Abstract
Priming of human NK cells with IL-2 is necessary to render them functionally competent upon NKG2D engagement. We examined the underlying mechanisms that control NKG2D responsiveness in NK cells and found that IL-2 up-regulates expression of the amino acid transporters solute carrier family 1 member 5 (SLC1A5)2 and CD98. Using specific inhibitors to block SLC1A5 and CD98 function, we found that production of IFNγ and degranulation by CD56bright and CD56dim NK cells following NKG2D stimulation were dependent on both transporters. IL-2 priming increased the activity of the mammalian target of rapamycin complex 1 (mTORC1) and inhibition of mTORC1 abrogated the ability of the IL-2-primed NK cells to produce IFNγ in response to NKG2D-mediated stimulation. This study identifies a series of IL-2-induced cellular changes that regulates the NKG2D responsiveness in human NK cells.
Introduction
NKG2D, an activating receptor expressed by NK cells, CD8+ T cells, subsets of CD4+ T cells, iNKT cells, and γδ T cells (1), recognizes a diverse array of ligands belonging to the MHC class I chain-related (MIC) protein family (i.e. MICA and MICB) and the UL16-binding protein family (i.e.ULBP1-6) (1). NKG2D ligands are normally absent or expressed at low levels on healthy resting cells, but are induced in many stressed, virally-infected, or transformed cells (2). The importance of the NKG2D receptor in immune surveillance is emphasized by the evasion strategies cancers and viruses employ to prevent the surface expression of NKG2D ligands (1). Moreover, NKG2D is implicated in the development and/or progression of autoimmune diseases, e.g. rheumatoid arthritis, diabetes, celiac disease, and Crohn's disease (1). NKG2D associates with the DAP10 adapter molecule, which is required for cell surface expression of NKG2D and promotes signaling through the PI3K and Grb2-Vav pathways to regulate NK cell-mediated cytotoxicity and cytokine production (2). However, the functional outcome of NKG2D stimulation depends on the activation state of the cells. NKG2D stimulation of freshly isolated human peripheral blood NK cells alone is insufficient to induce cytotoxicity and cytokine production even though the surface expression of NKG2D and intracellular signaling machinery are present (3, 4). In contrast, NK cells primed by IL-2 efficiently kill NKG2D ligand-expressing target cells and produce cytokines after NKG2D stimulation (5, 6). The mechanisms responsible for the IL-2-mediated change in NKG2D responsiveness remains undetermined.
Human NK cells are subdivided into two major subsets. CD56bright NK cells are immature, whereas CD56dim NK cells comprise a mature NK cell subset. CD56bright NK cells express the high-affinity IL-2 receptor, IL-2Rαβγ, whereas resting CD56dim NK cells lack IL-2Rα (7). When IL-2 binds to its receptor, JAK1 and JAK3 are phosphorylated, which recruit and activate STAT1, STAT3, and STAT5. The IL-2R also signals through PI3K, AKT, and the MAP kinase pathways (8). Gene expression profiling of resting and IL-2-primed human NK cells has shown that IL-2 treatment up-regulates expression of a wide range of genes (9). In this study, we found that IL-2 priming of human NK cells leads to a JAK3-dependent up-regulation of the glutamine transporter SLC1A5 (ASCT2) and the amino acid transporter SLC3A2/SLC7A5 (CD98). Furthermore, we show that the activity of these transporters and mTORC1 is essential for functional activation of both CD56bright and CD56dim NK cells in response to NKG2D stimulation.
Materials and Methods
Cells
PBMCs were isolated from blood obtained from the Blood Centers of the Pacific under an Institutional Review Board approved protocol (IRB# 10-00265) by density gradient centrifugation using Ficoll-Paque™ PLUS (GE Healthcare Bio-Sciences AB). NK cells were purified (>90-95%) using EasySep NK cell enrichment kits (Stem Cell technologies). NK cells were cultured in RPMI-1640 medium (Corning Cellgro, Mediatech Inc.) containing 10% FBS (Thermo Scientific), 1× MEM non-essential amino acids solution (Gibco), 1 mM sodium pyruvate (UCSF Cell Culture), 2 mM L-glutamine (UCSF Cell Culture), penicillin (100 IU/ml), streptomycin (100 μg/ml) (Corning Cellgro), and 200 U/ml human rIL-2 (generously provided by Prometheus Laboratories).
Plate-bound antibody stimulation of NK cells
NK cells were cultured in medium or with 200 U/ml IL-2 for 5, 10, 15, 20, or 24 h at 37°C and 5% CO2 prior to stimulation. Where indicated, the following inhibitors were added to cells 1 hr prior to stimulation: 10 mM L-glutamic acid γ-(p-nitroanilide) hydrochloride (GPNA) (Santa Cruz Biotechnology) (1M GPNA solution was prepared in DMSO and kept at 37°C immediately prior to its addition), 100 mM D-phenylalanine (Sigma-Aldrich) (dissolved in medium for 1 h at 37°C by vortexing every 5-10 min prior to addition); 100 nM rapamycin (Calbiochem) (109.4 μM rapamycin in DMSO stock was stored at -20° C, thawed, and diluted in medium prior to addition); 100 nM Torin-1 (ApexBio) (1.645 mM Torin-1 in DMSO stock was stored at -80°C, thawed, and diluted in medium before addition). Nunc maxisorp ELISA plates (Thermo Fisher Scientific) were washed twice with PBS and then coated with 5 μg/ml anti-NKG2D mAb (1D11, BioLegend) or 5 μg/ml control mouse IgG1 (MOPC-21, UCSF Monoclonal Antibody Core) in PBS for 24 h at 4°C. After coating, the plates were washed twice with PBS and blocked in complete medium for 10 min at room temperature (RT). For IFNγ measurements, GolgiSTOP was added to cells immediately prior to stimulation. Stimulation of 1.5 × 105 cells/well proceeded for 5 h at 37°C and 5% CO2.
Real-time PCR
NK cells were cultured in medium with or without 200 U/ml human rIL-2, 10 ng/ml human rIL-15 (R&D Systems), or 10 ng/ml human rIL-12 for 6 hrs. Where indicated, cells were primed for 6 h with 0.05% DMSO or 0.5 μM Jak3i (kindly provided by Drs. Jack Taunton, Dr. Art Weiss, and Geoff Smith) (1 mM Jak3i in DMSO was stored at -20° C, thawed, and diluted in medium prior to addition). Total RNA was purified using an RNeasy mini kit (Qiagen) and cDNA was generated from 0.05 μg RNA using SuperScript® III First-Strand Synthesis System (Invitrogen). Real-time PCR was performed with the SYBR green master mix reagent (Invitrogen) using standard conditions (primer Tm used: 55°C) and the following primer pairs: SLC1A5_Fwd: 5′-GTGTCCTCACTCTGGCCATC-3′; SLC1A5_Rev: 5′-TACAGGACCGGTCGACTAGC-3′; SLC7A5_Fwd: 5′-TCATCATCCGGCCT TCATCG-3′; SLC7A5_Rev: 5′-GAGCAGCAGCACGCAGA-3′; SLC3A2_Fwd: 5′-AGCTGGAG TTTGTCTCAGGC-3′; SLC3A2_Rev: 5′-GGCCAATCTCATCCCCGTAG-3′; HPRT_Fwd: 5′-GACCAGTCAACAGGGGACAT-3′; HPRT_Rev: 5′-CTTGCGACCTTGACCATCTT-3′. Samples were normalized to HPRT and the relative expression of the glutamine transporters was determined using the comparative CT (i.e. 2−ΔΔCT) method, using the ‘media plus DMSO’ sample as the reference sample.
Flow cytometry
mAbs used for cell surface staining were: FITC-anti-CD98 (MEM-108, BioLegend); FITC-anti-CD56 (HCD56, BioLegend); PerCP-Cy5.5-anti-CD56 (HCD56, BioLegend); AF700-anti-CD3 (HIT3a, BioLegend); and APC-anti-NKG2D (149810, R&D Systems). mAbs used for intracellular staining were: PE-anti-phospho-S6 (pS6) (D57.2.2E, Cell Signaling Technology); AF647-anti-phospho-STAT5 (pSTAT5) (47/Stat5 (pY694), BD Biosciences); AF647-anti-IFNγ; anti-ASCT2 (SLC1A5) (D7C12, Cell Signaling Technology); and AF647-conjugated goat anti-rabbit IgG (A-21245, Invitrogen). For surface staining, cells were incubated with the indicated mAbs or isotype-matched control antibodies (BioLegend) for 20 min on ice. During the pSTAT5 and pS6 measurements, the AF700-anti-CD3 and FITC-anti-CD56 were added 15 min prior to fixation in 1.5% paraformaldehyde in PBS. For intracellular IFNγ staining, cells were fixed and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences) and subsequently stained with Abs for 20 min on ice. For intracellular staining of pS6 and pSTAT5, cells were fixed in 1.5% paraformaldehyde in PBS for 10 min at room temperature (RT) and permeabilized in cold 100% methanol for 20 min on ice. Samples were either stored overnight at -20°C in methanol before staining or stained immediately following permeabilization with the anti-pS6 and -pSTAT5 for 30 min at RT. For intracellular staining of SLC1A5, cells were fixed in 1.5% paraformaldehyde in PBS for 10 min at RT, permeabilized in cold 100% methanol for 20 min on ice, and then stained with anti-SLC1A5 for 45 min at RT, followed by staining with anti-rabbit IgG for 20 min at RT. Samples were acquired on a LSRII (BD Biosciences) and analyzed with FlowJo software (Tree Star). Live single cells were gated based on forward and side light scatter profiles. NK cells were gated as CD3- CD56bright or CD3-CD56dim.
Results and Discussion
Maximal IFNγ production by NK cells in response to NKG2D stimulation requires IL-2 priming
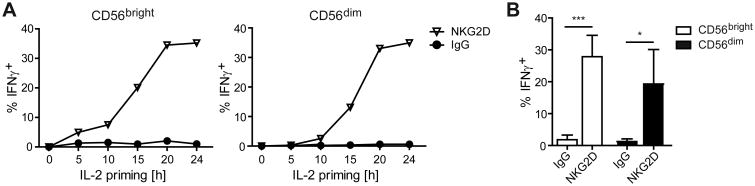
IL-2 priming renders human NK cells able to produce IFNγ in response to NKG2D stimulation (5, 6). To determine the kinetics of IL-2 priming needed to induce NKG2D responsiveness, we cultured freshly purified peripheral blood NK cells in medium with or without IL-2 for 5, 10, 15, 20, or 24 h. Cells were then stimulated with plate-bound anti-NKG2D for 5 h and IFNγ production was measured by flow cytometry. Few CD56bright NK cells and even fewer CD56dim NK cells produced IFNγ after only 10 h of IL-2 priming (Fig. 1A). After 15 h of IL-2 priming, intermediate levels of IFNγ+ cells were detected from both subsets, while peak IFNγ production required 20-24 h priming (Fig. 1A). While the relationship between priming time and IFNγ production was consistent between donors, donor variability was observed in the percentages of IFNγ+ cells at 24 h, ranging from 6%-35% in CD56bright NK cells and 5%-33% in CD56dim γ production in response to NKG2D stimulation, suggesting that IL-2 induced synthesis of new proteins might be necessary.
Figure 1. Maximal IFNγ production by NK cells in response to NKG2D stimulation requires IL-2 priming.
NK cells were enriched from PBMCs and cultured in medium± γ production was measured in CD56bright and CD56dim NK cells by flow cytometry. (A) Results are shown for one donor and are representative of four donors. (B) Data show mean ± s.d. from four donors. Statistical analysis was performed by 2-tailed unpaired student's t-test (*p<0.05, ***p<0.001).
IL-2 priming increases the expression of SLC1A5 and CD98 in NK cells
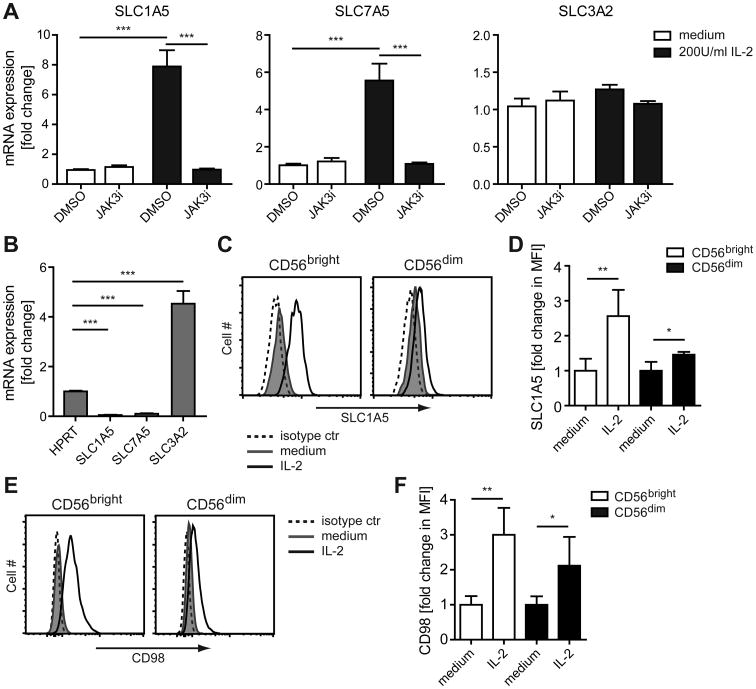
Searching the National Center for Biotechnology Information Gene Expression Omnibus database (10) accession number GSE8059 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE8059) to identify genes differentially expressed in human NK cells after IL-2 priming (9), we found two genes of interest: SLC1A5, which encodes a high affinity L-glutamine transporter (also designated ASCT2) (11), and SLC7A5, which together with SLC3A2 encodes a heterodimeric bidirectional antiporter (collectively designated CD98) that regulates the exchange of intracellular L-glutamine with extracellular amino acids such as L-leucine, L-phenylalanine, and L-tryptophan (11, 12). Consistent with the published dataset (9), we observed an increase in the SLC1A5 mRNA levels in NK cells after IL-2 priming (Fig. 2A). We also observed an increase in expression level of SLC7A5 after IL-2 priming, whereas no change was observed for SLC3A2 (Fig. 2A). However, SLC3A2 was expressed at a high level in resting NK cells compared to the housekeeping gene HPRT, in contrast to the low basal levels of SLC1A5 and SLC7A5 (Fig. 2B). Treatment with IL-15, which signals through components of the IL-2 receptor (13), similarly increased the expression of SLC1A5 and SLC7A5, and to a lower extent SLC3A2 (Fig. S1A). Treatment of NK cells with IL-12, which stimulates IFN-γ production by NK cells (14), also increased the expression of SLC1A5, SLC7A5, and SLC3A2 (Fig. S1A), although to a lesser degree than IL-15 and IL-2. IL-2 treatment leads to phosphorylation of STAT5 (pSTAT5) and the level of phosphorylation remained high throughout the course of IL-2 treatment (Fig. S1B). We used a selective covalent inhibitor of JAK3 (15), which completely blocks the phosphorylation of STAT5 in IL-2-primed NK cells (Fig. S1C), to examine its role in the IL-2-induced expression of SLC1A5 and SLC7A5. The induction of SLC1A5 and SLC7A5 expression in the IL-2-primed NK cells was abrogated after treatment with the JAK3 inhibitor, whereas the high basal expression of SLC3A2 was unaffected (Fig. 2A). Expression of both SLC1A5 and CD98 protein was increased in CD56bright and CD56dim NK cells after IL-2 priming (Fig. 2C, D and Fig. 2E, F, respectively), with the highest increase being observed in CD56bright NK cells. Thus, IL-2 priming of human NK cells induces expression of the amino acid transporters SLC1A5 and CD98 at the mRNA and protein level.
Figure 2. IL-2 priming increases the expression of SLC1A5 and CD98 in NK cells.
(A,B) NK cells were enriched from PBMCs and treated with DMSO (0.05%) or 500 nM Jak3i for 2 h. Cells were then cultured in medium ± 200 U/ml IL-2 for an additional 6 h. RNA was isolated and mRNA expression was analyzed by real-time PCR. (A) Show fold change of mRNA expression relative to the DMSO sample of the medium treated cells, calculated by ΔΔCT method, from three donors. (B) Show fold change of mRNA expression relative to the HPRT sample from three donors. (C-F) Enriched NK cells were cultured in medium ± 200 U/ml IL-2 for 24 h. (C, D) The intracellular SLC1A5 protein level and (E, F) surface expression of CD98 on CD56bright and CD56dim NK cells was measured by flow cytometry. (C, E) Results are shown for one donor and are representative of four donors. (D, F) Data show fold change in mean fluorescence intensity (MFI) ± s.d. relative to the average MFI of the medium treated samples from four donors. Statistical analysis was performed by 2-tailed unpaired student's t-test (*p<0.05, **p<0.01, ***p<0.001).
Inhibition of SLC1A5 and CD98 abrogates the NKG2D-mediated effector functions of IL-2-primed NK cells
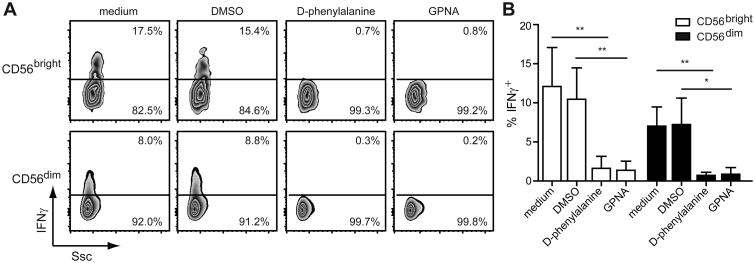
We treated IL-2-primed NK cells with inhibitors against SLC1A5 (i.e. GPNA) (12) or CD98 (i.e. D-phenylalanine) (12) for 1 h prior to NKG2D stimulation to determine their involvement in the NKG2D-induced production of IFNγ. Pre-treatment of IL-2-primed CD56brightand CD56dim NK cells with either inhibitor completely blocked the NK cells ability to produce IFNγ after NKG2D stimulation (Fig. 3). Furthermore, blocking of SLC1A5 and CD98 activity decreased theNKG2D-induced degranulation by the IL-2-primed NK cells (Fig. S2A). The lack of responsiveness was not due to a change in the surface level of NKG2D, as comparable levels were observed between the control-treated and inhibitor-treated cells (Fig. S2B). These results indicate that the activities of SLC1A5 and CD98 play an important role in NKG2D-mediated IFNγ production and degranulation.
Figure 3. Inhibition of SLC1A5 and CD98 abrogates the NKG2D-mediated effector functions of IL-2-primed NK cells.
NK cells enriched from PBMCs were primed with 200 U/ml IL-2 for 24 h. Cells were treated with medium, DMSO (1%), 100 mM D-phenylalanine, or 10 mM GPNA for 1 h prior to stimulation with plate-bound anti-NKG2D Abs for 5 h. IFNγ production was measured in CD56bright and CD56dim NK cells by flow cytometry. (A) Results are shown for one donor and are representative of four donors. (B) Data show mean ± s.d. from four donors. Statistical analysis was performed by 2-tailed unpaired student's t-test (**p<0.01, ***p<0.001).
IL-2 priming of NK cells increases S6 phosphorylation
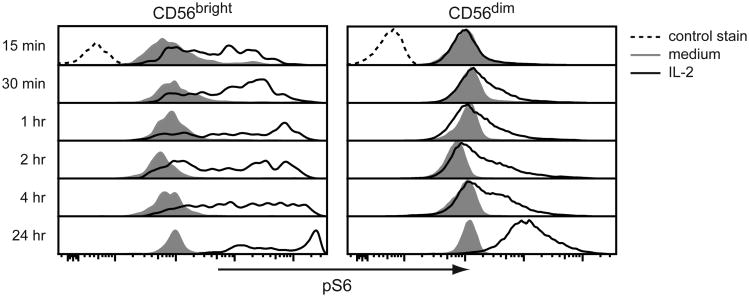
Uptake of L-glutamine by SLC1A5 closely followed by export of that L-glutamine in exchange for essential amino acids (EAA) by CD98 activates mTORC1 (12). To determine if increased expression of SLC1A5 and CD98 activates mTORC1 in the IL-2-primed NK cells, we measured the phosphorylation of S6, which is a downstream target of the mTORC1 pathway (16), by flow cytometry. Phosphorylated of S6 (pS6) was detected in both CD56bright and CD56dim NK cells early after IL-2 treatment, and the pS6 levels continued to increase over the 24 h treatment period (Fig. 4). Treatment of the IL-2 primed (24 h) CD56bright and CD56dim NK cells with GPNA or D-phenylalanine almost completely abrogated phosphorylation of S6, while only minimally affecting phosphorylation of STAT5 (Fig. S2C), confirming the essential roles of SLC1A5 and CD98 in S6 phosphorylation at this latter time-point.
Figure 4. IL-2 priming of NK cells increases S6 phosphorylation.
(A) NK cells enriched from PBMCs were cultured in medium ± 200 U/ml IL-2 for the indicated times. The intracellular level of phosphorylated S6 (pS6) was measured in CD56bright and CD56dim NK cells by flow cytometry. Results are shown for one donor and are representative of three donors.
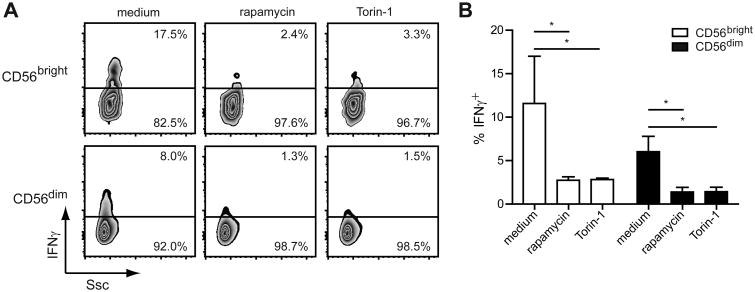
Inhibition of mTORC1 activity abrogates NKG2D-induced IFNγ production
mTORC1 is a critical regulator of mRNA translation (16) and controls IFNγ production by mouse NK cells in vivo after poly(I:C) injection (17) and in vitro by IL-15-primed mouse NK cells stimulated with IL-12 or plate-bound antibodies against the Ly49H (18). To examine its role in NKG2D-mediated IFNγ production, we treated IL-2-primed human NK cells with inhibitors against mTORC1 (i.e. Torin-1 and rapamycin (19)) for 1 h prior to stimulation. Treatment of the IL-2-primed CD56bright and CD56dim NK cells with either inhibitor significantly decreased IFNγ production (Fig. 5). However, inhibition of mTORC1 did not affect NK cell degranulation after NKG2D stimulation (Fig. S2A). The lack of responsiveness was again not due to a change in the surface level of NKG2D, as comparable levels were observed between the control-treated and inhibitor-treated cells (Fig. S2B). Together, these results highlight mTORC1's essential role in the NKG2D-mediated IFNγ production by IL-2-primed NK cells, but not the degranulation.
Figure 5. Inhibition of mTORC1 activity abrogates NKG2D-induced IFNγ production.
NK cells enriched from human PBMCs were primed with 200 U/ml IL-2 for 24 h. Cells were treated with medium, 100 nM rapamycin, or 100 nM Torin-1 for 1 h prior to stimulation with plate-bound anti-NKG2D Abs for 5 h. IFNγ production was measured in CD56bright and CD56dim NK cells by flow cytometry. (A) Results are shown for one donor and are representative of three donors. (B) Data show mean ± s.d. from three donors. Statistical analysis was performed by 2-tailed unpaired student's t-test (*p<0.05).
In this study we show that IL-2 priming of CD56bright and CD56dim NK cells leads to an up-regulation of the amino acid transporters SLC1A5 and CD98 and an increase in mTORC1 activity. The rate limiting step in mTORC1 activation by the bidirectional transport of L-glutamine has been shown to be the import of L-glutamine through SLC1A5 (12). The transport of L-glutamine out of the cells through CD98 appears to occur rapidly in the presence of EAA (12), which makes it difficult to the measure the intracellular levels of L-glutamine in IL-2-primed NK cells under physiological conditions. Thus, it remains to be determined whether the import of L-glutamine by SLC1A5 or its export by CD98 prevails in regulating the mTORC1 activation in IL-2-primed NK cells.
In summary, our findings reveal a crucial role for SLC1A5 and CD98 in controlling NKG2D responsiveness in human NK cells. Blocking either of the transporters completely abrogated the NKG2D-mediated IFNγ production by the IL-2-primed NK cells. Furthermore, activation of mTORC1 by the transporters was found to be critical for the ability of the IL-2-primed NK cells to produce IFNγ after NKG2D stimulation. In contrast to IFNγ production, mTORC1 activity was non-essential for NKG2D-mediated degranulation. The presence of lytic granules in many human NK cells is independent of the activation status (20), likely accounting for the continued ability of IL-2-primed NK cells to degranulate in the absence of mTORC1-promoted protein synthesis. Higher intracellular concentrations of L-Leucine or L-arginine has been shown to cause the exchange of GDP for GTP among Rag GTPases, thereby promoting translocation of mTORC1 to the lysosomal membranes and subsequent activation by the GTPase Rheb (21). Further characterization of the precise mechanism that regulates the mTORC1 activity in the IL-2-primed NK cells and whether additional glutamine-dependent pathways are involved will guide us towards a more comprehensive understanding of how amino acid transport regulates the NKG2D responsiveness in NK cells. A recent study by Nakaya et al. (22) reported that SLC1A5 and CD98 activity was required for activation of mTORC1 in mouse T cells following stimulation, and the absence of SLC1A5 resulted in fewer IFNγ-producing T cells after Listeria monocytogenes infection (22). Our data suggest a similar linkage between L-Glutamine transport, mTORC1 activation, and IFNγ production in human NK cells and thus provide novel insight into the cellular changes caused by IL-2 priming that controls the NKG2D responsiveness in NK cells.
Supplementary Material
Acknowledgments
We thank the Lanier laboratory for comments and discussions; Prometheus Laboratories, Inc. for providing human IL-2; and Drs. Jack Taunton, Art Weiss, and Geoff Smith for providing the Jak3 inhibitor.
Footnotes
Abbreviations: GPNA: L-Glutamic acid γ-(p-nitroanilide) hydrochloride; MFI: mean fluorescence intensity; MICA: MHC class I chainrelated A; mTORC1: mammalian target of rapamycin complex 1; RAET1: retinoic acid early transcript 1; SLC1A5: solute carrier family 1 member 5; ULBP: UL16-binding protein; rIL-2: recombinant IL-2; rIL18: recombinant IL-18; rIL-15: recombinant IL-15
References
- 1.Lanier LL. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol Res. 2015;3:575–582. doi: 10.1158/2326-6066.CIR-15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hubeshy ZB, Coleman A, Nelson M, Goodier MR. A rapid method for assessment of natural killer cell function after multiple receptor crosslinking. J Immunol Methods. 2011;366:52–59. doi: 10.1016/j.jim.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 8.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 9.Dybkaer K, Iqbal J, Zhou G, Geng H, Xiao L, Schmitz A, d'Amore F, Chan WC. Genome wide transcriptional analysis of resting and IL2 activated human natural killer cells: gene expression signatures indicative of novel molecular signaling pathways. BMC Genomics. 2007;8:230. doi: 10.1186/1471-2164-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pochini L, Scalise M, Galluccio M, Indiveri C. Membrane transporters for the special amino acid glutamine: structure/function relationships and relevance to human health. Front Chem. 2014;2:61. doi: 10.3389/fchem.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 15.Smith GA, Uchida K, Weiss A, Taunton J. Essential biphasic role for JAK3 catalytic activity in IL-2 receptor signaling. Nat Chem Biol. 2016;12:373–379. doi: 10.1038/nchembio.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly RP, Loftus RM, Keating SE, Liou KT, Biron CA, Gardiner CM, Finlay DK. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193:4477–4484. doi: 10.4049/jimmunol.1401558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandagopal N, Ali AK, Komal AK, Lee SH. The Critical Role of IL-15-PI3K-mTOR Pathway in Natural Killer Cell Effector Functions. Front Immunol. 2014;5:187. doi: 10.3389/fimmu.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thoreen CC, Sabatini DM. Rapamycin inhibits mTORC1, but not completely. Autophagy. 2009;5:725–726. doi: 10.4161/auto.5.5.8504. [DOI] [PubMed] [Google Scholar]
- 20.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15:555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakaya M, Xiao Y, Zhou X, Chang JH, Chang M, Cheng X, Blonska M, Lin X, Sun SC. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40:692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.