Abstract
Sepsis is a leading cause of death in the U.S. but the mechanisms underlying sepsis-induced immune dysregulation remain poorly understood. 2B4 (CD244, SLAM4) is a cosignaling molecule expressed predominantly on NK cells and memory CD8+ T cells that has been shown to regulate T cell function in models of viral infection and autoimmunity. Here we show that 2B4 signaling mediates sepsis lymphocyte dysfunction and mortality. 2B4 expression is increased on CD4+ T cells in both septic animals and human patients at early time points. Importantly, genetic loss or pharmacologic inhibition of 2B4 each significantly increased survival in a murine cecal ligation and puncture (CLP) model. Further, CD4-specific conditional knockouts showed that 2B4 functions on CD4+ T cell populations in a cell-intrinsic manner and modulates both adaptive and innate immune responses during sepsis. Our results illuminate a novel role for 2B4 coinhibitory signaling on CD4+ T cells in mediating immune dysregulation.
Keywords: 2B4(CD244), CD4+ T cells, sepsis
Introduction
Sepsis is the leading cause of death among critically ill patients in the United States (1). Importantly, no approved therapeutics are available for sepsis once antibiotics and supportive therapy fail. Recently, studies assessing the immune phenotypes and functionality of septic patients have shed light on the immune dysregulation that occurs during sepsis, and its ability to result in profound and protracted immune suppression (2). Previous studies have identified a role for cell-surface inhibitory receptors PD-1 and BTLA in controlling macrophage activation and antigen presenting cell function following sepsis (3, 4). However, the mechanisms underlying sepsis-induced immune suppression remain poorly understood.
It is well known that CD4+ T cells are heavily impacted during sepsis, in that both human septic patients and experimental mouse models of sepsis demonstrate profound CD4+ T cell apoptosis and loss of functionality (2, 5). Importantly, preventing CD4+ T cell apoptosis has been shown to reverse sepsis-induced mortality in mouse models. Here, we find that the coinhibitory molecule 2B4 is significantly upregulated on CD4+ T cells in both human septic patients and in an experimental model of mouse CLP. 2B4 (CD244, SLAMf4) is a member of the CD2 subset of immunoglobulin superfamily molecules. Previously best known for its role on NK cells (6), more recent work has shown that in certain settings 2B4 can be inducibly expressed on CD4+ and CD8+ T cells and possesses coinhibitory function on these cell populations (7, 8). We therefore sought to interrogate the role of 2B4 in mediating immune dysregulation and mortality during sepsis.
Materials and Methods
Mice
C57BL/6, CD4−/−, CD8−/− mice were originally purchased from the Jackson Laboratory and maintained at Emory University and used at. 8–12 week old. To generate CD42B4−/− chimeric mice, CD45.1+ mice received 1200 cGy irradiation and 16x106 CD4−/− Thy1.2+ bone marrow cells mixed with 4 x106 2B4−/− Thy1.1+ bone marrow cells. Control chimeric mice received WT bone marrow cells instead of 2B4−/−. CD82B4−/− chimeric mice were generated by the same method.
CLP model
Sepsis was induced by cecal ligation puncture (2×25G) following the method of Baker et al (9). Septic animals received s.q. antibiotics (50 mg/kg ceftriaxone and 35 mg/kg metronidazole) at 0, 12, 24 and 36 h after surgery. For anti-2B4 treatment, animals received anti-2B4 mAb (clone: 2B4, 250μg IP/dose, BioXcell) on days 0, 2, 4 and 6 after CLP. For NK cell depletion, anti-asialoGM1 (100μg IP) was given one day before CLP.
Human Patients
Healthy donor and septic patient PBMCs were isolated under Emory University IRB protocol #00002503. Blood was collected from septic patients within the first 24 hours of meeting the consensus clinical definition of sepsis. Patients’ demographics are provided in supplemental table 1.
Flow cytometry and intracellular cytokine staining (ICCS)
Murine 2B4 expression was assessed using clone eBio244F4 (eBioscience). CountBright™ Beads (Thermo Fisher) were used to determine absolute cell numbers. For ICCS, splenocytes were incubated with 30 ng/mL PMA and 400 ng/mL ionomycin in the presence of GolgiStop for 4 hours at 37 °C. Apoptotic cells were determined by Caspase3/7 staining (ThermoFisher).
Statistics
Student’s t-test, Mann-Whitney test, and log-rank test were used. Data presented are mean values ± SEM.
Results and Discussion
2B4 is upregulated on T cells but not NK cells following sepsis
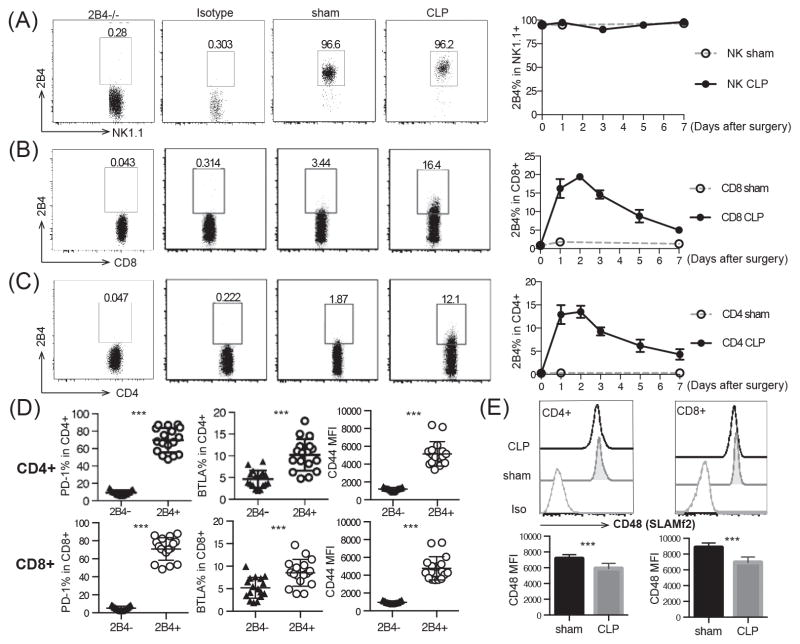
To interrogate the mechanisms underlying immune dysregulation during sepsis, WT mice were subjected to polymicrobial sepsis via CLP and expression of 2B4 was assessed by flow cytometry. As negative controls, 2B4−/− splenocytes and isotype controls were used. 2B4 expression was confirmed by staining with three distinct anti-2B4 clones (eBio244F4, m2B4(B6)458.1 and 2B4) and all yielded similar results (data not shown). Results indicated that NK cells isolated from both the sham surgery and CLP groups expressed high levels of 2B4 and maintained 2B4 expression over 7 days post surgery (Fig. 1A). Intriguingly, 2B4 expression on both CD8+ and CD4+ T cells was increased at 24 hours post-CLP in septic animals. The elevated expression of 2B4 on T cells was maintained for 3 days post-CLP and declined by day 4 (Fig. 1B, 1C). PD-1 and BTLA were both significantly higher on 2B4+ T cells, potentially indicative of an exhausted phenotype (Fig. 1D). In the murine CLP model, PD-1 and CTLA-4 begin to be upregulated on CD4+ and CD8+ T cells 48–96 hours post-CLP and steadily elevate until day 7 (10, 11). Contrastingly, our study found that 2B4 is expressed with distinct kinetics from PD-1 or CTLA-4, suggesting that 2B4 might have a unique and non-redundant coinhibitory function relative to PD-1 or CTLA-4, acting as an earlier inhibitory molecule during T cell activation.
Figure 1. 2B4 expression is upregulated on T cells following CLP.
WT mice underwent CLP or sham surgery and were sacrificed at the indicated time points. Splenocytes were harvested and stained for NK cells (A), CD8+ T cells (B) and CD4+ T cells (C). Isotype control and 2B4−/− animals were used to gate on 2B4+ cells. (D) PD-1, BTLA and CD44 expression was assessed at 24 h. (E) SLAMf2 expression was assessed at 24h..
2B4 up-regulation was mainly observed on CD44hi memory T cells during sepsis (Fig. 1D), which is consistent with previous findings and also suggests that 2B4 functions as a co-signaling receptor on memory cell populations. Analysis of expression of other SLAM family members revealed that although the MFI of SLAMf2 (CD48, the ligand of 2B4) decreased significantly on both CD4+ and CD8+ T cells following sepsis, SLAMf2 remained highly expressed on all T cells (Figure 1E), thus confirming ligand availability for 2B4-signaling on T cells during sepsis. Furthermore, SLAMf1, SLAMf3 and SLAMf6 failed to be upregulated on T cells following CLP (data not shown), a finding which further emphasizes the importance of 2B4 co-signaling in the regulation of T cell responses during sepsis. Congruent with previous findings that memory cell populations are more susceptible to sepsis-induced dysfunction (12), our data indicated that 2B4 expression on memory T cell populations might produce inhibitory signaling and lead to sepsis induced dysfunction.
2B4−/− animals display increased survival and effector T cells after sepsis
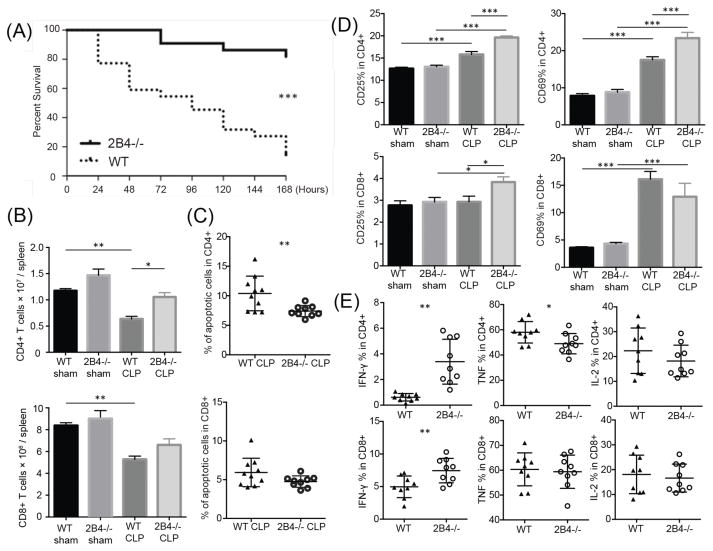
Given the above results, we sought to determine the effect of disrupting 2B4 signaling during sepsis. Strikingly, 2B4−/− mice were significantly protected from death during sepsis following CLP as compared to WT controls (82% survival compared to 13%, Fig. 2A). While no difference was found in the bacterial load in either peritoneal fluid or blood at 24 h post CLP, 2B4−/− mice possessed increased numbers of CD4+ T cells at 24 h relative to WT controls (Supplemental Fig. 1a, Fig. 2B). Analysis of caspase 3/7 activity in CD4+ T cells revealed that 2B4−/− CD4+ populations contained a lower frequency of apoptotic cells (Fig. 2C) and increased numbers of live cells relative to WT CD4+ cells (Supplemental Fig. 1b, 1c). To further determine the functionality of T cells in 2B4−/− vs. WT mice, surface markers and intracellular cytokines were assessed. 2B4−/− and WT animals exhibited no differences with regard to CD25 and CD69 expression in either the CD4+ or CD8+ T cell compartments prior to CLP. In contrast, CD25 was significantly upregulated in both the CD4+ and CD8+ T cell compartments in 2B4−/− CLP mice relative to WT CLP animals. Furthermore, CD69 was upregulated in 2B4−/− CLP mice relative to WT CLP animals in the CD4+ but not CD8+ T cell compartment (Fig. 2D). After CLP, increased secretion of IFN-γ was also observed in 2B4−/− CD4+ and CD8+ T cell populations relative to WT controls (Fig. 2E). Taken together, our data indicate that 2B4−/− CD4+ T cells exhibit a more activated phenotype and have higher functionality during sepsis, a finding that could underlie the increased survival observed in 2B4-deficient mice.
Figure 2. 2B4-deficient mice are protected from CLP and exhibit increased effector T cell functions during sepsis.
(A) CLP was performed on WT and 2B4-deficient mice and animals were monitored for survival for 7 days. (B) Splenocytes were harvested at 24 hours post-surgery and lymphocyte counts were assessed by flow cytometry. (C) Cell apoptosis was measured by Caspase 3/7+SYTOX− in both groups. (D) Frequencies of CD25+ and CD69+ CD4+ and CD8+ T cells in WT mice and 2B4-deficient mice are shown. (E) For ICCS, splenocytes were stimulated in PMA and ionomycin for 4 hours and cells were stained with IFN-γ, TNF and IL-2.
CD4+ -specific deletion of 2B4 enhances sepsis survival
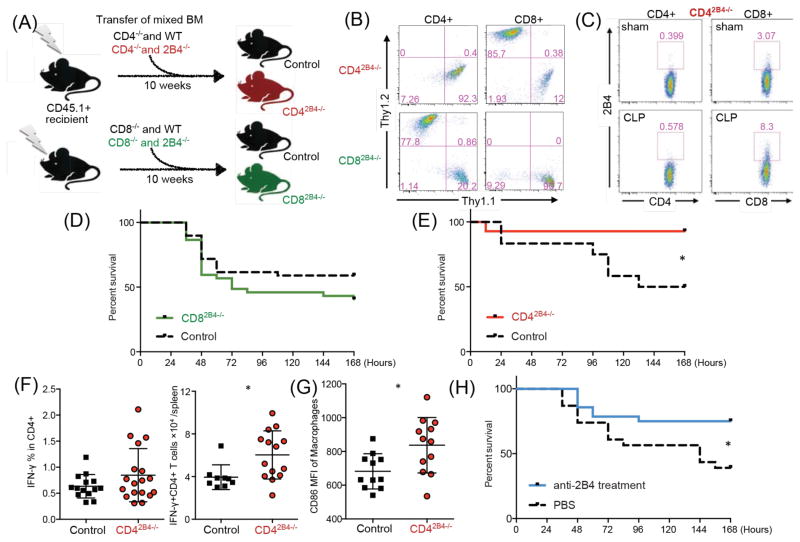
To elucidate which immune compartment contributes to the survival benefit obtained in 2B4−/− mice, we first targeted NK cells as they express high levels of 2B4. NK cells were depleted in 2B4−/− mice prior to CLP. No survival difference was observed between intact and NK depleted 2B4−/− mice (Supplemental Fig. 1d). This result indicated that NK cells likely did not contribute to the survival benefit in 2B4−/− animals. Next, to further dissect the function of 2B4 on different T cell compartments, we generated bone marrow (BM) chimeric conditional knockouts in which 2B4 is knocked out exclusively in either the CD4+ or CD8+ T cell compartment. Briefly, the CD4-specific 2B4 conditional knockouts were generated by a BM transplant of 80% CD4−/− Thy1.2+ (2B4-intact) BM mixed with 20% 2B4−/− Thy1.1+ BM (or 20% WT BM as control). After 10 weeks of reconstitution, all hematopoietic lineages except CD4+ T cells were reconstituted primarily from the CD4−/− WT BM. In contrast, CD4+ T cells were reconstituted from the 2B4−/− BM. The same procedure was done with CD8−/− BM, thus generating both CD4-and CD8-specific 2B4 conditional knockouts (CD42B4−/− and CD82B4−/−; Fig. 3A, 3B). There was no difference in the number of B or T cells between control chimera and CD42B4−/− chimera animals at baseline (Supplemental Fig. 1e), and we confirmed our chimera phenotype by subjecting CD42B4−/− chimeras to CLP and found that CD8+ but not CD4+ T cells upregulated 2B4 expression post-CLP (Figure 3C). While CD82B4−/− animals did not exhibit significantly altered survival relative to controls (Fig. 3D), CD42B4−/− chimeric mice exhibited significantly improved survival following CLP as compared to CD42B4+/+ control chimeras (Fig. 3E). Functional assays showed that CD42B4−/− mice exhibited increased numbers of IFN-γ secreting CD4+ T cells in splenocytes relative to controls (Fig. 3F), although they exhibited comparable bacterial loads (Supplemental Fig. 1f). To further investigate the functional effect of 2B4 specifically on CD4+ T cells on sepsis-induced immune dysregulation, we analyzed the innate immune compartments in CD42B4−/− mice and found that macrophages in these animals expressed elevated levels of CD86, suggesting increased activation of macrophages in the spleens of CD42B4−/− mice (Fig. 3G). Indeed, macrophage expression of CD86 has been correlated to reduced immune dysregulation and increased number of ICU-free days in human septic patients (13). In addition, reduced levels of CD86 mRNA were identified in lethal pediatric septic shock (14). Of note, increased activated IFN-γ-secreting macrophages did not correlate with higher serum TNF and IL-1β (Supplemental Fig. 1g). Taken together, our data strongly suggest that 2B4 signaling on CD4+ T cells results in distinct functionality relative to 2B4 signaling on CD8+ T cells, and significantly contributes to septic mortality.
Figure 3. Loss of 2B4 specifically on CD4+ T cells provides a survival benefit during sepsis, and blockade of 2B4 results in decreased mortality following CLP.
(A) Generation of CD42B4−/− and CD82B4−/− chimeric mice. (B) After 10 weeks of reconstitution, frequencies of CD4+ and CD8+ T cells assessed (gated on CD45.2+CD3+). (C) Representative 2B4 expression on T cells from CD42B4−/− chimeric mice after sham or CLP surgery. (D) Control chimeras and CD82B4−/− chimeras were subjected to CLP and monitored for survival. (E) Control chimera and CD42B4−/− chimeric animals were subjected to CLP and monitored for survival. (F) Chimeric splenocytes were harvested at 24 hours post-CLP and stimulated for ICCS. Frequencies and numbers of IFN-γ+ secreting CD4+ T cells are shown. (G) Splenic macrophages (CD11bhiCD11cloF4/80+) were assessed at 24 hours post-CLP for CD86 expression. (H) WT mice underwent CLP and were treated with either PBS or anti-2B4 mAb.
Blockade of 2B4 results in improved CLP survival
Next, in order to determine whether 2B4 could be pharmacologically targeted to improve mortality following sepsis, we blocked 2B4 signaling in septic animals with a monoclonal antibody to 2B4 (clone 2B4) given on days 0, 2, 4 and 6 post-CLP. We first confirmed that this clone results in 2B4 blockade and not depletion of 2B4-expressing cells in vivo by employing GFP+ 2B4-expressing retrogenic T cells transferred into WT animals (Supplemental Fig. 1h). Results showed significantly increased survival in the animals treated with anti-2B4 relative to PBS treated controls (Fig. 3H), suggesting that anti-2B4 might be a potential treatment during sepsis.
Septic patients exhibit increased 2B4 expression on CD4+ T cells
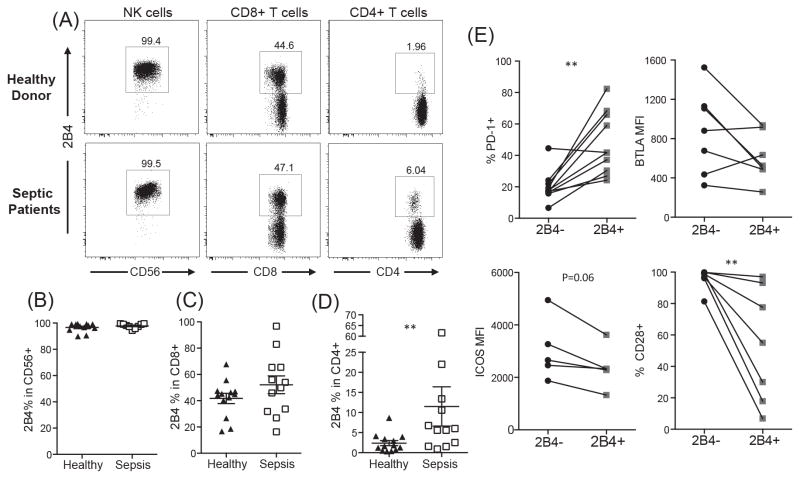
Finally, in order to interrogate the expression of 2B4 on distinct immune cell types in human septic patients, peripheral blood mononuclear cells (PBMCs) were collected from patients within the first 24 h of a diagnosis of clinically-defined sepsis (15). Our results showed no difference in 2B4 expression between septic patients and healthy donors within the NK cells and the CD8+ T cell compartment (Fig. 4A, Fig. 4B and 4C). However, while 2B4+ CD4+ T cells are rare in healthy human controls, we observed that 2B4 expression was significantly upregulated within the CD4+ T cell compartment of septic patients as compared to non-septic controls (Fig. 4D). Further immunophenotyping on human 2B4+ CD4+ T cells revealed that these cells possess an exhausted phenotype characterized by increased PD-1 expression and decreased expression of the co-stimulatory molecules ICOS and CD28 (Fig. 4E). Overall, these results combined with our mouse data highlight the critical role of 2B4 on CD4+ T cell populations during sepsis.
Figure 4. PBMC isolated from septic patients exhibit upregulation of 2B4 on CD4+ T cells.
PBMC isolated from septic patients and healthy donors were analyzed for 2B4 expression on CD4+ T cells, CD8+ T cells and NK cells. (A) Representative flow plots. (B–D) Summary data from n=13 healthy and n=12 septic patients per group. (E) Expression of PD-1, BTLA, ICOS, and CD28 on 2B4+ and 2B4− CD4+ T cells from septic patients.
The balance of costimulatory and coinhibitory molecules is critical in determining T cell function during infection. Here we show that increased 2B4 expression at early timepoints during sepsis can dampen T cell functionality. Importantly, blockade of 2B4 signaling improves sepsis mortality, thus identifying this pathway as a potential therapeutic target for sepsis immunotherapy. With increased attention on the immunosuppressive stage of sepsis, reversing sepsis-induced immune dysfunction has become a major direction of sepsis therapies. For example, anti-PD1 has been shown to improve sepsis survival in mice and has entered clinical trials for septic patients. However, it is increasingly apparent that different co-inhibitory molecules play distinct and non-redundant roles in inducing T cell dysfunction (16). Our data show that 2B4 expression kinetics are different from those of PD-1 and CTLA-4, indicating potential distinct roles of these coinhibitory molecules during sepsis. Recent work has also shown that the ability of anti-PD-1 to rescue anti-tumor T cells is dependent upon CD28 expression (17). Here, we found that 2B4+ CD4+ T cells express little surface CD28, suggesting that anti-PD-1 therapy may not effectively rescue the 2B4+ T cell compartment within septic patients, further highlighting the concept that targeting multiple co-inhibitory molecules during sepsis might be required to adequately reverse immune dysfunction.
Another important and novel finding of the current report is that 2B4 functions in a cell-intrinsic manner on CD4+ but not CD8+ T cells during sepsis and contributes to decreased macrophage activation. Previous work has shown that 2B4 functions either as a co-stimulatory or co-inhibitory molecule on NK cells and primarily as a co-inhibitory molecule on memory CD8+ T cells depending upon external stimulation and the down-stream adaptor proteins, SAP or SHP-1. Noticeably, distinct 2B4 expression patterns were observed in SPF-housed murine vs. healthy human CD8+ T cells, likely due to differential frequencies of memory and naive T cell populations. Indeed, our murine data showed that 2B4 is expressed mostly on CD44hi memory cell populations following CLP (Figure 1D). Because we have previously published that memory T cells are more susceptible to sepsis induce dysfunction as compared to naïve T cells (12), understanding the impact of 2B4 signaling specifically on memory T cell subsets remains an important goal. However, despite the differences in memory T cell composition between murine and human immune populations, our results show that 2B4 expression was significantly increased after sepsis on both human and mouse CD4+ T cells. Unlike 2B4 expression on human CD8+ T cells, which is high at baseline, our data indicate that sepsis selectively induces 2B4 expression on CD4+ T cells. Here, we have provided the first description of the critical co-inhibitory role of 2B4 on CD4+ T cells promoting the mortality mechanism of disease. However, further investigation is needed to identify the molecular signaling down-stream of 2B4 in CD4+ T cells during sepsis. In summary, our results suggest a novel therapeutic target for the treatment of septic individuals, and also highlight the unique roles of 2B4 within CD4+ and CD8+ T cell compartments.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and healthy donor members who contributed to this study, with special thanks to Leona Wells and Mona Brown for collecting patient samples.
This work was supported by R01GM113228 (M.L.F. and C.M.C), R01AI104699 (M.L.F.) T32GM095442 (C.M.C), R01GM072808 (C.M.C), R01GM104323 (C.M.C., M.L.F.), R01GM109779 (C.M.C., M.L.F.).
References
- 1.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 2.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shubin NJ, Chung CS, Heffernan DS, Irwin LR, Monaghan SF, Ayala A. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukoc Biol. 2012;92:593–603. doi: 10.1189/jlb.1211641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A, Yang WL, Matsuo S, Wang P. Differential alterations of tissue T-cell subsets after sepsis. Immunol Lett. 2015;168:41–50. doi: 10.1016/j.imlet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KM, McNerney ME, Stepp SE, Mathew PA, Schatzle JD, Bennett M, Kumar V. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J Exp Med. 2004;199:1245–1254. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu D, Krummey SM, Badell IR, Wagener M, Schneeweis LA, Stetsko DK, Suchard SJ, Nadler SG, Ford ML. 2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft-specific CD8+ T cell responses. J Exp Med. 2014;211:297–311. doi: 10.1084/jem.20130902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown DR, Calpe S, Keszei M, Wang N, McArdel S, Terhorst C, Sharpe AH. Cutting edge: an NK cell-independent role for Slamf4 in controlling humoral autoimmunity. J Immunol. 2011;187:21–25. doi: 10.4049/jimmunol.1100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker CC, I, Chaudry H, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 10.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue S, Bo L, Bian J, Unsinger J, Chang K, Hotchkiss RS. Dose-dependent effect of anti-CTLA-4 on survival in sepsis. Shock. 2011;36:38–44. doi: 10.1097/SHK.0b013e3182168cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serbanescu MA, Ramonell KM, Hadley A, Margoles LM, Mittal R, Lyons JD, Liang Z, Coopersmith CM, Ford ML, McConnell KW. Attrition of memory CD8 T cells during sepsis requires LFA-1. J Leukoc Biol. 2016;100:1167–1180. doi: 10.1189/jlb.4A1215-563RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolan A, Kobayashi H, Naveed B, Kelly A, Hoshino Y, Hoshino S, Karulf MR, Rom WN, Weiden MD, Gold JA. Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis. PLoS One. 2009;4:e6600. doi: 10.1371/journal.pone.0006600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong HR, Shanley TP, Sakthivel B, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, Kalyanaraman M, Tofil NM, Penfil S, Monaco M, Tagavilla MA, Odoms K, Dunsmore K, Barnes M, Aronow BJ S. S. S. I. Genomics of Pediatric. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics. 2007;30:146–155. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, Sica GL, Sharpe AH, Freeman GJ, Blazar BR, Turka LA, Owonikoko TK, Pillai R, Ramalingam SS, Araki K, Ahmed R. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017 doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.