30]. An Exer-3/6 Treadmill (Columbus Instruments, Columbus, OH), set at a 10-degree incline with the shock grid delivered 1.22 mA electric shocks at 2 Hz, was used for training and testing. The experimenter did not interact with mice until they met the criterion for fatigue-like behavior, defined as remaining in the designated fatigue zone of the treadmill for 5 consecutive seconds. The fatigue zone encompassed the rear of the treadmill belt and the shock grid. Once fatigued, mice were promptly removed and returned to their home cages. Distance run was the primary measure of performance.
For C57BL/6 mice, the TFT was performed as previously described [30]. Balb/c mice were physically larger and, in pilot studies, displayed greater facility for treadmill running than C57BL/6 mice, so we increased the size of the fatigue zone (fatigue zone for C57BL/6: 20 cm; Balb/c: 22 cm) and tested Balb/c mice using a modified treadmill testing speed protocol to achieve higher testing speeds faster (Table 1).
Table 1.
Treadmill testing speed protocol used for Balb/c mice.
| Time (min) | Speed (m/min) |
|---|---|
| 0 | 12 |
| 0.5 | 13 |
| 1 | 14 |
| 1.5 | 15 |
| 2 | 16 |
| 3 | 17 |
| 4 | 18 |
| 5 | 19 |
| 6 | 20 |
| 7 | 21 |
| 8 | 22 |
| 9 | 23 |
| 10 | 24 |
| 15 | 25 |
| 20 | 26 |
| 60 | 28 |
| 80 | 30 |
2.5. Treadmill Location
As a secondary measure of performance during the TFT, the location of each mouse was recorded at select times throughout each test. The treadmill was visually divided into three regions, the “top” (~16 cm of the treadmill belt, furthest from the shock grid), “bottom” (~20 or 22 cm [for C57BL/6 or Balb/c, respectively] in length, including the shock grid), and “middle” (~20 or 18 cm [for C57BL/6 or Balb/c, respectively] between the top and bottom). The location of each mouse running was recorded every 60 s for min 1 to 15, every 5 min for min 20 to 40, and every 10 min from min 50 through the end of testing. A score of 1 was given for each location recording in the bottom region, 2 for the middle, and 3 for the top. Scores for each mouse in a given test were averaged and the average location scores for each treatment group were analyzed. Mice that ran for less than 1 min (thus failing to yield any location data) were excluded from location score analysis.
2.6. Chemotherapy-induced fatigue model
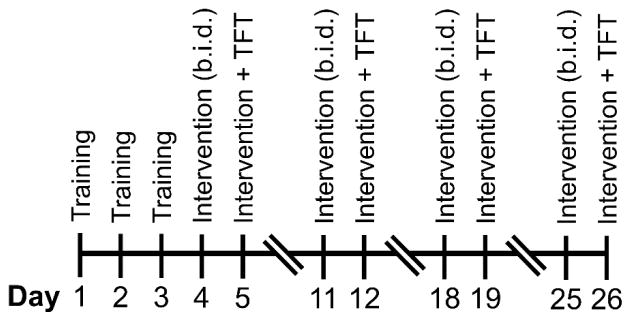
The timeline for chemotherapy-induced fatigue experiments is presented in Figure 1A. In short, female C57BL/6 mice were trained as previously described [30]. Mice were given PBS or 5-FU (i.p.) at a dose of 80 mg/kg on Day 7 and 60 mg/kg on Day 14. On the day before testing, mice received PBS or 1 mg/kg TAL (b.i.d., i.p.). Mice were given a single i.p. injection of PBS or 1 mg/kg TAL 30 min prior to the TFT.
Figure 1. Timelines for experiments.
(A) Chemotherapy-induced fatigue model. (B) Radiation-induced fatigue model. (C) Tumor-bearing fatigue model. TAL, taltirelin; 5-FU, 5-fluorouracil; TFT, treadmill fatigue test; C26, colon 26 carcinoma cell injection (5×105 cells injected, s.c.)
2.7. Radiation-induced fatigue model
The timeline for radiation-induced fatigue experiments is presented in Figure 1B. In short, male C57BL/6 mice were trained on the treadmill as described previously [30]. On Days 8–10, mice were exposed to 800 cGy of γ irradiation daily (RAD) or underwent a sham procedure, as previously described [20, 33]. On the day before testing, mice received PBS or 1 mg/kg TAL (b.i.d., i.p.). Mice were given a single i.p. injection of PBS or 1 mg/kg TAL 30 min prior to the TFT. Two mice died during the course of irradiation and were excluded from all analyses. To determine if the sham procedure affected TFT performance or response to TAL, we included and analyzed a Sham+TAL group. One mouse in the RAD+PBS group produced no treadmill location data because it ran for less than 1 min (the first time point when location is recorded). This mouse was therefore excluded from our analysis of treadmill location.
2.8. Cell preparation
Mouse C26 colon carcinoma cells were obtained from the Division of Cancer Treatment and Diagnosis Tumor Repository at the National Cancer Institute. Cells were cultured in RPMI 1640 containing 10% FBS and 1% penicillin/streptomycin (5% CO2). Upon the third passage, aliquots of C26 cells were frozen in liquid nitrogen. Cells were thawed 5 days prior to injection and grown as monolayer cultures to ≥ 50% confluence. To prepare cells for injection, cells were harvested with trypsin for 5 min (25°C, 5% CO2) before adding media to inactivate the trypsin. An aliquot of the cell suspension was counted using a Vi-Cell XR (Beckman Coulter, Indianapolis, IN). The remaining suspension was centrifuged at 125×G for 5 min, the supernatant removed, and cells resuspended in PBS at a concentration of 5×105 cells per 200 μL.
2.9. Tumor-bearing mouse fatigue model
The timeline for experiments using tumor-bearing mice is presented in Figure 1C. Female Balb/c mice were injected, under isoflurane anesthesia, s.c. between the scapulae with 200 μL of either PBS or 5×105 C26 cells suspended in PBS. Mice were trained on the treadmill before the development of visible tumors. On the day before testing, mice received PBS or 1 mg/kg TAL (b.i.d., i.p.). Mice were given a single i.p. injection of PBS or 1 mg/kg TAL 30 min prior to the TFT. Mice were euthanized the day after testing. Carcasses were weighed and tumors were dissected and weighed. To probe for cachexia, which may reduce a mouse’s ability to run, the left gastrocnemius muscle was dissected from each carcass and weighed. As the effects of TAL in tumor-free Balb/c mice was not of interest in this study, we did not include a PBS+TAL group.
As C26 tumor size affects fatigue [34], mice with small or no tumors on Day 31 (< 2% of body weight) were excluded from all analyses. The distance run by one mouse in the C26+PBS group was a significant outlier (distance: 1912 m, Grubbs’ test, P < 0.05); data from this mouse was excluded from all analyses.
2.10. Serum TSH Analysis
To compare the ability of TAL and TRH to stimulate TSH secretion, mice were administered (i.p. or orally by gavage) PBS, TAL (1 mg/kg), or TRH (0.2 mg/kg). Blood was collected 30 min after i.p. administration or 1 and 4 h after oral administration. Male and female WT and KO mice were used to examine the effects following i.p. treatment, but only WT mice were used to verify that a similar effect was observed after oral treatment. To collect blood at the 1 h time point, mice were anesthetized via isoflurane inhalation and blood was collected via retroorbital bleed. For the final blood collection (30 min or 4 h), mice were injected i.p. with ketamine/xylazine (10%/1%, v/v) to induce deep anesthesia and blood was collected via terminal retroorbital bleed. Blood samples were collected in BD Microtainer serum separator tubes (BD, Franklin Lakes, NJ). Tubes were centrifuged at 14,000 RPM for 15 min and serum was removed and stored at −80°C for later analysis. Serum TSH was measured using the Milliplex MAP Kit Mouse Pituitary Magnetic Bead Panel (EMD Millipore, Billerica, MA) per the manufacturer’s directions.
2.11. Statistical Analysis
ANOVA testing was performed using GraphPad Prism (version 7.02, GraphPad Software, La Jolla, CA). Post-hoc analyses were performed using Excel 2016 (Microsoft, Redmond, WA). For all experiments where a significant main effect of treatment was observed via ANOVA, specific post-hoc comparisons were made using two-tailed t-tests, with a Holm-Bonferroni correction for multiple comparisons. For chemotherapy-induced fatigue experiments using C57BL/6 mice, four post-hoc comparisons were: PBS+PBS vs PBS+TAL, PBS+PBS vs 5-FU+PBS, PBS+PBS vs 5-FU+TAL, and 5-FU+PBS vs 5-FU+TAL. For chemotherapy-induced fatigue experiments using WT and R1KO mice, six post-hoc comparisons were made: PBS+PBS vs PBS+TAL, PBS+PBS vs 5-FU+PBS, and PBS+PBS vs 5-FU+TAL within each genotype (WT or R1KO). For radiation-induced fatigue experiments, four post-hoc comparisons were made: Sham+PBS vs Sham+TAL, RAD+PBS vs RAD+TAL, Sham+PBS vs RAD+PBS, and Sham+TAL vs RAD+TAL. For tumor-bearing mouse experiments, three post-hoc comparisons were made: PBS+PBS vs C26+PBS, PBS+PBS vs C26+TAL, and C26+PBS vs C26+TAL. For the experiment in which mice were treated and tested weekly, two post-hoc comparisons were made: PBS vs TAL and PBS vs MPH. For the experiment using male and female WT and KO mice, sex was tested as a covariate in one-way ANOVAs; when sex was not a significant covariate, data from males and females were pooled for analysis. Data are presented as mean and SEM is used to show variance.
3. Results
3.1. TAL reversed chemotherapy-induced fatigue-like behavior
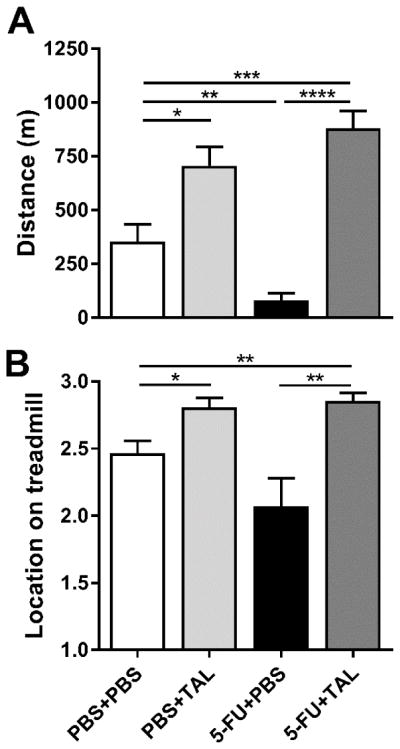
We used our recently reported TFT to assess fatigue-like behavior in mice [30]. In the TFT, the primary measure is the distance run by mice before meeting the criterion for fatigue-like behavior. The treatment regimen for inducing chemotherapy-induced fatigue and administering TAL is illustrated in Figure 1A. Treatment with 5-FU decreased the distance run in the TFT compared to controls (Figure 2A; ANOVA, main effect of treatment: F(3,55) = 23.92, P < 0.0001). TAL reversed this fatigue-like effect. Additionally, TAL caused mice to run nearer to the top of the treadmill during the test, as shown by average treadmill location (Figure 2B; ANOVA, main effect of treatment: F(3,51) = 9.458, P < 0.0001).
Figure 2. TAL reversed chemotherapy-induced fatigue-like behavior.
On Days 7 and 14, mice were given PBS or 5-FU (at 80 and 60 mg/kg, respectively). Mice were given PBS or TAL b.i.d. on Day 14 and a single injection of PBS or TAL 30 min prior to measurements on Day 16 in the TFT. (A) Distance run by mice in the treadmill fatigue test. (B) Location of mice on the treadmill during testing (“1” is the bottom portion of the treadmill; “3” is the top, furthest from the shock grid). Data represent the mean+SEM of 14–15 or 12–15 mice per group for panels A and B, respectively. TAL, taltirelin; 5-FU, 5-fluorouracil; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, t-test with Holm-Bonferroni correction
3.2. TAL effects were mediated by the TRH1 receptor
WT and R1KO mice were given 5-FU and TAL or PBS in the same manner as the C57BL/6 mice in the above experiments, then underwent the TFT. TAL increased the distance run by WT, but not R1KO mice (Figure 3; ANOVA, main effect of treatment, sexes pooled: F(7,119) = 8.031, P < 0.0001). Additionally, we confirmed that the pituitary response (i.e., TRH- or TAL-induced TSH secretion) was present in WT and R2KO, but not R1KO or R1R2KO, mice (data not shown). Thus, like stimulation of TSH secretion, the effects of TAL in the TFT were mediated by the TRH1 receptor.
Figure 3. TAL effect in the treadmill fatigue test is mediated by the TRH1 receptor.
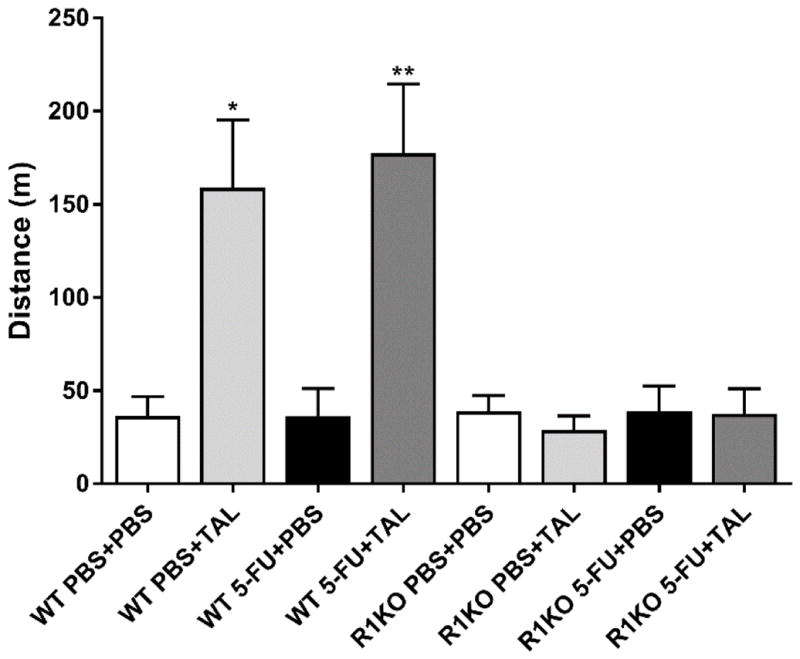
Distance run in the TFT by Wild-type (WT) or TRH1 receptor knockout (R1KO) mice. Mice were treated with PBS+PBS, PBS+TAL, 5-FU+PBS, or 5-FU+TAL. On Days 7 and 14, 5-FU (at 80 and 60 mg/kg, respectively) or PBS was administered. TAL or PBS was administered b.i.d. on Day 15 and 30 min prior to the treadmill fatigue test on Day 16. Data represent the mean+SEM of 13–19 mice per group. TAL, taltirelin; 5-FU, 5-fluorouracil; *P < 0.05, **P < 0.01, t-test compared to PBS+PBS control with Holm-Bonferroni correction
3.3. TAL, but not MPH, reduced fatigue-like behavior when given repeatedly and is orally effective
To determine if either intervention was effective when given repeatedly, female C57BL/6 mice were treated with TAL or MPH and underwent the TFT weekly (see Figure 4 for timeline). TAL increased the distance run in the TFT at every time point (Table 2). MPH had no effect on distance run at all time points. TAL also increased the average treadmill running location score, indicating that TAL-treated mice consistently ran near the top of the treadmill, whereas PBS-treated control mice ran near the middle of the treadmill belt and MPH-treated mice ran near the middle or top (Table 2). Additionally, in all tests, more PBS- or MPH-treated mice were unwilling to run longer than six min (i.e., “non-runners”) than TAL-treated mice. TAL was also effective orally in the TFT (n = 12/group; PBS: 64.2 ± 17.8; TAL [10 mg/kg]: 239 ± 32.5; two-tailed Student’s t-test, P = 0.0001).
Figure 4. Timeline for weekly treadmill fatigue testing to assess repeated intervention efficacy.
Mice were trained to run on the treadmill; given i.p. injections of one of three interventions: PBS, TAL (1 mg/kg), or MPH (6 mg/kg); and tested via treadmill fatigue test (TFT) once weekly. Results of testing are presented in Table 2.
Table 2.
Distance run, location, and task compliance of mice during weekly treadmill fatigue tests.
| Week | PBS (n=12) | TAL (n=11) | MPH (n=11) | Main effect of treatment | ||
|---|---|---|---|---|---|---|
|
| ||||||
| F value | P value | |||||
| Distance (m) | 1 | 296 ± 52 | 779 ± 118** | 333 ± 66 | F(2,31) = 10.64 | P = 0.0003 |
| 2 | 443 ± 104 | 954 ± 110** | 228 ± 78 | F(2,31) = 13.96 | P < 0.0001 | |
| 3 | 247 ± 92 | 837 ± 156** | 172 ± 57 | F(2,31) = 10.99 | P = 0.0002 | |
| 4 | 129 ± 54 | 907 ± 157** | 278 ± 311 | F(2,31) = 14.60 | P < 0.0001 | |
|
| ||||||
| Location | 1 | 2.1 ± 0.11 | 2.9 ± 0.03*** | 2.5 ± 0.12* | F(2,31) = 17.62 | P < 0.0001 |
| 2 | 2.1 ± 0.16 | 2.9 ± 0.04*** | 2.3 ± 0.10 | F(2,30) = 14.36 | P < 0.0001 | |
| 3 | 1.9 ± 0.22 | 2.7 ± 0.15* | 2.2 ± 0.12 | F(2,29) = 5.175 | P = 0.0120 | |
| 4 | 1.9 ± 0.30 | 2.9 ± 0.06* | 2.2 ± 0.12 | F(2,26) = 10.45 | P = 0.0005 | |
|
| ||||||
| Number of Non-Runners | 1 | 1 | 0 | 2 | ||
| 2 | 2 | 0 | 4 | |||
| 3 | 6 | 1 | 3 | |||
| 4 | 7 | 1 | 3 | |||
Data for the PBS group in this table were previously published and reproduced with permission from ref 30.
P < 0.05,
P < 0.01,
P < 0.001 compared to PBS, post-hoc t-tests with a Holm-Bonferroni correction.
3.4. TAL reversed radiation-induced fatigue-like behavior
Peripheral irradiation caused a significant decrease in body weight by the end of the study (Figure 5A; ANOVA, main effect of treatment: F(3,42) = 12.79, P < 0.0001), likely due to decreased food consumption. As expected, irradiation caused fatigue-like behavior. TAL treatment increased the distance run by both sham and irradiated mice; that is, it reversed the fatigue-like effect of irradiation (Figure 5B; ANOVA, main effect of treatment: F(3,42) = 17.85, P < 0.0001). The average location of mice on the treadmill was not changed by irradiation. TAL caused sham-treated and irradiated mice to run nearer to the top of the treadmill than PBS-treated counterparts (Figure 5C; ANOVA, main effect of treatment: F(3,41) = 5.896, P = 0.0019; t-tests with Holm-Bonferroni correction: Sham+PBS vs Sham+TAL, P = 0.048; RAD+PBS vs RAD+TAL, P = 0.06).
Figure 5. TAL reversed radiation-induced fatigue-like behavior.
(A) Body weight of mice before performing the treadmill fatigue test. (B) Distance run in the treadmill fatigue test. (C) Location of mice on the treadmill during testing (“1” is the bottom portion of the treadmill; “3” is the top, furthest from the shock grid). Data represent the mean+SEM of 12 mice for Sham+PBS and Sham+TAL groups, 11 mice for the RAD+TAL group, or 10–11 mice for the RAD+PBS group. TAL, taltirelin; RAD, peripheral irradiation; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, t-test with Holm-Bonferroni correction
3.5. TAL reversed fatigue-like behavior in tumor-bearing mice
We used mouse colon 26 carcinoma cells (C26) to produce tumors in mice. Tumor burden was variable between mice, but similar overall between C26 tumor-bearing mice given PBS (C26+PBS) and C26 tumor-bearing mice administered TAL (C26+TAL) groups (PBS: 4.76 ± 0.51; TAL: 4.42 ± 0.50 % body weight). Tumor-bearing mice did not display a significant change in body weight by the end of the study, although there was a slight trend toward decreased body weight in both tumor-bearing groups (Figure 6A). Analysis of the gastrocnemius muscle, however, revealed significant muscle wasting (Figure 6B; ANOVA, main effect of treatment: F(2,59) = 14.77, P < 0.0001). As expected, the C26+PBS group displayed fatigue-like behavior. TAL reversed this fatigue-like behavior (Figure 6C; ANOVA, main effect of treatment: F(2,59) = 6.128, P = 0.0038). The average location of mice on the treadmill displayed a trend toward decreasing in tumor-bearing mice compared to controls, and TAL also reversed this effect (Figure 6D; ANOVA, main effect of treatment: F(2,59) = 6.727, P = 0.0023).
Figure 6. TAL reversed fatigue-like behavior in tumor-bearing mice.
On Day 1, mice were injected with C26 cells or PBS. On Day 30, fatigue-like behavior was measured using the treadmill fatigue test. (A) Mouse body weight at the end of the study. (B) Weight of the left gastrocnemius muscle on Day 31. (C) Distance run in the TFT. (D) Location of mice on the treadmill during testing (“1” is the bottom portion of the treadmill; “3” is the top, furthest from the shock grid). Data represent the mean+SEM of 25, 20, and 17 mice in the PBS+PBS, C26+PBS, and C26+TAL groups, respectively. TAL, taltirelin; C26, colon 26 carcinoma; *P < 0.05, **P < 0.01, ****P < 0.0001, t-test with Holm-Bonferroni correction
4. Discussion
The current study demonstrated that fatigue-like behavior in mice, whether caused by chemotherapy, peripheral irradiation, or tumor burden/muscle wasting, was alleviated by TAL, a TRH analog. Additionally, we found that TAL displayed anti-fatigue effects when given orally and when administered repeatedly, and verified that it acted through the TRH1 receptor. Moreover, we observed no adverse effects of TAL, even in mice treated for 4 weeks. Taken together, these data provide strong preclinical support for further investigation of TAL as a potential anti-CRF drug by demonstrating its therapeutic potential to alleviate fatigue from different causes.
Interestingly, TAL typically improved treadmill running location scores in the TFT. As noted in published treadmill testing protocols, mice typically run near the top of the treadmill when they are capable of running faster and, conversely, near the shock grid when unable or unwilling to run faster [30, 35]. Thus, the observation that TAL-treated mice ran near the top of the treadmill, while controls ran near the middle, supports the conclusion that TAL reduced fatigue.
We found that the TRH1 receptor is necessary for the anti-fatigue effects of TAL. This is important as it confirms that the effects on fatigue are mediated specifically by a TRH receptor and are not caused by off-target effects. This is consistent with our prior study using TRH receptor knockout mice, which showed that other effects of TAL, which are also most likely mediated by receptors in the central nervous system, require the TRH1, but not TRH2, receptor [11]. Although studies have examined the effects of TRH analogs with greater or lesser in vitro potencies for the TRH1 or TRH2 receptor [36, 37], to our knowledge, the current study and Thirunarayanan et al. [11] are the only to employ TRH receptor knockout mice to determine which TRH receptor mediates a given response to TRH. Thus, we conclude that the TRH1 receptor is the primary mediator of the anti-fatigue effects of TRH receptor agonists. Future studies evaluating the anti-fatigue effects of additional TRH analogs using TRH receptor knockout mice may provide additional validation for this hypothesis.
TRH receptor agonists appear to have antidepressant-like effects in rodents [11, 38] and may have antidepressant effects in humans [39]. As depression may accompany fatigue in cancer patients [40, 41] and a previous study reports that a mouse model of chronic social stress-induced depression increases fatigue-like behavior in their treadmill testing protocol [42], a drug like TAL may be a particularly desirable therapy, potentially alleviating both depression and fatigue. Moreover, repeated TAL administration consistently reduced fatigue-like behavior in healthy mice and previous studies found no behavioral tolerance for its other effects (e.g., increasing motor activity in rats [43] or reducing ataxia in mice [44]). As such, we anticipate that, if initially effective, TAL would remain efficacious with prolonged treatment in humans. This is particularly important, as CRF can occur at various times throughout and for extended periods after completing chemotherapy or radiation therapy [45].
One limitation of this study is that our experiment using WT and KO mice appeared to show a “floor” effect, that is, control mice only run a short distance. The background strain of these mice, 129S1, has shown low compliance and poor performance in other behavioral tasks [46] and in pilot studies in our lab. Thus, it was not surprising to find that these mice ran too short of a distance to detect a decrease induced by 5-FU treatment. Although this prevents us from concluding that TAL affected chemotherapy-induced fatigue-like behavior in R1KO mice, the results nonetheless demonstrate that the observed TAL effects require the TRH1 receptor. Future testing using WT and R1KO mice on a genetic background better suited for behavioral testing (e.g., C57BL/6) may provide further validation for our conclusion.
Peripheral irradiation of mice causes a decrease in voluntary wheel running [20, 33] and, in this study, showed a similar fatigue-like effect in the TFT. This provides further validation that this mouse model induces fatigue and establishes a foundation for future studies to use either or both assays to measure fatigue-like behavior in mice. Importantly, these findings support the use of this model to delineate the changes irradiation induces to cause fatigue, allowing relevant biomarkers to be identified and novel targets for treating or preventing radiation-induced fatigue to be discovered.
The effects of TAL in our cancer model are noteworthy. TAL reversed fatigue-like behavior in our study, but did not reduce muscle wasting. The gastrocnemius muscle, important for running and likely to affect running in the TFT, showed a similar decrease in mass as in previous studies using this model, which typically showed a 10–20% decrease [21, 47, 48]. In contrast, ibuprofen, an anti-inflammatory drug that may help alleviate cachexia in clinical populations [49], significantly reduces cachexia and improves grip strength in tumor-bearing mice [21, 47] without affecting fatigue-like behavior (as assessed via voluntary wheel running) [21]. These findings, taken together, suggest that fatigue-like behavior and inflammation/cachexia are independent processes.
C26 cells differ in growth rate and cytokine production depending on the number of passages and storage conditions [50]. It should therefore be noted that we used C26 cells that had been passaged four times prior to injection and stored in liquid nitrogen prior to use. The later passage may account for differences from previous studies, which used C26 cells at a lower passage and reported the appearance of tumors sooner than we typically observed in our mice, and, consequently, required euthanasia of mice by Day 21 after injection [21, 27, 47]. In contrast, our mice did not bear excessively large tumors (≥ 10% of body weight) until at least 31 days post-injection and did not exhibit significant weight loss. However, as our mice showed similar fatigue-like behavior and similar muscle wasting as reported in the literature, any differences due to the number of passages do not appear to have fundamentally changed the findings of this study.
5. Conclusions
We found that fatigue-like behavior—whether induced by chemotherapy treatment, tumor burden, or irradiation—was consistently alleviated by TAL. A major strength of this study is that these effects have been shown across multiple mouse strains and both sexes, using female Balb/c and female/male C57BL/6 mice, and female/male knockout mice on a 129S1 background and are likely to be generalizable to other strains. In the future, we plan to evaluate TAL as a potential therapy for other preclinical fatigue models, and, ultimately, to determine whether these anti-fatigue effects translate to clinical populations.
Acknowledgments
Source of funding
This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health [grant number 1Z01 DK011006]; and the National Institute of Nursing Research at the National Institutes of Health [grant number ZIA NR000020-06].
The authors wish to thank Sumiyya Raheem for her technical assistance in the radiation-induced fatigue experiment and the animal caretakers for their care of the mice used in these studies.
Abbreviations
- 5-FU
5-fluorouracil
- ANOVA
one-way analysis of variance
- C26
colon 26 carcinoma cells
- CRF
cancer-related fatigue
- MPH
methylphenidate
- PBS
phosphate-buffered saline
- TAL
taltirelin
- TFT
treadmill fatigue test
- TRH
thyrotropin-releasing hormone
Footnotes
Conflicts of interest
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Yennurajalingam S, Sriram Y, Escalante CP, del Giglio A, Kober KM, Kamath J, Palesh O, Mustian K Multinational Association of Supportive Care in Cancer Fatigue Study Group-Biomarker Working Group. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. 2015;23:2461–2478. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, Schnipper HH, Lacchetti C, Ligibel JA, Lyman GH, Ogaily MS, Pirl WF, Jacobsen PB. American Society of Clinical Oncology, Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32:1840–1850. doi: 10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitbart W, Alici Y. Psychostimulants for cancer-related fatigue. J Natl Compr Canc Netw. 2010;8:933–942. doi: 10.6004/jnccn.2010.0068. [DOI] [PubMed] [Google Scholar]
- 5.Yennurajalingam S, Bruera E. Review of clinical trials of pharmacologic interventions for cancer-related fatigue: focus on psychostimulants and steroids. Cancer J. 2014;20:319–324. doi: 10.1097/PPO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 6.Minton O, Richardson A, Sharpe M, Hotopf M, Stone PC. Psychostimulants for the Management of Cancer-Related Fatigue: A Systematic Review and Meta-Analysis. Journal of Pain and Symptom Management. 2011;41:761–767. doi: 10.1016/j.jpainsymman.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, Mohr D, Palesh OG, Peppone LJ, Piper BF, Scarpato J, Smith T, Sprod LK, Miller SM. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamath J, Feinn R, Winokur A. Thyrotropin-releasing hormone as a treatment for cancer-related fatigue: a randomized controlled study. Support Care Cancer. 2012;20:1745–1753. doi: 10.1007/s00520-011-1268-8. [DOI] [PubMed] [Google Scholar]
- 9.Bassiri RM, Utiger RD. Metabolism and excretion of exogenous thyrotropin-releasing hormone in humans. J Clin Invest. 1973;52:1616–1619. doi: 10.1172/JCI107339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinoshita K, Yamamura M, Sugihara J, Matsuoka Y. Taltirelin Hydrate (TA-0910): An Orally Active Thyrotropin-Releasing Hormone Mimetic Agent with Multiple Actions. CNS Drug Reviews. 1998;4:25–41. doi: 10.1111/j.1527-3458.1998.tb00039.x. [DOI] [Google Scholar]
- 11.Thirunarayanan N, Nir EA, Raaka BM, Gershengorn MC. Thyrotropin-releasing hormone receptor type 1 (TRH-R1), not TRH-R2, primarily mediates taltirelin actions in the CNS of mice. Neuropsychopharmacology. 2013;38:950–956. doi: 10.1038/npp.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita K, Fujitsuka T, Yamamura M, Matsuoka Y. Effects of TA-0910, a novel orally active thyrotropin-releasing hormone analog, on the gait of ataxic animals. Eur J Pharmacol. 1995;274:65–72. doi: 10.1016/0014-2999(94)00712-g. [DOI] [PubMed] [Google Scholar]
- 13.Yamamura M, Kinoshita K, Nakagawa H, Tanaka T, Maeda K, Ishida R. Pharmacological study of TA-0910, a new thyrotropin-releasing hormone (TRH) analog, (I): Effects on the central nervous system by oral administration. Jpn J Pharmacol. 1990;53:451–461. doi: 10.1254/jjp.53.451. [DOI] [PubMed] [Google Scholar]
- 14.The Uniprot Consortium. UniProt: a hub for protein information. Nucl Acids Res. 2015;43:D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heuer H, Schäfer MK, O’Donnell D, Walker P, Bauer K. Expression of thyrotropin-releasing hormone receptor 2 (TRH-R2) in the central nervous system of rats. J Comp Neurol. 2000;428:319–336. [PubMed] [Google Scholar]
- 16.Sun Y, Lu X, Gershengorn MC. Thyrotropin-releasing hormone receptors -- similarities and differences. J Mol Endocrinol. 2003;30:87–97. doi: 10.1677/jme.0.0300087. [DOI] [PubMed] [Google Scholar]
- 17.Zombeck JA, Fey EG, Lyng GD, Sonis ST. A clinically translatable mouse model for chemotherapy-related fatigue. Comp Med. 2013;63:491–497. [PMC free article] [PubMed] [Google Scholar]
- 18.Weymann KB, Wood LJ, Zhu X, Marks DL. A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain Behav Immun. 2014;37:84–94. doi: 10.1016/j.bbi.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahoney SE, Davis JM, Murphy EA, McClellan JL, Gordon B, Pena MM. Effects of 5-fluorouracil chemotherapy on fatigue: role of MCP-1. Brain Behav Immun. 2013;27:155–161. doi: 10.1016/j.bbi.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renner M, Feng R, Springer D, Chen MK, Ntamack A, Espina A, Saligan LN. A murine model of peripheral irradiation-induced fatigue. Behav Brain Res. 2016;307:218–226. doi: 10.1016/j.bbr.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norden DM, McCarthy DO, Bicer S, Devine RD, Reiser PJ, Godbout JP, Wold LE. Ibuprofen ameliorates fatigue- and depressive-like behavior in tumor-bearing mice. Life Sci. 2015;143:65–70. doi: 10.1016/j.lfs.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aulino P, Berardi E, Cardillo VM, Rizzuto E, Perniconi B, Ramina C, Padula F, Spugnini EP, Baldi A, Faiola F, Adamo S, Coletti D. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer. 2010;10:363. doi: 10.1186/1471-2407-10-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts BM, Frye GS, Ahn B, Ferreira LF, Judge AR. Cancer cachexia decreases specific force and accelerates fatigue in limb muscle. Biochem Biophys Res Commun. 2013;435:488–492. doi: 10.1016/j.bbrc.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griswold DP, Corbett TH. A colon tumor model for anticancer agent evaluation. Cancer. 1975;36:2441–2444. doi: 10.1002/1097-0142(197512)36:6<2441::aid-cncr2820360627>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y, Eda H, Tanaka T, Udagawa T, Ishikawa T, Horii I, Ishitsuka H, Kataoka T, Taguchi T. Experimental cancer cachexia induced by transplantable colon 26 adenocarcinoma in mice. Cancer Res. 1990;50:2290–2295. [PubMed] [Google Scholar]
- 26.Pigna E, Berardi E, Aulino P, Rizzuto E, Zampieri S, Carraro U, Kern H, Merigliano S, Gruppo M, Mericskay M, Li Z, Rocchi M, Barone R, Macaluso F, Di Felice V, Adamo S, Coletti D, Moresi V. Aerobic Exercise and Pharmacological Treatments Counteract Cachexia by Modulating Autophagy in Colon Cancer. Sci Rep. 2016;6:26991. doi: 10.1038/srep26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talbert EE, Metzger GA, He WA, Guttridge DC. Modeling human cancer cachexia in colon 26 tumor-bearing adult mice. J Cachexia Sarcopenia Muscle. 2014;5:321–328. doi: 10.1007/s13539-014-0141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng H, Schimpf BA, Rohde AD, Pavlova MN, Gragerov A, Bergmann JE. Thyrotropin-releasing hormone receptor 1-deficient mice display increased depression and anxiety-like behavior. Mol Endocrinol. 2007;21:2795–2804. doi: 10.1210/me.2007-0048. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Zupan B, Raaka BM, Toth M, Gershengorn MC. TRH-receptor-type-2-deficient mice are euthyroid and exhibit increased depression and reduced anxiety phenotypes. Neuropsychopharmacology. 2009;34:1601–1608. doi: 10.1038/npp.2008.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dougherty JP, Springer DA, Gershengorn MC. The Treadmill Fatigue Test: A Simple, High-throughput Assay of Fatigue-like Behavior for the Mouse. Journal of Visualized Experiments. 2016 doi: 10.3791/54052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahoney SE, Davis JM, Murphy EA, McClellan JL, Pena MM. Dietary quercetin reduces chemotherapy-induced fatigue in mice. Integr Cancer Ther. 2014;13:417–424. doi: 10.1177/1534735414523315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eto K, Kim SK, Nabekura J, Ishibashi H. Taltirelin, a thyrotropin-releasing hormone analog, alleviates mechanical allodynia through activation of descending monoaminergic neurons in persistent inflammatory pain. Brain Res. 2011;1414:50–57. doi: 10.1016/j.brainres.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 33.Wolff BS, Renner MA, Springer DA, Saligan LN. A Mouse Model of Fatigue Induced by Peripheral Irradiation. J Vis Exp. 2017 doi: 10.3791/55145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norden DM, Bicer S, Clark Y, Jing R, Henry CJ, Wold LE, Reiser PJ, Godbout JP, McCarthy DO. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain Behav Immun. 2015;43:76–85. doi: 10.1016/j.bbi.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conner JD, Wolden-Hanson T, Quinn LS. Assessment of Murine Exercise Endurance Without the Use of a Shock Grid: An Alternative to Forced Exercise. Journal of Visualized Experiments. 2014 doi: 10.3791/51846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meena CL, Ingole S, Rajpoot S, Thakur A, Nandeker PP, Sangamwar AT, Sharma SS, Jain R. Discovery of a low affinity thyrotropin-releasing hormone (TRH)-like peptide that exhibits potent inhibition of scopolamine-induced memory impairment in mice. RSC Adv. 2015;5:56872–56884. doi: 10.1039/c5ra06935a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monga V, Meena CL, Rajput S, Pawar C, Sharma SS, Lu X, Gershengorn MC, Jain R. Synthesis, receptor binding, and CNS pharmacological studies of new thyrotropin-releasing hormone (TRH) analogues. ChemMedChem. 2011;6:531–543. doi: 10.1002/cmdc.201000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd RL, Pekary AE, Sattin A, Amundson T. Antidepressant effects of thyrotropin-releasing hormone analogues using a rodent model of depression. Pharmacol Biochem Behav. 2001;70:15–22. doi: 10.1016/s0091-3057(01)00555-x. [DOI] [PubMed] [Google Scholar]
- 39.Szuba MP, Amsterdam JD, Fernando AT, Gary KA, Whybrow PC, Winokur A. Rapid antidepressant response after nocturnal TRH administration in patients with bipolar type I and bipolar type II major depression. J Clin Psychopharmacol. 2005;25:325–330. doi: 10.1097/01.jcp.0000169037.17884.79. [DOI] [PubMed] [Google Scholar]
- 40.Fox SW, Lyon DE. Symptom clusters and quality of life in survivors of lung cancer. Oncol Nurs Forum. 2006;33:931–936. doi: 10.1188/06.ONF.931-936. [DOI] [PubMed] [Google Scholar]
- 41.So WKW, Marsh G, Ling WM, Leung FY, Lo JCK, Yeung M, Li GKH. The symptom cluster of fatigue, pain, anxiety, and depression and the effect on the quality of life of women receiving treatment for breast cancer: a multicenter study. Oncol Nurs Forum. 2009;36:E205–214. doi: 10.1188/09.ONF.E205-E214. [DOI] [PubMed] [Google Scholar]
- 42.Azzinnari D, Sigrist H, Staehli S, Palme R, Hildebrandt T, Leparc G, Hengerer B, Seifritz E, Pryce CR. Mouse social stress induces increased fear conditioning, helplessness and fatigue to physical challenge together with markers of altered immune and dopamine function. Neuropharmacology. 2014;85:328–341. doi: 10.1016/j.neuropharm.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 43.Asai H, Asahi T, Yamamura M, Yamauchi-Kohno R, Saito A. Lack of behavioral tolerance by repeated treatment with taltirelin hydrate, a thyrotropin-releasing hormone analog, in rats. Pharmacol Biochem Behav. 2005;82:646–651. doi: 10.1016/j.pbb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Kinoshita K, Fukushima T, Kodama Y, Sugihara J, Yamamura M, Matsuoka Y. Chronic anti-ataxic actions of the novel thyrotropin-releasing hormone (TRH) analog, TA-0910, during and after repeated administration in Rolling mouse Nagoya: behavioral and pharmacokinetic studies. Biol Pharm Bull. 1997;20:36–39. doi: 10.1248/bpb.20.36. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz AL, Nail LM, Chen S, Meek P, Barsevick AM, King ME, Jones LS. Fatigue patterns observed in patients receiving chemotherapy and radiotherapy. Cancer Invest. 2000;18:11–19. doi: 10.3109/07357900009023057. [DOI] [PubMed] [Google Scholar]
- 46.Lightfoot JT, Leamy L, Pomp D, Turner MJ, Fodor AA, Knab A, Bowen RS, Ferguson D, Moore-Harrison T, Hamilton A. Strain screen and haplotype association mapping of wheel running in inbred mouse strains. J Appl Physiol. 2010;109:623–634. doi: 10.1152/japplphysiol.00525.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarthy DO, Whitney P, Hitt A, Al-Majid S. Indomethacin and ibuprofen preserve gastrocnemius muscle mass in mice bearing the colon-26 adenocarcinoma. Res Nurs Health. 2004;27:174–184. doi: 10.1002/nur.20019. [DOI] [PubMed] [Google Scholar]
- 48.Khamoui AV, Park BS, Kim DH, Yeh MC, Oh SL, Elam ML, Jo E, Arjmandi BH, Salazar G, Grant SC, Contreras RJ, Lee WJ, Kim JS. Aerobic and resistance training dependent skeletal muscle plasticity in the colon-26 murine model of cancer cachexia. Metab Clin Exp. 2016;65:685–698. doi: 10.1016/j.metabol.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 49.McMillan DC, Wigmore SJ, Fearon KCH, O’Gorman P, Wright CE, McArdle CS. A prospective randomized study of megestrol acetate and ibuprofen in gastrointestinal cancer patients with weight loss. Br J Cancer. 1999;79:495–500. doi: 10.1038/sj.bjc.6690077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norden DM, Devine R, McCarthy DO, Wold LE. Storage Conditions and Passages Alter IL-6 secretion in C26 adenocarcinoma cell lines. MethodsX. 2015;2:53–58. doi: 10.1016/j.mex.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]