Abstract
An accurate, rapid and selective method was developed to quantify cyclocreatine in mouse and rat plasma using hydrophilic interaction (HILIC) ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). The plasma samples were prepared by protein precipitation with acetonitrile:methanol (70:30). Chromatographic separation was performed on a HILIC BEH amide column (2.1 mm × 50 mm, 1.7 μm) with a 3 min gradient elution at a flow rate of 0.5 mL/min. For mass spectrometric detection, selected reaction monitoring (SRM) was used; the SRM transitions were m/z 144 → 98 and m/z 144 → 56 for cyclocreatine and m/z 148 → 102 for the internal standard (D4-cyclocreatine) in the positive ionization mode. No endogenous components interfered with the analysis of cyclocreatine and the internal standard in mouse and rat plasma. Plasma calibration curves were constructed in the range of 0.01–25 μM. The correlation coefficient of the calibration curves was greater than 0.99. The mean intraday assay accuracy for all quality control (QC) replicates was between 93 and 105%. The mean intraday assay precision (CV%) was 1.9–11% for all QC levels. The HILIC–UPLC-MS/MS method was successfully applied in pharmacokinetic (PK) studies of cyclocreatine in mice and rats for the first time. After a single 30 mg/kg oral administration in mice and rats, the AUC0-∞ (area under the curve) was 84.1 μg·hr/mL and 91.7±18.0 μg·hr/mL, respectively.
1. Introduction
Creatine transporter deficiency (CTD) is an X-linked inherited metabolic disorder characterized by cerebral creatine deficiency and affects about 1% of males with non-syndromic mental disability [1]. CTD symptoms include intellectual, language and speech impairment, seizures, and movement and behavioral disturbances. Cyclocreatine (1-Carboxymethyl-2-iminoimidazolidine), an analog of creatine, is under investigation as a potential drug candidate [2] for the treatment of CTD. Previously, cyclocreatine was given to humans as a chemotherapeutic adjunct under an investigational new drug Phase I safety study [3].
Cyclocreatine is a polar molecule with a molecular weight of 143 Da. Its polarity and low molecular weight create challenges in developing of quantitative bioanalytical assay for pharmacokinetic (PK) studies. To date, only one bioanalytical method was published for cyclocreatine quantitation [4]. In that method, a cation-exchange column was used to assay cyclocreatine and its analogues in mammalian skeletal muscles using an UV detector. However, that method is not compatible with mass spectrometry which is widely used in bioanalysis due to high selectivity and sensitivity. Several analytical methods for creatine have been described, including the use of ion-pair reversed-phase liquid chromatography [5], capillary electrophoresis [6], isotope dilution liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry [7], and reverse phase LC-MS/MS [8]. However, the reverse phase LC-MS/MS method [8] had poor chromatographic retention and a derivatization method needed to be employed during sample preparation [9] to retain the hydrophilic analytes.
Hydrophilic interaction chromatography (HILIC) is a valuable alternative to reverse phase liquid chromatography (RPLC) for the analysis of highly polar and hydrophilic compounds and its separation mechanism is quite different from that of RPLC [10]. HILIC enables the use of both highly hydrophilic stationary phases to retain polar analytes and high organic content mobile phases with volatile buffers. The latter makes HILIC readily compatible with mass spectrometry. By providing good chromatographic retention for polar compounds, HILIC reduces the potential matrix interferences in bioanalysis. HILIC methods do not need the derivatization steps for polar compounds and thus reduce sample preparation time. Hydrophilic interaction chromatography and tandem mass spectrometry (HILIC-MS/MS) was used in analysis of creatine, purine and pyrimidine derivatives in cow’s urine samples to achieve good retention and better separation [11]. However, high throughput quantification of cyclocreatine in plasma using UPLC-MS/MS for pharmacokinetic studies has not yet been reported. Therefore, a robust HILIC–UPLC MS/MS assay for the determination of cyclocreatine in plasma for drug metabolism and PK studies was developed and is described here.
2. Experimental
2.1. Chemicals and reagents
LC-MS grade acetonitrile (ACN) and methanol (MeOH) were purchased from Fisher Scientific (Pittsburgh, PA). Deionized water was purified by a Milli-Q Ultrapure Water Purification System from EMD Millipore Corporation (Billerica, MA). Drug-free control blank mouse and rat plasma (K2EDTA) was obtained from Bioreclamation IVT (Westbury, NY). Cyclocreatine (Fig. 1), ammonium acetate and formic acid were purchased from Sigma Aldrich (St. Louis, MO). Stable labeled internal standard (IS), D4-cyclocreatine [2-(2-imino-4,4,5,5-D4-imidazolidin-1-yl)acetic acid], was synthesized in-house by a chemist at the National Center for Advancing Translational Sciences (NCATS, Rockville, MD).
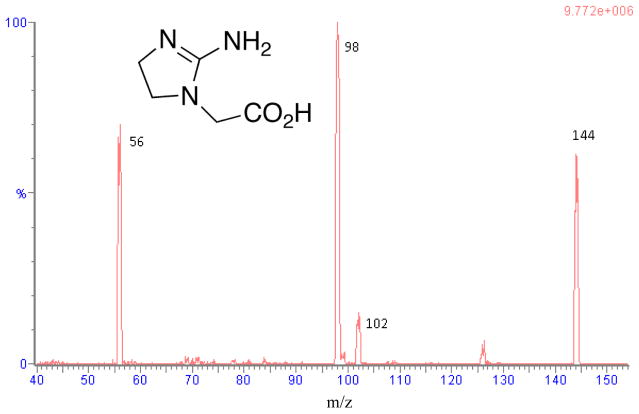
Figure 1.
Product ion spectrum of cyclocreatine
2.2. Preparation of calibration standards and quality control samples
Stock solutions of cyclocreatine and D4-cyclocreatine (IS) at 100 mM were prepared by dissolving each compound in water and stored at −20°C. Calibration standards were prepared in drug-free blank mouse and rat plasma over a range of 0.01 – 25 μM. The quality controls (QC) were prepared in blank mouse and rat plasma at 0.025, 0.1, 1.0 and 25 μM.
2.3. Plasma sample extraction
Calibration standards were freshly prepared on the day of sample analysis in control blank mouse or rat plasma. All frozen PK study samples and quality control samples were thawed at room temperature prior to analysis. Once thawed, samples were thoroughly vortexed. In a 2-mL 96-well plate, a 200 μL aliquot of internal standard working solution (2.0 μM in ACN/MeOH:7/3) was added to each well, then a 10 μL aliquot of standards, QCs and PK study samples was added. The 2-mL 96-well plate was capped, vortexed and centrifuged at 3000 rpm for 30 min at 4°C. 150 μL of supernatant was transferred to a 350-μL 96-well plate using a TECAN Freedom Evo 200 robotic system (Morrisville, NC) and 2 μL of supernatant was injected for HILIC-UPLC-MS/MS analysis. Samples were kept in the autosampler at 10°C during sample analysis.
2.4. HILIC-UPLC–MS/MS conditions
HILIC-UPLC separation was carried out using a Waters Acquity I-class system (Waters Corp., Milford, MA). The HILIC column was a Waters Acquity BEH amide (1.7 μm, 2.1 × 50 mm) and was maintained at 60°C. The mobile phases were 10 mM ammonium acetate in 5% ACN/H2O (v/v) (A) and 10 mM ammonium acetate in 95% ACN/H2O (v/v) (B). The flow rate was 0.5 mL/min. The optimal UPLC elution gradient was: 0–0.2 min 99% B; 0.2–3 min 99 → 50% B; hold at 30%B for 0.5 min and 3.5–4.0 min 99% B.
A Waters Xevo TQ-S triple quadrupole mass spectrometer was operated in positive electrospray ionization (+ESI) mode for assay development. Mass spectrometric conditions were optimized through infusion of each compound at 5 μL/min. Under these conditions, cyclocreatine and the internal standard yielded predominantly protonated molecules at m/z 144 and m/z 148, respectively. Each of the precursor ions was subjected to collision-induced dissociation (CID) in order to generate product ions. The product ions of cyclocreatine at m/z 98 and 56, and internal standard at m/z 102 were chosen for the selected reaction monitoring (SRM) (Fig. 1). Experimental parameters were optimized as follows: 150 L/h cone gas, 550 L/h desolvation gas, 150°C source temperature, 550°C desolvation temperature, 30 V cone voltage and 1 kV of capillary voltage. The collision gas argon was pressurized at 5.5 × 10−5 Torr. The optimized collision energy (CE) for cyclocreatine was 17 and 21 V for m/z 144 → 98 and 144→ 56, respectively, and CE for D4-cyclocreatine was 18 V for m/z 148 → 102. The results were analyzed by 1/x2 weighted least-squares linear regression using TargetLynx (Waters Corp., Milford, MA).
2.5. Pharmacokinetic studies
Male C57BL/6 mice (~ 30 g, total 12 mice with n=4/sampling time point) and male Sprague-Dawley rats (~300 g, total 4 rats) were obtained from Charles River Laboratories (Wilmington, MA). Mice and rats were housed at the centralized animal facilities at the NIH (Bethesda, MD) with a 12 h light-dark cycle. The housing temperature and relative humidity were controlled at 22°C and 55%, respectively. The animals had free access to water and food. All experimental procedures were approved by the Animal Care and Use Committee (ACUC) of the NIH Division of Veterinary Resources (DVR). An oral dose (PO) of 30 mg/kg with a dosing volume of 10 mL/kg was administered via gavage. The formulation used was 20 mM citric buffer (pH~3). All dosing solutions were freshly prepared on the day of administration. The blood samples (~ 100 μL) were collected in K2EDTA tubes at 0.167, 0.5, 1, 2, 3, 5, 7 and 24 hr after drug administration, and plasma (~ 40 μL) was harvested after centrifugation at 3000 rpm for 10 min. All plasma samples were stored at −80°C until analysis.
The pharmacokinetic parameters were calculated using the non-compartmental approach (Model 200) of the pharmacokinetic software Phoenix WinNonlin, version 6.2 (Certara, St. Louis, MO). The area under the plasma concentration versus time curve (AUC) was calculated using the linear trapezoidal method. The slope of the apparent terminal phase was estimated by log linear regression using at least 3 data points and the terminal rate constant (λ) was derived from the slope. AUC0-∞ was estimated as the sum of the AUC0-t (where t is the time of the last measurable concentration) and Ct/λ. The apparent terminal half-life (t½) was calculated as 0.693/λ.
3. Results and discussions
3.1. Method development
A HILIC-UPLC-MS/MS method was developed and optimized with conditions suitable for cyclocreatine retention and MS/MS detection. Mobile phases with different volatile buffers were tested. A mobile phase with 10 mM ammonium acetate in 5% acetonitrile/water (eluent A) and 10 mM ammonium acetate in 95%acetonitrile/water (eluent B) showed the best overall cyclocreatine retention and peak shape. The use of sub-2-μm particles improved performance in LC separations. The 1.7 μm particles, rather than conventional 3.5 or 5-μm, enable faster mass transfer in a wider range of linear velocity, and hence greater efficiency. Short gradient times also benefited from low UPLC system void volume. The peak width in UPLC is typically in the range of 2 sec which provides high chromatographic resolution.
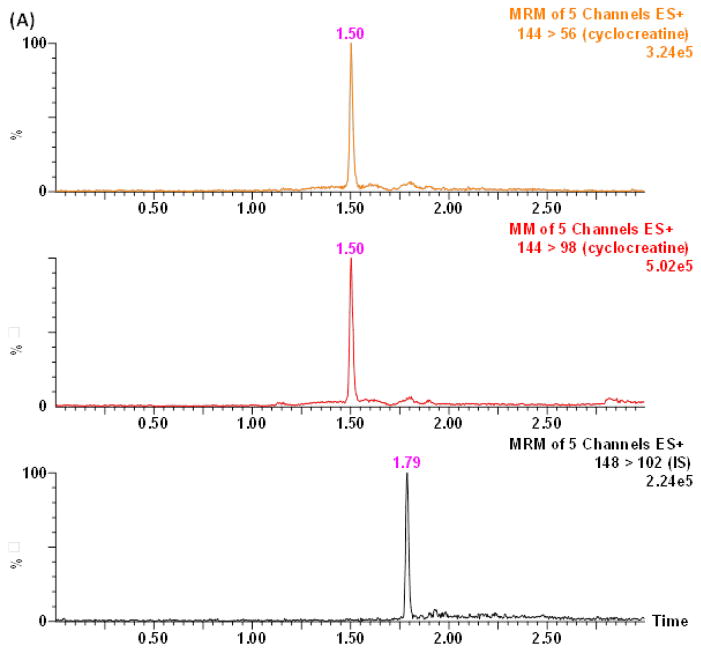
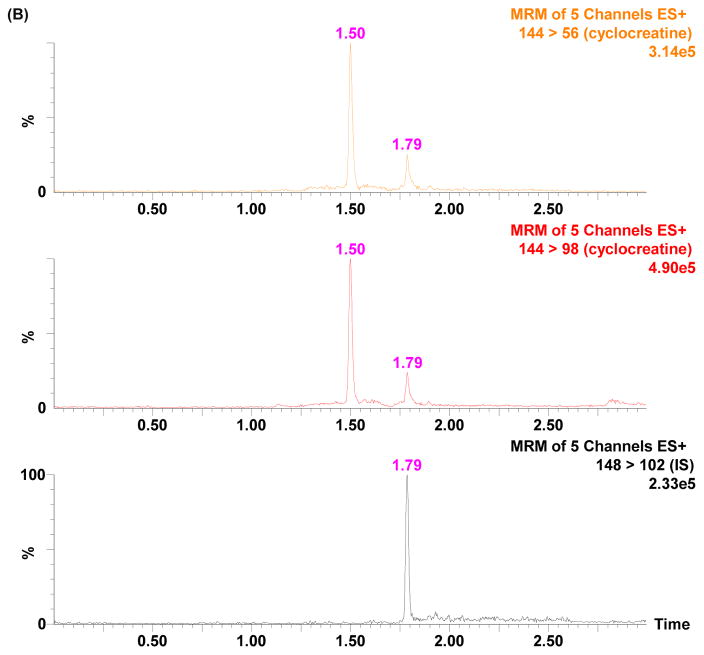
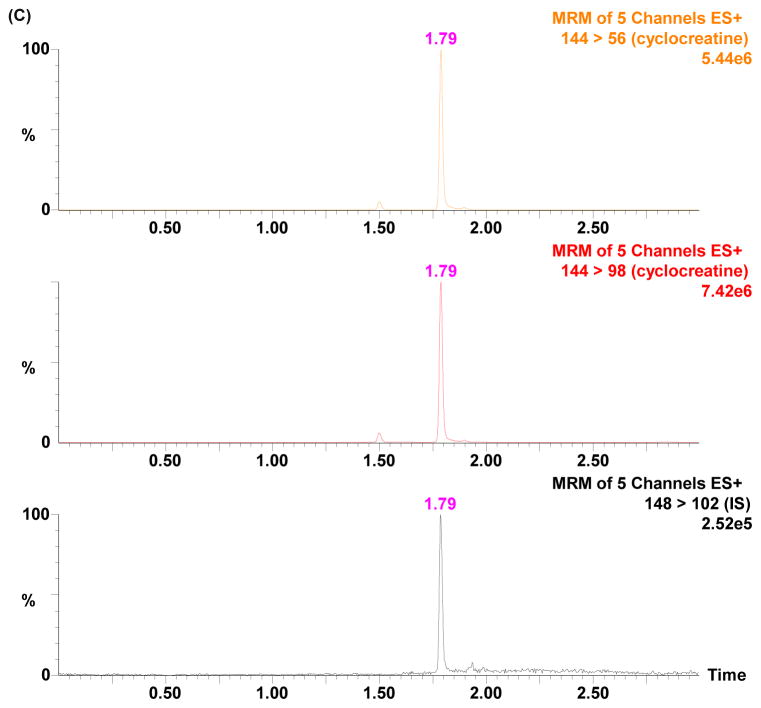
Tandem mass spectrometry (MS/MS) offers high selectivity and sensitivity. Mass spectrometry parameters were optimized to achieve selectivity and sensitivity in positive ion mode for cyclocreatine. The protonated molecular ions of cyclocreatine and D4-cyclocreatine were observed in the ESI-MS positive ionization mode. The cyclocreatine precursor ion was fragmented in the collision cell and yielded the most abundant product ion m/z 98 (Fig. 1). This allowed the use of selected reaction monitoring (SRM) for cyclocreatine and D4-cyclocreatine at m/z 144 → 98 and m/z 148 → 102, respectively, to increase the detection specificity and sensitivity. SRM of m/z 144 → 56 was monitored for cyclocreatine as the qualifying ion to verify the absence of endogenous interference. The retention time of cyclocreatine and D4-cyclocreatine was 1.79 min using the optimized HILIC-UPLC gradient elution method. The developed HILIC-UPLC-MS/MS method offers the advantages of improved chromatographic resolution, decreased analysis time, and reduced consumption of solvent. The analytical run time of the present method is 4 min. Representative chromatograms of rat plasma samples depict the internal standard only, the lower limit of quanti cation (LLOQ), and a PK sample in Figs. 2A–2C. Cyclocreatine is not present in the D4-cyclocreatine sample (Fig. 2A) at 1.79 min. The signal to noise ratio (S/N) was >10 at the LLOQ (0.01 μM) (Fig. 2B).
Figure 2.
Typical SRM chromatograms of cyclocreatine and IS (D4-cyclocreatine) in rat plasma: (A) blank plasma spiked with IS; (B) blank plasma spiked with 0.01 μM cyclocreatine (LLOQ) and IS; (C) a plasma sample obtained at 1 h after PO administration of 30 mg/kg of cyclocreatine
3.2. Method evaluation and performance
3.2.1 Selectivity and linearity
To investigate whether endogenous compounds from mouse and rat plasma could interfere with the detection of cyclocreatine or the internal standard, control drug-free mouse and rat K2EDTA plasma was prepared as double blank (containing neither analyte nor internal standard) and single blank (containing internal standard only) samples. Samples were processed and analyzed according to the above described procedures. In SRM chromatograms of double and single blank samples, no co-eluting peaks >20% of the analyte peak areas at the LLOQ were found and no co-eluting peak >5% of the internal standard were detected. These results indicated the absence of both endogenous interference and the crosstalk of internal standard in the cyclocreatine SRM transitions. An endogenous chromatographic peak at 1.50 min in rat plasma was separated from cyclocreatine using the optimized HILIC-UPLC conditions (Fig. 2B). The calibration range was 0.01 μM (LLOQ) to 25 μM (ULOQ, upper limit of quantitation) in plasma with a 2 μL injection. A linear regression with 1/x2 weighting was used to construct the calibration curve. The responses of cyclocreatine standards ranging from 0.01 to 25 μM in mouse and rat plasma were linear with a correlation coefficient greater than 0.99. Blank samples were injected in between high-concentration QC samples to monitor the carryover effect [12]. There was no observable carryover effect in this HILIC-UPLC-MS/MS method.
3.2.2 Precision and accuracy
Precision and accuracy of the assay were established by analyzing QC samples. Five replicates of each QC sample were measured in each analytical run. The CV was used to report the intraday (n=5) and interday (n=15) precision. The intraday and interday precision and accuracy of this method is shown in Tables 1 and 2. The intraday precision (CV%) was 1.9–11% for all QC concentration levels, while the accuracy was between 93 and 105%. The intrerday precision (CV%) was 1.3–5.1% for all QC concentration levels, while the accuracy was between 95 and 103%. These data suggested that the developed HILIC-UPLC-MS/MS method generated accurate and reproducible results.
Table 1.
Intraday (n=5) and interday (n=15, three runs) accuracy and precision results in mouse plasma
| QC Concentration (μM) | Intraday (n=5) | Interday (n=15, three runs) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Conc. | CV (%) | Accuracy (%) | Conc. | CV (%) | Accuracy (%) | |
| LLOQ (0.025) | 0.0259 | 11 | 104 | 0.0238 | 4.9 | 95 |
| LQC (0.100) | 0.100 | 2.3 | 100 | 0.0983 | 1.3 | 98 |
| MQC (1.00) | 1.05 | 1.9 | 105 | 1.02 | 1.9 | 102 |
| HQC (25.0) | 23.9 | 4.0 | 95 | 23.7 | 2.3 | 95 |
Table 2.
Intraday (n=5) and interday (n=15, three runs) accuracy and precision results in rat plasma
| QC Concentration (μM) | Intraday (n=5) | Interday (n=15, three runs) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Conc. | CV (%) | Accuracy (%) | Conc. | CV (%) | Accuracy (%) | |
| LLOQ (0.025) | 0.0255 | 6.5 | 102 | 0.0247 | 5.1 | 99 |
| LQC (0.100) | 0.0931 | 1.9 | 93 | 0.0989 | 1.7 | 99 |
| MQC (1.00) | 0.968 | 7.7 | 97 | 1.03 | 2.0 | 103 |
| HQC (25.0) | 23.3 | 3.0 | 93 | 24.1 | 2.8 | 96 |
3.3. Application to pharmacokinetic studies
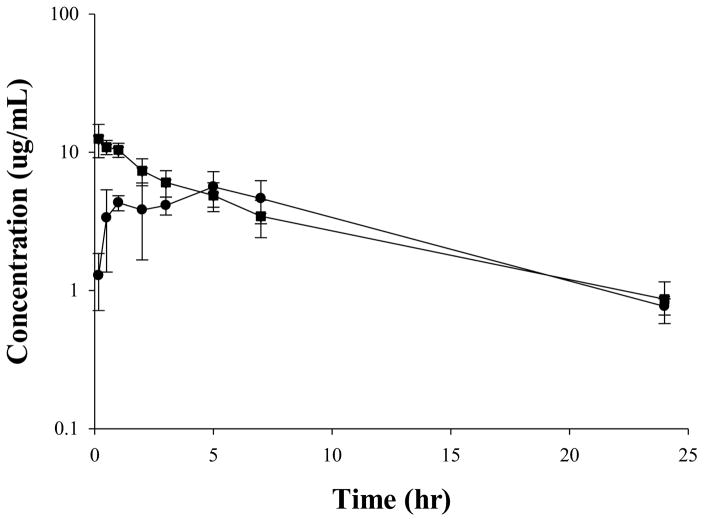
The developed HILIC UPLC–MS/MS method was applied to the pharmacokinetic studies of cyclocreatine in male C57BL/6 mice and male Sprague-Dawley (SD) rats. The plasma concentration–time profiles of cyclocreatine after 30 mg/kg PO in mice and rats are presented in Fig. 3 and the corresponding PK parameters are presented in Table 3.
Figure 3.
Mean plasma concentration–time profile of cyclocreatine after 30 mg/kg PO in male C57 mice (n=12, 4 samples at each time point, ●) and male Sprague-Dawley rats (n=4, ■). Data are expressed as mean ± SD.
Table 3.
Average plasma pharmacokinetic parameters of cyclocreatine in male C57 mice (n=12) and male Sprague-Dawley (SD) rats (n=4) after oral administration (30 mg/kg).
| 30 mg/kg PO (C57 mice) | 30 mg/kg PO (SD rats) | |
|---|---|---|
| AUC0 → ∞ (μg h/mL) | 84.1 | 91.7 ± 18.0 |
| t(1/2) (h) | 6.6 | 7.9± 0.9 |
| Tmax (h) | 5 | 0.58±0.48 |
| Cmax (μg/mL) | 5.62 | 13.3±2.6 |
After a single 30 mg/kg PO administration in C57BL/6 mice, the mean Cmax value was 5.62 μg/mL and the AUC0-∞ value (calculated from the mean concentration of 4 samples at each time point) was 84.1 μg·hr/mL. The half-life (t1/2) was about 6.6 hr. After a single 30 mg/kg PO administration in SD rats, the mean Cmax value was 13.3±2.6 μg/mL and the mean AUC0-∞ value was 91.7±18.0 μg·hr/mL. The mean half-life (t1/2) was 7.9 ± 0.9 hr. At the same dose of 30 mg/kg PO, the in vivo exposure (Cmax and AUC) of cyclocreatine appeared to be higher in rats compared to that in mice.
4. Conclusion
The objective of the present study was to develop a reliable and fast UPLC–MS/MS method for an initial PK assessment of cyclocreatine in mice and rats. The optimized HILIC-UPLC-MS/MS method provided good chromatographic retention for cyclocreatine, separated cyclocreatine from the endogenous interference, and reduced the sample preparation and analysis time. Additional validations will be needed for toxicology and clinical studies. Nevertheless, the method evaluation demonstrated that the HILIC-UPLC-MS/MS method is rapid, simple, specific, precise and accurate for the quantification of cyclocreatine. This method was applied successfully to the PK studies of cyclocreatine in mice and rats. The PK results suggested that cyclocreatine was well absorbed after PO administrations and the half-life was 7–8 hr in rodents. These PK results will guide future safety studies of cyclocreatine as a potential therapeutics for the treatment of CTD.
Highlights.
Rapid, selective, accurate and reproducible HILIC UPLC–MS/MS method to quantify cyclocreatine was developed.
Method provided good retention of cyclocreatine with no interference from mouse and rat plasma.
The pharmacokinetic parameters of cyclocreatine after oral administration are reported.
Acknowledgments
We thank Elias Gonzalez and Eric Nimako (DVR/OD/NIH) for conducting the in-life portion of the PK studies.
This work was supported by the Intramural Research Program of the National Center for Advancing Translational Sciences, the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van de Kamp JM, Mancini GM, Salomons GS. X-linked creatine transporter deficiency: clinical aspects and pathophysiology. J Inherit Metab Dis. 2014;37:715–33. doi: 10.1007/s10545-014-9713-8. [DOI] [PubMed] [Google Scholar]
- 2.Kurosawa Y, Degrauw TJ, Lindquist DM, Blanco VM, Pyne-Geithman GJ, Daikoku T, et al. Cyclocreatine treatment improves cognition in mice with creatine transporter deficiency. J Clin Invest. 2012;122:2837–46. doi: 10.1172/JCI59373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teicher BA, Menon K, Northey D, Liu J, Kufe DW, Kaddurah-Daouk R. Cyclocreatine in cancer chemotherapy. Cancer Chemother Pharmacol. 1995;35:411–6. doi: 10.1007/s002800050255. [DOI] [PubMed] [Google Scholar]
- 4.Wiseman RW, Moerland TS, Chase PB, Stuppard R, Kushmerick MJ. High-performance liquid chromatographic assays for free and phosphorylated derivatives of the creatine analogues beta-guanidopropionic acid and 1-carboxy-methyl-2-iminoimidazolidine (cyclocreatine) Anal Biochem. 1992;204:383–9. doi: 10.1016/0003-2697(92)90255-6. [DOI] [PubMed] [Google Scholar]
- 5.Ally A, Park G. Rapid determination of creatine, phosphocreatine, purine bases and nucleotides (ATP, ADP, AMP, GTP, GDP) in heart biopsies by gradient ion-pair reversed-phase liquid chromatography. J Chromatogr. 1992;575:19–27. doi: 10.1016/0378-4347(92)80499-g. [DOI] [PubMed] [Google Scholar]
- 6.See HH, Schmidt-Marzinkowski J, Pormsila W, Morand R, Krahenbuhl S, Hauser PC. Determination of creatine and phosphocreatine in muscle biopsy samples by capillary electrophoresis with contactless conductivity detection. Anal Chim Acta. 2012;727:78–82. doi: 10.1016/j.aca.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Fernandez M, Rodriguez-Gonzalez P, Anon Alvarez ME, Rodriguez F, Menendez FV, Garcia Alonso JI. Simultaneous determination of creatinine and creatine in human serum by double-spike isotope dilution liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry (GC-MS) Anal Chem. 2015;87:3755–63. doi: 10.1021/acs.analchem.5b00769. [DOI] [PubMed] [Google Scholar]
- 8.Wang JM, Chu Y, Li W, Wang XY, Guo JH, Yan LL, et al. Simultaneous determination of creatine phosphate, creatine and 12 nucleotides in rat heart by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;958:96–101. doi: 10.1016/j.jchromb.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Boenzi S, Rizzo C, Di Ciommo VM, Martinelli D, Goffredo BM, la Marca G, et al. Simultaneous determination of creatine and guanidinoacetate in plasma by liquid chromatography-tandem mass spectrometry (LC-MS/MS) J Pharm Biomed Anal. 2011;56:792–8. doi: 10.1016/j.jpba.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Hao Z, Xiao B, Weng N. Impact of column temperature and mobile phase components on selectivity of hydrophilic interaction chromatography (HILIC) J Sep Sci. 2008;31:1449–64. doi: 10.1002/jssc.200700624. [DOI] [PubMed] [Google Scholar]
- 11.Boudra H, Doreau M, Noziere P, Pujos-Guillot E, Morgavi DP. Simultaneous analysis of the main markers of nitrogen status in dairy cow’s urine using hydrophilic interaction chromatography and tandem mass spectrometry detection. J Chromatogr A. 2012;1256:169–76. doi: 10.1016/j.chroma.2012.07.094. [DOI] [PubMed] [Google Scholar]
- 12.Zeng W, Musson DG, Fisher AL, Wang AQ. A new approach for evaluating carryover and its influence on quantitation in high-performance liquid chromatography and tandem mass spectrometry assay. Rapid Commun Mass Spectrom. 2006;20:635–40. doi: 10.1002/rcm.2353. [DOI] [PubMed] [Google Scholar]