Abstract
Members of the IL-1 family play protective and regulatory roles in immune defense against the opportunistic mold Aspergillus fumigatus. Here, we investigated the IL-1 family member IL-33 in lung defense against A. fumigatus. IL-33 was detected in the naïve lung, which further increased after exposure to A. fumigatus in a Dectin-1 independent manner. Mice deficient in the receptor for IL-33 (Il1rl1−/−) unexpectedly demonstrated enhanced lung clearance of A. fumigatus. IL-33 functioned as a negative regulator of multiple inflammatory cytokines, as IL-1α, IL-1β, IL-6, IL-17A and IL-22 were significantly elevated in fungal-exposed Il1rl1−/− mice. Subsequently, IL-33 administration to normal mice attenuated fungal-induced IL-17A and IL-22, but not IL-1α, IL-1β and IL-6 production. IL-33 mediated regulation of IL-17A and IL-22 did not involve the modulation of IL-23 but rather prostaglandin E2 (PGE2); PGE2 was significantly increased in fungal-exposed Il1rl1−/− mice and normal mice produced less PGE2 after fungal exposure when administered IL-33, suggesting that IL-33 mediated regulation of IL-17A and IL-22 occurred at the level of PGE2. This was confirmed by in vivo cyclooxygenase 2 (COX-2) inhibition, which attenuated fungal-induced IL-17A and IL-22, as well as IL-1α, IL-1β and IL-6 production, in Il1rl1−/− mice resulting in impaired fungal clearance. We also show that a PGE2 receptor agonist increased, whereas a PGE2 synthase inhibitor decreased, the levels of IL-17A and IL-22, but not IL-1α, IL-1β and IL-6. This study establishes novel mechanisms of induction of innate IL-17A/IL-22 production via PGE2 and regulation of the PGE2-IL-17A-IL-22 axis via IL-33 signaling during lung fungal exposure.
Introduction
Concerns over the rise in invasive fungal infections (IFIs) over the last several decades due to modern medical interventions (new, more effective immunosuppressive drugs/regimens) are mounting (1). IFIs caused by Aspergillus fumigatus remain one of the most lethal human infectious diseases. Invasive aspergillosis (IA) is particularly insidious due to a combination of increasing antifungal drug resistance, extremely high mortality and an inherent difficulty in diagnosis (2); (3). Although IA is the most devastating aspect of A. fumigatus exposure and accounts for 20–40% of IFIs in solid organ and hematopoietic transplantation (4) (5), colonization with, or sensitization to, A. fumigatus has dramatic effects on lung function in asthmatics (6) and individuals with cystic fibrosis (7).
The IL-1 family of cytokines is comprised of eleven members, seven with pro-inflammatory characteristics (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β and IL-36γ) and four with anti-inflammatory characteristics (IL-1Ra, IL-36Ra, IL-37 and IL-38). Early studies in experimental models of A. fumigatus infection documented the induction of IL-1 family members, such as IL-1β (8) (9). We have further shown that the fungal beta-glucan receptor Dectin-1 drives IL-1α and IL-1β production in vivo during IA (10). A recent study has demonstrated that IL-1R was essential for survival with IL-1α promoting leukocyte recruitment and IL-1β promoting anti-fungal activity (11). Studies in humans have shown that A. fumigatus strongly induces IL-36γ and IL-36Ra, but not IL-36α, which were dependent on Dectin-1 and TLR4 (12). Inhibiting IL-36 signaling was found to abrogate the induction of protective Th17 and Th1 responses. With respect to immunopathogenic effects of IL-1 family members during IA, IL-1α and IL-1β are thought to be the primary drivers of inflammation in CGD via decreased autophagy and increased inflammasome activation (13). Finally, analysis of polymorphisms within the IL-1 family revealed that a SNP in IL-1Ra VNTR2 (rs380092), IL-1α-889C (rs1800587) and IL-1β (rs1143627) correlates with incidence or risk of developing IA in haematological patients (14).
IL-33 is recognized as one of eleven members of the IL-1 cytokine family, due to its chromosome cluster, structure and processing (reviewed in (15)). IL-33 has been studied in a variety of inflammatory conditions, including allergic asthma, colitis, rheumatoid arthritis and cardiovascular diseases (reviewed in (16)). Likewise, IL-33 has been investigated in a variety of viral (influenza, RSV), bacterial (sepsis, Gram-negative keratitis, Gram-positive skin infection) and fungal (C. albicans peritonitis, fungal asthma) infection models (17). By and large, IL-33 mediates the induction of type 2/Th2 responses and suppression of Th1 and inflammatory responses. However, IL-33 has the potential to mediate a diverse array of functions, such as neutrophil recruitment and ROS/RNI induction, depending on the site and type of insult (17). Although IL-33 has yet to be investigated in IA, blockade of IL-33R signaling has been shown to improve fungal asthma severity, putatively through the modulation of pro-allergic responses (18). In the current study, we defined the role of IL-33 in lung defense against IA. Here, we show that IL-33 is detrimental for lung antifungal defense due to its function as a negative regulator of IL-17A and IL-22 via suppression of COX-2/PGE2.
Materials and Methods
Mice
WT BL/6 mice, 6 to 8 weeks of age, were obtained from The Jackson Laboratory (Bangor, ME) or Taconic (Hudson, NY). Il1rl1−/− (ST2/IL-33R) mice were a kind gift from Dr. Andrew McKenzie, Cambridge University. Il1r1−/− mice were obtained from Jackson. All animals were housed in a specific pathogen-free, Association for Assessment and Accreditation of Laboratory Animal Care-certified facility and handled according to Public Health Service Office of Laboratory Animal Welfare policies after review by the UAB Institutional Animal Care and Use Committee.
Preparation of A. fumigatus; in vivo challenge; lung fungal burden assessment
A. fumigatus isolate 13073 (ATCC, Manassas, VA) was maintained on potato dextrose agar for 5–7 days at 37°C. Conidia were harvested by washing the culture flask with 50 ml of sterile phosphate buffered saline supplemented with 0.1% Tween 20. The conidia were then passed through a sterile 40 µm nylon membrane to remove hyphal fragments and enumerated on a hemacytometer. For challenge, mice were lightly anesthetized with isoflurane and administered 7 × 107 A. fumigatus conidia in a volume of 50 µl intratracheally as previously described (10) (19). Briefly, mice are held in a vertical, upright position and the tongue is withdrawn from the mouth using forceps. A pipette is used to deliver the 50 µl inoculum to the caudal oropharynx in which normal breathing results in fluid aspiration into the lungs (20). For lung fungal burden analysis, the left lungs were collected at 48 h post-exposure and homogenized in 1 ml of PBS. Total RNA was extracted from 0.1 ml of unclarified lung homogenate using the MasterPure™ yeast RNA purification kit (Epicentre Biotechnologies, Madison, WI), which includes a DNAse treatment step to eliminate genomic DNA as previously reported (21). Total RNA was also extracted from serial 1:10 dilutions of live A. fumigatus conidia (101 – 109) and DNAse treated to form a standard curve. Lung A. fumigatus burden was analyzed with real time PCR measurement of the A. fumigatus 18S rRNA (GenBank accession number AB008401 (22)) and quantified using a standard curve of A. fumigatus conidia as previously described (21). As a validation of the real-time PCR method, heat-killed A. fumigatus did not yield a signal by real-time PCR and were unable to grow on potato dextrose agar plates (21). In addition, no amplification controls (i.e. no reverse transcriptase included in the cDNA reaction) yielded a signal of <0.001% by real-time PCR, indicating that the DNAse treatment step efficiently eliminated contaminating A. fumigatus DNA (as DNA is not predicative of organism viability (23)).
Lung cell isolation and culture; inflammatory cytokine analysis; PGE2 analysis
Mice were anesthetized with intra-peritoneal ketamine/xylazine and sacrificed by exsanguination 48 h post-infection. Both lungs were collected and minced in IMDM media (Sigma, St. Louis, MO) supplemented with 1% pen-strep-glut (Mediatech, Herndon, VA), 10% heat inactivated FBS (Invitrogen, Carlsbad, CA) and 0.4 mg/ml polymyxin B (Thermo Fisher), followed by incubation for 60 min with tissue culture-grade type IV collagenase (1 mg/ml; Sigma, St. Louis, MO) in a 37°C orbital shaker at 100 rpm. The cell suspension was filtered through sterile 70 µm and 40 µm nylon filters and red blood cells lysed with ACK buffer (Lonza, Walkersville, MD) to create lung cell preparations. For lung cell cultures, cells were enumerated on a hemacytometer and plated at 1 × 106 cells in a volume of 0.2 ml. Supernatants were collected after 24 h, clarified by centrifugation and stored at −80°C. Supernatants were analyzed for protein levels of 32 cytokines and chemokines using Bio-Plex multiplex suspension cytokine array (Bio-Rad Laboratories), according to the manufacturer’s instructions (20) (21). The data were analyzed using Bio-Plex Manager software (Bio-Rad Laboratories). IL-22 levels were quantified by ELISA (R&D Systems) (10). PGE2 levels were quantified using the Prostaglandin E2 Parameter Assay Kit (Cat. #KGE004B, R&D Systems). In some experiments, neutrophil, inflammatory monocyte and eosinophil levels in lung digest cells were analyzed by flow cytometry. Cells were washed and Fc receptors were blocked with Mouse BD Fc Block™ (BD Biosciences, San Diego, CA) at 4°C for 20 min. Thereafter, cells were stained with a single-color LIVE/DEAD® Fixable Dead Cell Stain (Invitrogen) followed by labeling with cell-specific markers (antibodies from BD Biosciences, Biolegend and eBiosciences) (24).
In vivo treatments (IL-33, celecoxib, IL-17A/IL-22 neutralization)
In specific experiments, WT BL/6 mice were treated with recombinant murine IL-33 intratracheally. Specifically, mice were challenged with A. fumigatus and 6 and 24 h thereafter, administered IL-33 (1 µg in 50 µl; R&D Systems) or PBS intratracheally. In other experiments, WT BL/6 and Ilrl1−/− mice were administered celecoxib (Cayman Chemical; 100 mg/kg in 250 µl intraperitoneally) or vehicle (ethanol). Mice received celecoxib or vehicle 24 h prior to A. fumigatus challenge and then again at 6 h and 24 h post-challenge. For both treatments, 48 h after A. fumigatus challenge, mice were sacrificed and the lungs processed for cell isolation, culture and inflammatory cytokine, IL-22 and PGE2 analysis. For in vivo IL-17A and IL-22 neutralization, Ilrl1−/− mice were challenged intratracheally with 7 × 107 A. fumigatus conidia in 50 µl and 24 h thereafter, mice were administered 50 µg of rat anti-mouse IL-17A (Cat. #MAB421) or IL-22 (Cat. #MAB5821) or rat IgG isotype control antibody or polyclonal goat anti-mouse IL-17A (Cat. #AF-421-NA) or IL-22 (Cat. #AF582) or goat IgG isotype control antibody (Cat. #AB-108-C) (all antibodies from R&D Systems) intratracheally. Forty-eight hours after challenge, mice were sacrificed, the left lungs were collected and fungal burden assessed as described above.
In vitro treatments (PGE2 inhibition, EP receptor agonist)
In specific experiments, WT BL/6 mice were challenged with A. fumigatus and 48 h thereafter, lungs collected and enzymatically digested. Cells were enumerated and plated at 1 × 106 cells in a volume of 0.2 ml in the presence of vehicle, an inhibitor of microsomal prostaglandin E synthase-1 (mPGES-1; Cayman Chemical) or misoprostol, a global EP receptor (PGE2 receptor) agonist (Sigma, St. Louis, MO). Supernatants were collected after 24 h, clarified by centrifugation and inflammatory cytokine and IL-22 levels were quantified by Bio-Plex and ELISA (R&D Systems), respectively (10).
Statistics
Data were analyzed using GraphPad Prism® Version 5.0 statistical software (GraphPad Software, San Diego, CA). Comparisons between groups when data were normally distributed were made with the two-tailed unpaired Student’s t test. Significance was accepted at a value of p < 0.05.
Results
The lack of IL-33 signaling results in enhanced A. fumigatus lung clearance
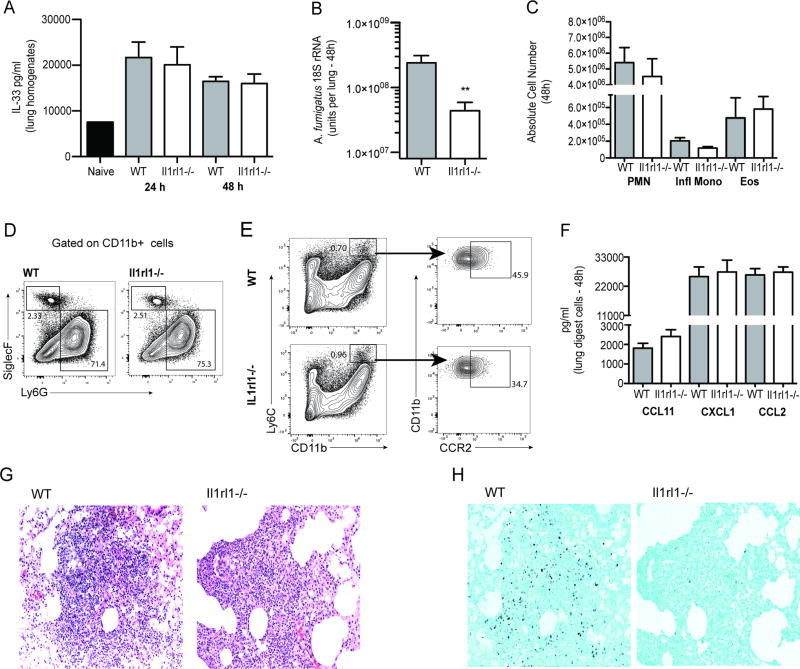
IL-33 is detrimental for lung infection with the Th1-requring fungal pathogen Crytococcus neoformans as a result of its known propensity to induce type 2/Th2 responses (25). IL-33 is protective in a model of C. albicans peritoneal sepsis via the induction of heightened neutrophil responses (26). Elimination of A. fumigatus from the lung is highly dependent on neutrophils (27). Moreover, there is evidence to suggest that innate type 2 responses may also be beneficial in A. fumigatus lung clearance (28). Collectively, host immune responses initiated by IL-33 would appear to favor A. fumigatus lung clearance. In initial studies, we demonstrate that IL-33 is increased in response to acute A. fumigatus challenge (Figure 1A), however unlike our previous report with fungal asthma (29), IL-33 did not require Dectin-1 mediated beta-glucan recognition for induction (Figure 1A). To our surprise, mice deficient in the IL-33 receptor (ST2/T1/IL1-RL1; Il1rl1−/−) demonstrated lower A. fumigatus burden in the lung 48 h post-challenge (Figure 1B), suggesting that IL-33 induction functions as a negative regulator of fungal clearance. We did not observe a difference in fungal burden prior to 48 h post-challenge (data not shown). Intriguingly, augmented fungal clearance in Il1rl1−/− mice at 48 h post-challenge was not a consequence of differential lung cellularity (Figure 1C), as we observed no significant changes in neutrophils and eosinophils (gated on CD11b+ cells followed by Ly6G+ cells as neutrophils and Siglec-F+ cells as eosinophils) (Figure 1D) or inflammatory monocytes (gated on CD11b+ Ly6C+ cells followed by gating on CCR2+ cells) (Figure 1E) (all of which have been implicated in innate lung clearance of A. fumigatus (10) (30) (31). Similar data was observed at 12 and 24 h post-infection (Supplemental Figure 1). This observation is supported by no differences in the levels of various chemokines produced by lung digest cells (Figure 1F) and similar levels of inflammatory cells and inflammation on H&E-stained lung tissue sections (Figure 1G). Sequential GMS-stained lung tissue sections also demonstrated lower levels of A. fumigatus organisms in Il1rl1−/− mice (right) compared to WT (left) mice (Figure 1H). Thus, despite the induction of IL-33 after acute A. fumigatus exposure, clearance of the organism from the lung is enhanced in the absence of IL-33 signaling.
Figure 1. The lack of IL-33 signaling results in enhanced A. fumigatus lung clearance.
(A) C57BL/6 wild-type and Dectin-1 deficient (Clec7a−/−) mice were challenged intratracheally with A. fumigatus conidia (Af) and 48 h post-exposure, whole lungs were collected and IL-33 levels quantified in clarified lung homogenates by ELISA. The Figure illustrates cumulative data from two to three independent studies (n = 3–5 mice per group, per study). (B) C57BL/6 wild-type (WT) and Il1rl1−/− (IL-1RL1; ST2 deficient) mice were challenged intratracheally with A. fumigatus conidia and 48 h after exposure, lung fungal burden was assessed by real-time PCR analysis of A. fumigatus 18S rRNA levels. The Figure illustrates cumulative data from two independent studies (n = 4–5 mice per group, per study). Data are expressed as mean A. fumigatus 18S rRNA + SEM. (C) Cumulative flow cytometric data from two independent studies (n = 2–4 mice per group, per study). Data are expressed as absolute number of live cells in lung digests. (D/E) Lung cells were isolated via enzymatic digestion, Fc-blocked, stained with a live/dead staining kit and thereafter stained with fluorochrome-conjugated CD11c, CD11b, Ly6G, Ly6C and Siglec F. Representative flow cytometric plots are included for (D) neutrophils and eosinophils (gated on CD11b+ cells followed by Ly6G+ cells as neutrophils and Siglec-F+ cells as eosinophils) and (E) inflammatory monocytes (gated on CD11b+ Ly6C+ cells followed by gating on CCR2+ cells). (F) C57BL/6 wild-type (WT) and Il1rl1−/− (IL-1RL1; ST2 deficient) mice were challenged intratracheally with A. fumigatus conidia and 48 h after exposure, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured in triplicate for 24 h. CCL11/eotaxin, CXCL1/KC and CCL3/MIP-1α levels were quantified in clarified co-culture supernatants by Bio-Plex. The Figure illustrates cumulative data from four independent studies (n = 1–2 mice per group, per study). (G) Representative H&E-stained lung sections from wild-type (left) and Il1rl1−/− (right) mice challenged intratracheally with A. fumigatus conidia for 48 h. Original magnification of 200X. (H) Representative GMS-stained lung sections from wild-type (left) and Il1rl1−/− (right) mice challenged intratracheally with A. fumigatus conidia for 48 h. Original magnification of 200X. For all graphs, ** represents a P value of < 0.01, respectively (Unpaired two-tailed Student’s t test).
IL-33 signaling negatively affects IL-17A and IL-22 after A. fumigatus exposure
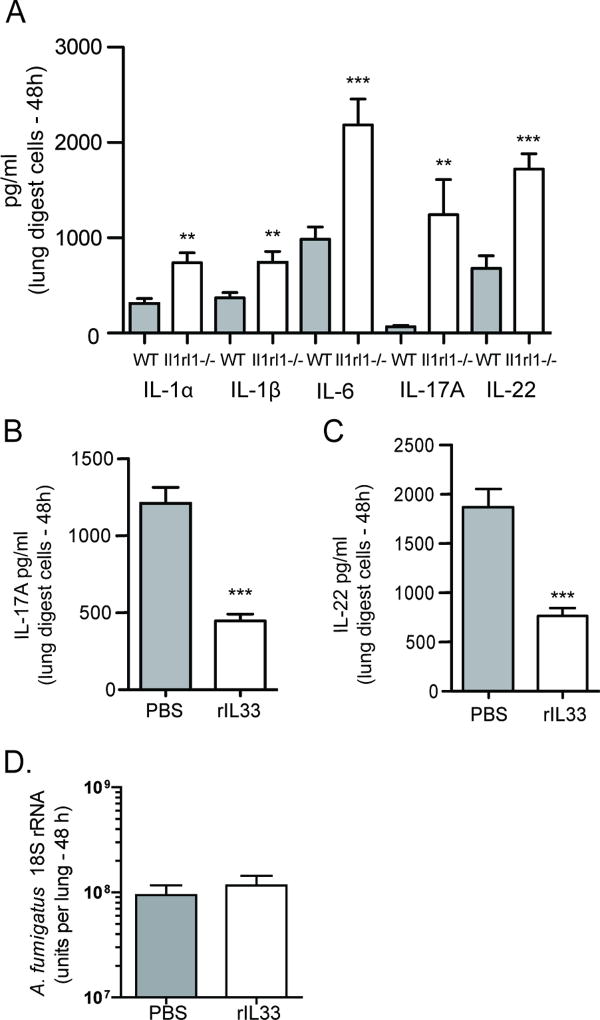
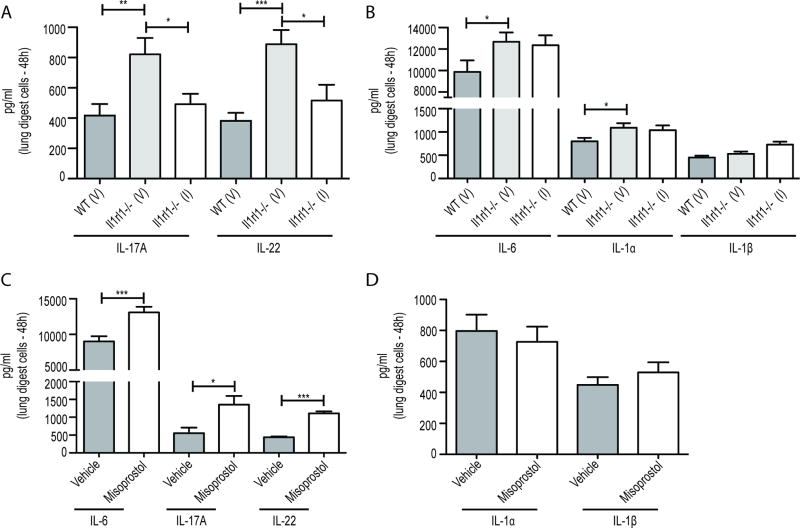
As we did not observe major cellular changes in the lung between A. fumigatus exposed WT and Il1rl1−/− mice, we examined whether there was a difference in inflammatory factors that could explain improved fungal clearance in the absence of IL-33 signaling. We observed significantly higher levels of IL-1α, IL-1β and IL-6, a profile that favors the development of IL-17A and IL-22, which were also produced at higher levels by lung digest cells from Il1rl1−/− mice (Figure 2A). Higher inflammatory cytokine levels in the absence of IL-33 signaling suggested that IL-33 functioned as a negative regulator of these mediators. To confirm this, we challenged mice with A. fumigatus followed by treating them with IL-33, which resulted in a ~ 65% reduction in IL-17A (Figure 2B) and ~ 50% reduction in IL-22 production (Figure 2C). Although this suggests that IL-33 treatment could have a negative effect on the clearance of A. fumigatus from the lung, we did not observe an effect of IL-33 treatment on fungal burden (Figure 2D). Thus, the absence of IL-1RL1/ST2 signaling results in augmented lung clearance of A. fumigatus in the presence of heightened IL-17A and IL-22 production, indicating that signaling through IL-1RL1 serves as a negative regulator of these mediators.
Figure 2. IL-33 signaling negatively affects IL-17A and IL-22 production after A. fumigatus exposure.
C57BL/6 wild-type (WT) and Il1rl1−/− (IL-1RL1; ST2 deficient) mice were challenged intratracheally with A. fumigatus conidia and 48 h after exposure, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured in triplicate for 24 h. (A) IL-1α, IL-1β, IL-6, IL-17A and IL-22 levels were quantified in clarified co-culture supernatants by Bio-Plex or ELISA. The Figure illustrates cumulative data from four independent studies (n = 1–2 mice per group, per study). (B/C) C57BL/6 wild-type mice were challenged with A. fumigatus and 6 and 24 h thereafter, administered IL-33 (1 µg in 50 µl) or PBS intratracheally. Forty-eight h after exposure, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured in triplicate for 24 h. (B) IL-17A and (C) IL-22 levels in co-culture supernatants were quantified by Bio-Plex or ELISA. The Figure illustrates cumulative data from three independent studies (n = 1–2 mice per group, per study). For all graphs, ** or *** represent a P value of < 0.01 or 0.001, respectively (Unpaired two-tailed Student’s t test). (D) C57BL/6 wild-type mice were challenged with A. fumigatus and 6 and 24 h thereafter, administered IL-33 (1 µg in 50 µl) or PBS intratracheally. Forty-eight h after exposure, lung fungal burden was assessed by real-time PCR analysis of A. fumigatus 18S rRNA levels. The Figure illustrates cumulative data from four independent studies (n = 3–4 mice per group, per study). Data are expressed as mean A. fumigatus 18S rRNA + SEM.
PGE2, but not IL-23, is increased in the absence of IL-33 signaling
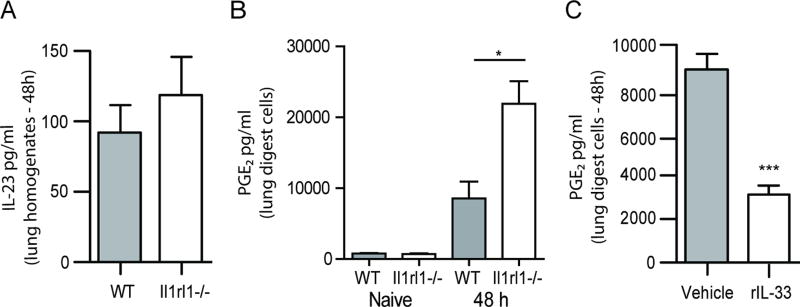
IL-23 is the most recognized positive regulator of IL-17A and IL-22 production and we have previously reported that IL-23 drives IL-17A and IL-22 production in the lung after A. fumigatus exposure (24) (19). We therefore speculated that the increased production of IL-17A and IL-22 in the absence of IL-33 signaling was most likely the result of increased IL-23. However, Il1rl1−/− mice had similar levels of IL-23 in the lung as WT mice (Figure 3A). There was also no difference in IL-23 production by A. fumigatus stimulated BMDCs between WT and Il1rl1−/− mice (Supplemental Figure 2). This data suggested that IL-17A and IL-22 were elevated in Il1rl1−/− mice via an IL-23 independent mechanism. PGE2 has been recently described as potentiator of innate IL-22 production (32), therefore we questioned whether PGE2 was modulated in the absence of IL-33 signaling. Results show that at baseline, lung cells from naïve WT and Il1rl1−/− mice produced similar levels of PGE2 (Figure 3B). However, upon exposure to A. fumigatus, lung cells from Il1rl1−/− mice demonstrated significantly higher PGE2 production (Figure 3B). This data suggests IL-33 signaling regulates PGE2 production during A. fumigatus exposure. We next examined mice treated with IL-33 after A. fumigatus exposure and found that, like IL-17A and IL-22, PGE2 levels were significantly lower (Figure 3C). Thus, IL-33 is a negative regulator of PGE2, which may function as a positive regulator of IL-17A and IL-22 production during lung fungal exposure.
Figure 3. PGE2, but not IL-23, is increased in the absence of IL-33 signaling.
(A) C57BL/6 wild-type (WT) and Il1rl1−/− (IL-1RL1; ST2 deficient) mice were challenged intratracheally with A. fumigatus conidia and 48 h after exposure, whole lungs were collected and IL-23 levels quantified in clarified lung homogenates by ELISA. The Figure illustrates cumulative data from two independent studies (n = 4–5 mice per group, per study). (B) C57BL/6 wild-type (WT) and Il1rl1−/− (IL-1RL1; ST2 deficient) mice were challenged intratracheally with A. fumigatus conidia and 48 h after exposure, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured in triplicate for 24 h. PGE2 levels were quantified in clarified co-culture supernatants by EIA. The Figures illustrates cumulative data from three independent studies (n = 1–2 mice per group, per study). (C) C57BL/6 wild-type mice were challenged with A. fumigatus and 6 and 24 h thereafter, administered IL-33 (1 µg in 50 µl) or PBS intratracheally. Forty-eight h after exposure, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured in triplicate for 24 h. PGE2 levels were quantified in clarified co-culture supernatants by EIA. The Figure illustrates cumulative data from three independent studies (n = 1–2 mice per group, per study). For all graphs, * or *** represent a P value of < 0.05 or 0.001, respectively (Unpaired two-tailed Student’s t test).
Cyclooxygenase-2 inhibition results in attenuated inflammatory cytokine production in Il1rl1−/− mice after A. fumigatus exposure
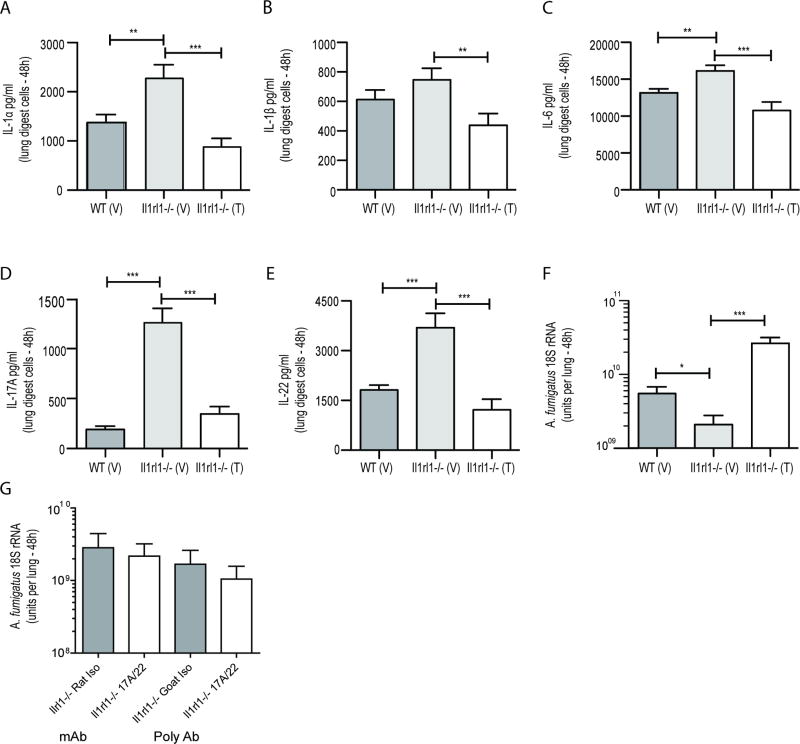
Prostaglandin-endoperoxide synthase 2 (i.e. cyclooxygenase 2, COX-2) initiates the conversion of arachidonic acid to multiple prostaglandins, including PGE2. A recent study in humans reported that individuals with various systemic inflammatory syndromes demonstrated decreased COX-2 and PGE2 expression coupled with lower IL-22 levels (32). Employing an experimental murine model of LPS-induced systemic inflammation, this report demonstrated that PGE2-EP4 signaling promoted homeostasis of type 3 innate lymphoid cells (ILC3s) and drove them to produce IL-22 (32). To determine if this axis also functioned in the induction of lung-derived IL-22, as well as other inflammatory mediators, we examined the effects of COX-2 inhibition. Treating A. fumigatus exposed Il1rl1−/− mice with Celecoxib/Celebrex®, a COX-2 selective NSAID, significantly lowered IL-1α (Figure 4A), IL-1β (Figure 4B), IL-6 (Figure 4C), IL-17A (Figure 4D) and IL-22 (Figure 4E) production. Consequently, the attenuation of these mediators resulted in less efficient fungal clearance in Il1rl1−/− mice (Figure 4F). Despite our previous reports showing a role for IL-17A and IL-22 in lung defense against A. fumigatus (24) (19), neutralization of both mediators in Il1rl1−/− mice unexpectedly did not affect their ability to clear A. fumigatus from the lung (Figure 4G). The effects of COX-2 inhibition were also observed in WT mice (for example, IL-22 levels were 504 ± 67 pg/ml vs. 166 ± 61 pg/ml in vehicle-treated (n = 4) vs. Celebrex-treated mice (n = 4), respectively; P = 0.001). Thus, inhibition of COX-2 signaling in the absence of IL1RL1 during A. fumigatus exposure compromises lung production of multiple inflammatory mediators leading to impaired fungal clearance.
Figure 4. Cyclooxygenase-2 inhibition results in attenuated inflammatory cytokine production in Il1rl1−/− mice after A. fumigatus exposure.
(A/B/C/D/E) C57BL/6 wild-type (WT) and Il1rl1−/− (IL-1RL1; ST2 deficient) mice were administered Celecoxib intraperitoneally (100 mg/kg in 250 µl; denoted as “T” for treated) or Vehicle (ethanol; denoted as “V” for vehicle) 24 h prior to A. fumigatus challenge and then again at 6 h and 24 h post-challenge. Thirty-six to forty-eight h after A. fumigatus challenge, mice were sacrificed, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured for 24 h. (A) IL-1α, (B) IL-1β, (C) IL-6, (D) IL-17A and (E) IL-22 levels in co-culture supernatants were quantified by Bio-Plex or ELISA. The Figure illustrates cumulative data from four independent studies (n = 3–4 mice per group, per study). (F) Mice were treated as in (A) and 48 h after exposure, lung fungal burden was assessed by real-time PCR analysis of A. fumigatus 18S rRNA levels. The Figure illustrates cumulative data from two independent studies (n = 4 mice per group, per study). For all graphs, * or *** represent a P value of < 0.05 or 0.001, respectively (Unpaired two-tailed Student’s t test). (G) Il1rl1−/− (IL-1RL1; ST2 deficient) mice were challenged intratracheally with A. fumigatus conidia and 24 h thereafter, mice were administered 50 µg of rat anti-mouse IL-17A or IL-22 (R&D Systems) or rat IgG isotype control antibody or goat anti-mouse IL-17A or IL-22 (R&D Systems) or goat IgG isotype control antibody. Forty-eight hours after challenge, mice were sacrificed, the left lungs were collected and fungal burden assessed. The Figure illustrates cumulative data from two to three independent studies (n = 3–4 mice per group). Data are expressed as mean A. fumigatus 18S rRNA + SEM.
PGE2 supports production of IL-17A and IL-22, but not IL-6, IL-1α and IL-1β, by lung cells from A. fumigatus exposed mice
Although PGE2 is a product of COX-2 signaling, it is unclear whether PGE2 itself affects inflammatory mediator production. To address this, we cultured lung cells form infected mice with an inhibitor of microsomal prostaglandin E synthase-1 (mPGES-1), which converts the COX-2 product PGH2 into the biologically active PGE2. This demonstrated that specific inhibition of PGE2 attenuated IL-17A and IL-22 production (Figure 5A), but not IL-6, IL-1α and IL-1β (Figure 5B). Conversely, treating lung cells form infected mice with the global EP receptor (PGE2 receptor) agonist misoprostol increased production of IL-6, IL-17A and IL-22 production (Figure 5C) over vehicle treated lung cells, but not IL-1α and IL-1β (Figure 5D). Misoprostol was not effective at inducing these mediators in lung cells from naïve mice (Supplemental Figure 3). Collectively, our data reveals that PGE2 specifically promotes the production of IL-17A and IL-22 during lung fungal exposure.
Figure 5. PGE2 supports IL-17A and IL-22, but not IL-6, IL-1α and IL-1β, by lung cells from A. fumigatus exposed mice.
(A/B) C57BL/6 wild-type (WT) and Il1rl1−/− (IL-1RL1; ST2 deficient) mice were challenged with A. fumigatus conidia and 48 h after exposure, mice were sacrificed, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured in the presence of the mPGES inhibitor (denoted as “I” for inhibitor) or vehicle (DMSO; denoted as “V” for vehicle) in triplicate for 24 h. (A) IL-17A and IL-22 levels and (B) IL-6, IL-1α and IL-1β levels in co-culture supernatants were quantified by Bio-Plex or ELISA. The Figure illustrates cumulative data from three independent studies (n = 1–2 mice per group, per study). (C/D) C57BL/6 wild-type (WT) mice were exposed and cells isolated as in (A). Cells were cultured in the presence of misoprostol or vehicle in triplicate for 24 h. (C) IL-6, IL-17A and IL-22 levels and (D) IL-1α and IL-1β levels in co-culture supernatants were quantified by Bio-Plex or ELISA. The Figure illustrates cumulative data from two independent studies (n = 1–2 mice per group, per study). For all graphs, * or *** represent a P value of < 0.05 or 0.001, respectively (Unpaired two-tailed Student’s t test).
Increased IL-1α and IL-1β signaling is not the mechanism associated with enhanced PGE2 and IL-22 production in Il1rl1−/− mice after A. fumigatus exposure
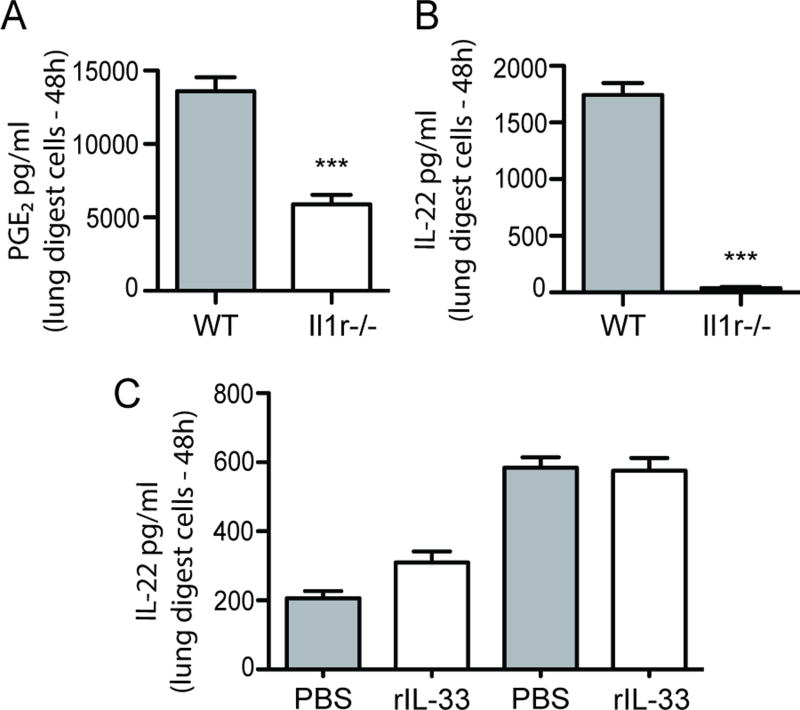
Data thus far indicates that the level of PGE2 directly correlates with the level of IL-17A and IL-22 after A. fumigatus exposure. However, it is not clear why PGE2 is elevated in Il1rl1−/− mice after A. fumigatus exposure. In addition to IL-17A and IL-22, data in Figure 2A showed that IL-1α and IL-1β were also significantly elevated in Il1rl1−/− mice after A. fumigatus exposure. IL-1β has previously been implicated in PGE2 induction (33). In turn, mice deficient in the receptor for IL-1α and IL-1β demonstrated ~ 60% reduction in PGE2 levels (Figure 6A) as well as a profound reduction in the production of IL-22 (Figure 6B), suggesting that the increase in IL-1α and/or IL-1β in the absence of IL-33 signaling was potentially responsible for the increase in PGE2 and thus IL-22 production. To confirm this, we determined whether IL-1α and IL-1β levels were modulated in mice that received IL-33 after A. fumigatus challenge. Despite IL-33 signaling impairing PGE2 (Figure 3C) and IL-22 (Figure 2C) production, IL-33 administration had no effect on the levels of IL-1α or IL-1β (Figure 6C). There was also no synergy between IL-1β and PGE2 receptor stimulation, as the addition of IL-1β in conjunction with misoprostol in lung cells from A. fumigatus infected mice did not augment production of IL-22 (Supplemental Figure 3). Thus, the mechanism behind elevated PGE2 in the absence of IL-33 signaling is not a result of enhanced IL-1α and IL-1β production.
Figure 6. Increased IL-1R signaling is not the mechanism associated with enhanced PGE2 and IL-22 in Il1rl1−/− mice after A. fumigatus exposure.
C57BL/6 wild-type (WT) and Il1r1−/− (IL-1R1 deficient) mice were challenged intratracheally with A. fumigatus conidia and 48 h after exposure, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured in triplicate for 24 h. (A) PGE2 levels were quantified in clarified co-culture supernatants by EIA and (B) IL-22 levels were quantified in clarified co-culture supernatants by ELISA. The Figures illustrates cumulative data from four independent studies (n = 1–2 mice per group, per study). (C) C57BL/6 wild-type mice were challenged with A. fumigatus and 6 and 24 h thereafter, administered IL-33 (1 µg in 50 µl) or PBS intratracheally. Forty-eight h after exposure, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured in triplicate for 24 h. IL-1α and IL-1β levels were quantified in clarified co-culture supernatants by Bio-Plex. The Figure illustrates cumulative data from two independent studies (n = 1–2 mice per group, per study). For all graphs, *** represents a P value of < 0.001, respectively (Unpaired two-tailed Student’s t test).
Discussion
In this body of work, we demonstrate that despite induction of IL-33 in the lung in response to A. fumigatus, IL-33 signaling negatively regulated immune responsiveness to A. fumigatus, as evidenced by mice deficient in IL-33 receptor signaling (ST2; Il1rl1−/− mice) displaying lower fungal burden post-challenge. Assessment of potential mechanisms to explain enhanced fungal clearance in the absence of IL-33 signaling revealed a specific profile of elevated cytokines, IL-1α, IL-1β, IL-6, IL-17A and IL-22. Despite observing elevated levels of these mediators, they did not correlate with any changes in lung cellularity, an observation itself supported by the lack of changes in the levels of multiple chemokines. As a result, we focused on the IL-17A/IL-22 axis, due to their known abilities to induce soluble antimicrobial responses from the lung epithelium (34) and our previous work documenting a protective role for both IL-17A and IL-22 in host defense against A. fumigatus (24) (19). An intriguing finding from the current study was that IL-33 functioned as a negative regulator of IL-17A and IL-22. To date, there have been relatively few cytokines identified as negative regulators of IL-17A and IL-22. The most well-recognized include TGF-β, which promotes IRF4-mediated suppression of IL-17A and IL-22 (35). Others include IL-27, which promotes c-Maf mediated inhibition of IL-17A and IL-22, and IL-25, which favors type 2 cytokine induction and consequently, IL-17A and IL-22 suppression. Overall, our findings suggest the addition of IL-33 to this list, as the absence of IL-1RL1/ST2 signaling results in heightened IL-17A and IL-22 production, whereas increasing IL-33 levels results in decreased IL-17A and IL-22 production, indicating that IL-33 signaling through IL-1RL1 serves as a negative regulator of IL-17A and IL-22.
Based on our previous work (24) (19), our hypothesis behind elevated IL-17A and IL-22 levels in the absence of IL-33 signaling was that IL-33 negatively regulated IL-23, which we have shown to drive IL-17A and IL-22 production in the lung (24) (19). However, this was found not to be the case, resulting in a search for an IL-23 independent mechanism of enhanced IL-17A and IL-22 production. A role for PGE2 in the generation of IL-17A and IL-22 has been controversial. It has been reported that PGE2 may enhance IL-17A production from previously polarized Th17 cells or memory Th17 cells (36) (37), however recent data shows that PGE2 has the complete opposite (i.e. negative) effect on Th17 generation from naïve T cells (38). In this latter study, the fungal pathogen C. neoformans was shown to produce its own PGE2, which negatively affected IL-17A production but had no effect on IL-22 (38). Other studies showed that anti-CD3 activation of human PBMCs in the presence of exogenous PGE2 promoted IL-17A production but significantly dampened IL-22 production (39). Human PBMCs stimulated with the fungus Candida albicans demonstrated increased IL-17A and IL-22 production that were slightly affected by the NSAID diclofenac (P < 0.05 for IL-17A, IL-22 was slightly lower but not statistically significant (40)). In this report, EP receptor agonists were moderately effective at restoring NSAID-inhibited IL-17A production, although the effects on IL-22 were not determined. However, as described earlier, a recent report demonstrated that the COX2/PGE2 axis functioned in the generation of innate IL-22 production in both humans and mice (32). Here, using an experimental model of LPS-induced systemic inflammation, this report demonstrated that PGE2-EP4 signaling promoted homeostasis of type 3 innate lymphoid cells (ILC3s) and drove them to produce IL-22. We found that similar to IL-17A and IL-22, PGE2 levels were elevated in Il1rl1−/− mice and treating mice with IL-33 resulted in the lowering of both IL-17A and IL-22 and PGE2 levels in the lung. Although IL-17A and IL-22 levels were reduced after IL-33 treatment (~ two-thirds and one-half, respectively), they were not completely eliminated. Moreover, IL-33 treatment had no effect on the levels of IL-1α, IL-1β and IL-6. Consequently, we did not observe an effect of IL-33 treatment on A. fumigatus lung clearance. Based on these results, we hypothesize that IL-33 treatment does not facilitate a level of immunosuppression that compromises fungal clearance. In contrast, when targeting PGE2 via COX-2 inhibition, IL-17A and IL-22 levels, as well as IL-1α, IL-1β and IL-6 levels, in Il1rl1−/− mice were significantly reduced, which correlated with reduced A. fumigatus lung clearance. An unexpected finding from our work was that neutralization of IL-17A and IL-22 in Il1rl1−/− mice did not impair A. fumigatus lung clearance. Based on observations with IL-33 treatment (reduced IL-17A and IL-22 levels, intact IL-1α, IL-1β and IL-6 levels and no effect on fungal clearance) and Celecoxib/Celebrex® treatment (reduced IL-17A, IL-22, IL-1α, IL-1β and IL-6 levels and a negative effect on fungal clearance), the mechanism of resistance in Il1rl1−/− mice appears to be quite complex. Specifically, intact (IL-33 treatment) or elevated (Il1rl1−/− mice) IL-1α, IL-1β and/or IL-6 may compensate in some manner when IL-17A and IL-22 levels are manipulated. It would be of interest in future studies to cross Il1rl1−/− mice with Il1r−/− mice, which would eliminate IL-1α and IL-1β signaling in tandem with IL-33 signaling and may result in mice deficient in both IL-17A and IL-22 induction as well as IL-1α and IL-1β signaling.
When targeting PGE2 via COX-2 inhibition, IL-17A and IL-22 levels, as well as IL-1α, IL-1β and IL-6 levels, in Il1rl1−/− mice were significantly reduced, further suggesting that COX-2 mediated PGE2 contributed in some way to the heighted IL-17A and IL-22 production observed in these mice. To confirm that PGE2 had the specific ability to induce IL-17A and IL-22, addition of a PGE2 agonist to lung cells from A. fumigatus exposed mice increased IL-6, IL-17A and IL-22 production, but not IL-1α, IL-1β. Conversely, specific inhibition of PGE2 had a negative effect on IL-17A and IL-22 production from lung cells, but not IL-1α, IL-1β and IL-6 production. The novelty in our findings is not only that the COX-2/PGE2 axis drives innate IL-17A and IL-22 production in a mucosal tissue (i.e. the lung), suggesting a conserved mechanism of IL-17A and IL-22 induction, but also identifies putative upstream regulators of the PGE2-IL-17A-IL-22 axis, i.e. IL-33 signaling. A question arising from our findings is how does IL-33 signaling regulate PGE2 induction and thus control the level of inflammatory cytokines during fungal exposure. IL-1 signaling was a prime candidate responsible for increased PGE2 in Il1rl1−/− mice, as IL-1α and IL-1β were elevated in these mice after fungal exposure and IL-1 signaling is known to induce PGE2 (41) (42). However, despite a profound reduction in IL-17A and IL-22 levels, IL-1 receptor deficiency resulted in only a moderate reduction in PGE2 production. Moreover, treating mice with IL-33 did not attenuate the levels of IL-1α and IL-1β despite significantly lower PGE2 levels. Collectively, these results suggest that other IL-1 independent factors were responsible for elevated PGE2 levels in Il1rl1−/− mice. To this end, we assessed additional factors that were increased in A. fumigatus exposed Il1rl1−/− mice, yet were decreased in mice that were treated with IL-33. This analysis identified the cytokines IL-3, IL-17A and IFN-γ. Although published reports suggest that each of these may induce PGE2 (43) (44) (45) (46) (47), neutralization of IL-3, IL-17A or IFN-γ in lung cell cultures from Il1rl1−/− mice did not result in attenuated PGE2 or IL-22 production (Supplemental Figure 4). Based on these observations, we hypothesize that a soluble factor such as a cytokine is an unlikely candidate for heightened PGE2 production in the absence of IL-33 signaling. IL1RL1 (and thus IL-33) has been shown to regulate TLR2 responses, as macrophages from Il1rl1−/− mice show hyperresponsiveness when stimulated with bacterial lipoprotein (48). As we have previously reported a small role for TLR2 in alveolar macrophages responses to A. fumigatus (49), it is possible that enhanced TLR2 signaling in Il1rl1−/− mice contribute to higher PGE2 and IL-17A and IL-22 induction in some manner. Indeed, fungal recognition via TLR2 can drive PGE2 production (50). To this end, we cannot exclude the possibility that IL-33 may affect the expression or function of other PRRs, namely the beta-glucan receptor Dectin-1. Another study has shown that macrophages from mice deficient in Atf3, an ATF/CREB basic leucine zipper (bZip) transcription factor induced during cellular stress have enhanced COX-2 signaling and PGE2 production after stimulation with zymosan (51). Ongoing studies are interrogating the role of IL-33 in Atf3 induction as well as its effect on Dectin-1.
In conclusion, this is the first report identifying a novel role for IL-33 in the regulation of IL-17A and IL-22 production in the lung after fungal exposure. The absence of IL-33 signaling resulted in enhanced IL-17A and IL-22 production in the presence of enhanced PGE2 levels, whereas the opposite was true in mice administered IL-33. Inhibition of COX-2 signaling in vivo resulted in lower IL-17A and IL-22 induction and impaired fungal clearance. A specific agonist of PGE2 receptors augmented, while specific inhibition of PGE2 generation impaired, IL-17A and IL-22 production. Overall, the current body of work adds depth to our understanding of the role specific IL-1 family members play in IL-17A and IL-22 regulation and innate lung defense. Moreover, these findings lay the foundation for determining whether IL-33 signaling affects PGE2 and/or IL-17A and IL-22 induction in other IL-17A/IL-22 dependent models of inflammation, such as colitis and psoriasis, or infection models requiring IL-17A/IL-22 for defense, such as Gram-negative bacterial pneumonia and influenza.
Supplementary Material
Acknowledgments
This work was supported was supported by Public Health Service grants HL096702, HL122426 and HL136211.
References
- 1.Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. Science. 2012;336:647. doi: 10.1126/science.1222236. [DOI] [PubMed] [Google Scholar]
- 2.Tsitsikas DA, Morin A, Araf S, Murtagh B, Johnson G, Vinnicombe S, Ellis S, Suaris T, Wilks M, Doffman S, Agrawal SG. Impact of the revised (2008) EORTC/MSG definitions for invasive fungal disease on the rates of diagnosis of invasive aspergillosis. Med Mycol. 2012;50:538–542. doi: 10.3109/13693786.2011.630040. [DOI] [PubMed] [Google Scholar]
- 3.Liss B, Vehreschild JJ, Bangard C, Maintz D, Frank K, Gronke S, Michels G, Hamprecht A, Wisplinghoff H, Markiefka B, Hekmat K, Vehreschild MJ, Cornely OA. Our 2015 approach to invasive pulmonary aspergillosis. Mycoses. 2015;58:375–382. doi: 10.1111/myc.12319. [DOI] [PubMed] [Google Scholar]
- 4.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 5.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 6.Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A, Fairs A, Greenberger PA, Kariuki B, Kita H, Kurup VP, Moss RB, Niven RM, Pashley CH, Slavin RG, Vijay HM, Wardlaw AJ. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. 2012;129:280–291. doi: 10.1016/j.jaci.2011.12.970. [DOI] [PubMed] [Google Scholar]
- 7.Burgel PR, Baixench MT, Amsellem M, Audureau E, Chapron J, Kanaan R, Honore I, Dupouy-Camet J, Dusser D, Klaassen CH, Meis JF, Hubert D, Paugam A. High prevalence of azole-resistant Aspergillus fumigatus in adults with cystic fibrosis exposed to itraconazole. Antimicrob Agents Chemother. 2012;56:869–874. doi: 10.1128/AAC.05077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, Dinauer MC. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med. 1997;185:207–218. doi: 10.1084/jem.185.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duong M, Ouellet N, Simard M, Bergeron Y, Olivier M, Bergeron MG. Kinetic study of host defense and inflammatory response to Aspergillus fumigatus in steroid-induced immunosuppressed mice. J Infect Dis. 1998;178:1472–1482. doi: 10.1086/314425. [DOI] [PubMed] [Google Scholar]
- 10.Werner J, Metz AE, Horn D, Faro-Trindade I, Schoeb TR, Hewitt MM, Schwiebert LM, Brown GD, Steele C. Requisite role for the Dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caffrey AK, Lehmann MM, Zickovich JM, Espinosa V, Shepardson KM, Watschke CP, Hilmer KM, Thammahong A, Barker BM, Rivera A, Cramer RA, Obar JJ. IL-1alpha signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS Pathog. 2015;11:e1004625. doi: 10.1371/journal.ppat.1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gresnigt MS, Rosler B, Jacobs CW, Becker KL, Joosten LA, van der Meer JW, Netea MG, Dinarello CA, van de Veerdonk FL. The IL-36 receptor pathway regulates Aspergillus fumigatus-induced Th1 and Th17 responses. Eur. J Immunol. 2013;43:416–426. doi: 10.1002/eji.201242711. [DOI] [PubMed] [Google Scholar]
- 13.De LA, Smeekens SP, Casagrande A, Iannitti R, Conway KL, Gresnigt MS, Begun J, Plantinga TS, Joosten LA, van der Meer JW, Chamilos G, Netea MG, Xavier RJ, Dinarello CA, Romani L, van de Veerdonk FL. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc. Natl. Acad. Sci U. S. A. 2014;111:3526–3531. doi: 10.1073/pnas.1322831111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sainz J, Perez E, Gomez-Lopera S, Jurado M. IL1 gene cluster polymorphisms and its haplotypes may predict the risk to develop invasive pulmonary aspergillosis and modulate C-reactive protein level. J Clin Immunol. 2008;28:473–485. doi: 10.1007/s10875-008-9197-0. [DOI] [PubMed] [Google Scholar]
- 15.Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17:122–131. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 16.De la Fuente M, Macdonald TT, Hermoso MA. The IL-33/ST2 axis: Role in health and disease. Cytokine Growth Factor Rev. 2015;26:615–623. doi: 10.1016/j.cytogfr.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Rostan O, Arshad MI, Piquet-Pellorce C, Robert-Gangneux F, Gangneux JP, Samson M. Crucial and diverse role of the interleukin-33/ST2 axis in infectious diseases. Infect Immun. 2015;83:1738–1748. doi: 10.1128/IAI.02908-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramaprakash H, Shibata T, Duffy KE, Ismailoglu UB, Bredernitz RM, Moreira AP, Coelho AL, Das AM, Fursov N, Chupp GL, Hogaboam CM. Targeting ST2L potentiates CpG-mediated therapeutic effects in a chronic fungal asthma model. Am J Pathol. 2011;179:104–115. doi: 10.1016/j.ajpath.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, Chan YR, Ouyang W, Brown GD, Weaver CT, Steele C. Dectin-1-Dependent Interleukin-22 Contributes to Early Innate Lung Defense against Aspergillus fumigatus. Infect Immun. 2012;80:410–417. doi: 10.1128/IAI.05939-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemzek JA, Ebong SJ, Kim J, Bolgos GL, Remick DG. Keratinocyte growth factor pretreatment is associated with decreased macrophage inflammatory protein-2alpha concentrations and reduced neutrophil recruitment in acid aspiration lung injury. Shock. 2002;18:501–506. doi: 10.1097/00024382-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Mattila PE, Metz AE, Rapaka RR, Bauer LD, Steele C. Dectin-1 Fc targeting of Aspergillus fumigatus beta-glucans augments innate defense against invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2008;52 doi: 10.1128/AAC.01274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowman JC, Abruzzo GK, Anderson JW, Flattery AM, Gill CJ, Pikounis VB, Schmatz DM, Liberator PA, Douglas CM. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob Agents Chemother. 2001;45:3474–3481. doi: 10.1128/AAC.45.12.3474-3481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hummel M, Spiess B, Roder J, von KG, Durken M, Kentouche K, Laws HJ, Morz H, Hehlmann R, Buchheidt D. Detection of Aspergillus DNA by a nested PCR assay is able to improve the diagnosis of invasive aspergillosis in paediatric patients. J Med Microbiol. 2009;58:1291–1297. doi: 10.1099/jmm.0.007393-0. [DOI] [PubMed] [Google Scholar]
- 24.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, Brown GD, Steele C. Neutrophils produce IL-17A in a Dectin-1 and IL-23 dependent manner during invasive fungal infection. Infect Immun. 2011;79:3966–3977. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flaczyk A, Duerr CU, Shourian M, Lafferty EI, Fritz JH, Qureshi ST. IL-33 signaling regulates innate and adaptive immunity to Cryptococcus neoformans. J Immunol. 2013;191:2503–2513. doi: 10.4049/jimmunol.1300426. [DOI] [PubMed] [Google Scholar]
- 26.Le HT, Tran VG, Kim W, Kim J, Cho HR, Kwon B. IL-33 priming regulates multiple steps of the neutrophil-mediated anti-Candida albicans response by modulating TLR and dectin-1 signals. J Immunol. 2012;189:287–295. doi: 10.4049/jimmunol.1103564. [DOI] [PubMed] [Google Scholar]
- 27.Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982;69:617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatia S, Fei M, Yarlagadda M, Qi Z, Akira S, Saijo S, Iwakura Y, van RN, Gibson GA, St Croix CM, Ray A, Ray P. Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype. PLoS One. 2011;6:e15943. doi: 10.1371/journal.pone.0015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lilly LM, Gessner MA, Dunaway CW, Metz AE, Schwiebert L, Weaver CT, Brown GD, Steele C. The beta-Glucan Receptor Dectin-1 Promotes Lung Immunopathology during Fungal Allergy via IL-22. J Immunol. 2012;189:3653–3660. doi: 10.4049/jimmunol.1201797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, Rosenfeld J, Leiner I, Chen CC, Ron Y, Hohl TM, Rivera A. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog. 2014;10:e1003940. doi: 10.1371/journal.ppat.1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lilly LM, Scopel M, Nelson MP, Burg AR, Dunaway CW, Steele C. Eosinophil deficiency compromises lung defense against Aspergillus fumigatus. Infect. Immun. 2014;82:1315–1325. doi: 10.1128/IAI.01172-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffin R, O’Connor RA, Crittenden S, Forster T, Yu C, Zheng X, Smyth D, Robb CT, Rossi F, Skouras C, Tang S, Richards J, Pellicoro A, Weller RB, Breyer RM, Mole DJ, Iredale JP, Anderton SM, Narumiya S, Maizels RM, Ghazal P, Howie SE, Rossi AG, Yao C. Prostaglandin E(2) constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science. 2016;351:1333–1338. doi: 10.1126/science.aad9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catley MC, Chivers JE, Cambridge LM, Holden N, Slater DM, Staples KJ, Bergmann MW, Loser P, Barnes PJ, Newton R. IL-1beta-dependent activation of NF-kappaB mediates PGE2 release via the expression of cyclooxygenase-2 and microsomal prostaglandin E synthase. FEBS Lett. 2003;547:75–79. doi: 10.1016/s0014-5793(03)00672-0. [DOI] [PubMed] [Google Scholar]
- 34.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CM, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015;33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 37.Napolitani G, Acosta-Rodriguez EV, Lanzavecchia A, Sallusto F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-gamma production by memory CD4+ T cells. Eur. J Immunol. 2009;39:1301–1312. doi: 10.1002/eji.200838969. [DOI] [PubMed] [Google Scholar]
- 38.Valdez PA, Vithayathil PJ, Janelsins BM, Shaffer AL, Williamson PR, Datta SK. Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells. Immunity. 2012;36:668–679. doi: 10.1016/j.immuni.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chizzolini C, Chicheportiche R, Alvarez M, de RC, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smeekens SP, van de Veerdonk FL, van der Meer JW, Kullberg BJ, Joosten LA, Netea MG. The Candida Th17 response is dependent on mannan- and beta-glucan-induced prostaglandin E2. Int. Immunol. 2010;22:889–895. doi: 10.1093/intimm/dxq442. [DOI] [PubMed] [Google Scholar]
- 41.Endo Y, Blinova K, Romantseva T, Golding H, Zaitseva M. Differences in PGE2 production between primary human monocytes and differentiated macrophages: role of IL-1beta and TRIF/IRF3. PLoS One. 2014;9:e98517. doi: 10.1371/journal.pone.0098517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanabe N, Maeno M, Suzuki N, Fujisaki K, Tanaka H, Ogiso B, Ito K. IL-1 alpha stimulates the formation of osteoclast-like cells by increasing M-CSF and PGE2 production and decreasing OPG production by osteoblasts. Life Sci. 2005;77:615–626. doi: 10.1016/j.lfs.2004.10.079. [DOI] [PubMed] [Google Scholar]
- 43.Kuroda E, Noguchi J, Doi T, Uematsu S, Akira S, Yamashita U. IL-3 is an important differentiation factor for the development of prostaglandin E2-producing macrophages between C57BL/6 and BALB/c mice. Eur J Immunol. 2007;37:2185–2195. doi: 10.1002/eji.200737041. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Liu L, Zhang Q, Liu S, Ge D, You Z. Interleukin-17 Indirectly Promotes M2 Macrophage Differentiation through Stimulation of COX-2/PGE2 Pathway in the Cancer Cells. Cancer Res Treat. 2014;46:297–306. doi: 10.4143/crt.2014.46.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li JK, Nie L, Zhao YP, Zhang YQ, Wang X, Wand SS, Liu Y, Zhao H, Cheng L. IL-17 mediates inflammatory reactions via p38/c-Fos and JNK/c-Jun activation in an AP-1-dependent manner in human nucleus pulposus cells. J Transl Med. 2016;14:77. doi: 10.1186/s12967-016-0833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vila-Del SV, Fresno M. Involvement of TNF and NF-kappa B in the transcriptional control of cyclooxygenase-2 expression by IFN-gamma in macrophages. J Immunol. 2005;174:2825–2833. doi: 10.4049/jimmunol.174.5.2825. [DOI] [PubMed] [Google Scholar]
- 47.Hegyi B, Kudlik G, Monostori E, Uher F. Activated T-cells and pro-inflammatory cytokines differentially regulate prostaglandin E2 secretion by mesenchymal stem cells. Biochem Biophys Res Commun. 2012;419:215–220. doi: 10.1016/j.bbrc.2012.01.150. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Buckley JM, Redmond HP, Wang JH. ST2 negatively regulates TLR2 signaling, but is not required for bacterial lipoprotein-induced tolerance. J Immunol. 2010;184:5802–5808. doi: 10.4049/jimmunol.0904127. [DOI] [PubMed] [Google Scholar]
- 49.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villamon E, Roig P, Gil ML, Gozalbo D. Toll-like receptor 2 mediates prostaglandin E(2) production in murine peritoneal macrophages and splenocytes in response to Candida albicans. Res Microbiol. 2005;156:115–118. doi: 10.1016/j.resmic.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Hellmann J, Tang Y, Zhang MJ, Hai T, Bhatnagar A, Srivastava S, Spite M. Atf3 negatively regulates Ptgs2/Cox2 expression during acute inflammation. Prostaglandins Other Lipid Mediat. 2015;116–117:49–56. doi: 10.1016/j.prostaglandins.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.