Abstract
Many countries, like Denmark, have tailored Disease Management Programs (DMPs) based on patients having single chronic diseases [defined institutionally as “program diseases” (PDs)], which can complicate treatment for those with multiple chronic diseases. The aims of this study were (a) to assess the prevalence and overlap among acutely hospitalized older medical patients of PDs defined by the DMPs, and (b) to examine transitions between different departments during hospitalization and mortality and readmission within two time intervals among patients with the different PDs. We conducted a registry study of 4649 acutely hospitalized medical patients ≥65 years admitted to Copenhagen University Hospital, Hvidovre, Denmark, in 2012, and divided patients into six PD groups (type 2 diabetes, chronic obstructive pulmonary disease, cardiovascular disease, musculoskeletal disease, dementia and cancer), each defined by several ICD-10 codes predefined in the DMPs. Of these patients, 904 (19.4%) had 2 + PDs, and there were 47 different combinations of the six different PDs. The most prevalent pair of PDs was type 2 diabetes with cardiovascular disease in 203 (22.5%) patients, of whom 40.4% had an additional PD. The range of the cumulative incidence of being readmitted within 90 days was between 28.8% for patients without a PD and 46.6% for patients with more than one PD. PDs overlapped in many combinations, and all patients had a high probability of being readmitted. Hence, developing strategies to create a new generation of DMPs applicable to older patients with comorbidities could help clinicians organize treatment across DMPs.
Keywords: Disease management program, Older hospitalized patients, Multimorbidity
Introduction
The prevalence of chronic diseases increases with age (van den Akker et al. 1998; Fortin et al. 2005; Denton and Spencer 2010) and systematic reviews have found a prevalence of multimorbidity (multiple chronic diseases) of up to 98% in persons aged 60 or older (Marengoni et al. 2011; Fortin et al. 2012). The high number of people with multimorbidity puts pressure on the existing hospital system, which in Denmark as in many other countries is organized into specialized wards according to organ systems. Patients with diseases in more than one organ system risk incoherent trajectories in association with the hospitalization because the coordination among different departments and different parts of the health service may be perceived as problematic (Boyd et al. 2007; Boult and Wieland 2010). Thus, prioritizing collaboration and communication around patients with chronic conditions is important.
To improve management of chronic diseases, the Danish Health and Medicines Authority has recommended Disease Management Programs (DMPs) tailored to Danish health care (Danish Health and Medicines Authority 2012a), similar to several other countries (Lugtenberg et al. 2011). The DMPs are standardized descriptions of the multidisciplinary, multisectional, coordinated and evidence-based healthcare work. This work includes prevention, diagnosis, treatment, rehabilitation and follow-up, cooperation and coordination between the acute and primary care settings based on a specific patient group (Danish Health and Medicines Authority 2012a).
The DMPs address single diseases which are in accordance with the focus on treatment of single diseases in medicine (Tinetti and Fried 2004). Greater attention to multiple chronic diseases (multimorbidity) (Boyd and Fortin 2011) is warranted, and several countries have included multimorbidity in their DMPs (Vitry and Zhang 2008; Lugtenberg et al. 2011). Multimorbidity is often discussed in general without concrete actions (Lugtenberg et al. 2011), and the evidence for treatment and rehabilitation still relies on a single disease concept (Danish Health and Medicines Authority 2012a). Most studies are designed for examining single conditions, and individuals with multimorbidity are therefore often excluded (Starfield 2001; Fortin et al. 2006). International studies problematize the DMPs’ single disease focus when treating patients with multimorbidity, stating that DMPs provide limited guidance on the combined use of treatments (Tinetti and Fried 2004; Vitry and Zhang 2008; Boult and Wieland 2010; Boyd and Fortin 2011; Lugtenberg et al. 2011; Cox et al. 2011; Mutasingwa et al. 2011; Hughes et al. 2013). Several professional societies and researchers around the world have started developing guidelines or DMPs for older patients with multimorbidity (Fabbri et al. 2012; Uhlig et al. 2014; Weiss et al. 2014; Bernabeu-Wittel et al. 2014), but the complexity of the different treatment regimens and the interactions between the DMPs makes the work difficult. More knowledge is needed about how the patients’ different chronic diseases occur together and the trajectories for these patients.
In Denmark, DMPs have primarily been implemented in the primary care setting and in outpatient clinics. Improving patient care requires implementing coordinated health care across all sectors to increase our understanding of complex medical patients and their management. As it is today, however, hospitals are organized in specialized wards based on single diseases. Therefore, it is important to investigate the extent of multimorbidity (based on DMPs) in the hospital setting and explore the trajectories of these patients. The aims of this study thus were (a) to study the prevalence and overlap of program diseases (PDs) defined by the DMPs among acutely hospitalized older medical patients, and (b) to examine transitions among different departments during hospitalization and mortality and readmission within two time intervals in patients with different PDs.
Design and methods
Setting
In Denmark, the publicly funded healthcare system covers all primary and specialist services uniformly for all citizens. Amager and Hvidovre Hospital, University of Copenhagen, covers 10 municipalities with approximately 460,000 citizens and has approximately 14,000 medical admissions each year. Of these, 85% are acute. The emergency department (ED) at Amager and Hvidovre Hospital consists of a traditional ED and a medical unit, where patients referred by general practitioners or by ambulance due to an emergency call can be hospitalized for up to 3 days before discharge or transfer to a specialized medical ward. All Danish citizens have a unique personal identification number, the Central Personal Register number (CPR number) (Pedersen 2011). Because of the CPR number, linkage at the individual level among nationwide and local registries is feasible.
Study population
Because of the large number of patients hospitalized acutely, the study population included all medical patients with a Danish CPR number, aged ≥ 65 years old, who were acutely admitted to the medical unit at the ED at Amager and Hvidovre Hospital from January 1, 2012, to December 31, 2012. Patients were divided into eight groups: one group with no PD, six groups of patients, respectively, having only one of the six PDs [type 2 diabetes, chronic obstructive pulmonary disease (COPD), cardiovascular or musculoskeletal disease, dementia or cancer], and a group of patients with two or more PDs.
Data collection
Data were collected from the Danish National Patient Registry, a nationwide population registry in Denmark (Lynge et al. 2011), from the Danish Civil Registration System (Pedersen 2011), and from the local registry of Amager and Hvidovre Hospital, University of Copenhagen. The World Health Organization’s updated International Classification of Diseases 10th edition (ICD-10) classification system was used to define PDs. The system is divided into 21 chapters representing different organ systems and categories of health problems (World Health Organization 2014).
Outcomes
PD: A PD is predefined by the Danish Health and Medicines Authority by the presence of at least one of the following ICD-10 codes: type 2 diabetes (E10–E14), COPD (J44), cardiovascular disease (I20–I21, I25.1 and I50), musculoskeletal (G550–G553, G558, L88, L71, L97, M431, M471, M472, M478E, M480, M482, M485B, M510–M514, M511F, M533, M533B, M539, M543–M545 and S336A), dementia (G30.0, G30.1, G30.8, G31.0B, G31.8, G31.8E, G31.9, I69.4 and I69.3) and cancer (C00–C99) according to the Danish DMPs (Capital Region, Denmark 2009a, b, 2011, 2012a, b; Danish Health and Medicines Authority 2012b). ICD-10 codes were extracted from the Danish National Patient Registry based on both the patient’s first acute hospital admission in 2012 and a 10-year prevalence of the ICD-10 codes registered prior to the index admission based on recommendations in Schram et al. (2008).
Transitions during hospitalization: Information regarding the patients’ hospital departments during hospitalization was recorded from the local registry of Amager and Hvidovre Hospital, based on data from the first acute hospital admission in 2012. One transition was defined as a transition from the medical unit at the ED to a specialized ward, and two or more transitions were defined as a transition from the medical unit at the ED to a specialized ward and an additional transition to another specialized ward.
Mortality
Time of death was recorded from the Danish Civil Registration System and from admission and discharge dates from the Danish National Patient Register. Time to death was recorded in three different time intervals: during hospitalization, to reflect the quality of care during hospitalization; 7 days after admission, to reflect the quality of care and the discharge process; and 90 days after discharge, to reflect the patients’ general health.
Readmissions
Admission and discharge dates were recorded from the Danish National Patient Register. To avoid overestimating the number of readmissions, a hospital readmission was defined as an acute admission more than 4 h after discharge. Both acute readmission dates up to 7 and 90 days after discharge were obtained from the register. An interval of 7 days was chosen to reflect the quality of care in the previous hospitalization and discharge process, and 90 days was chosen to reflect the patients’ general health.
Descriptive data
From the Danish Civil Registration System, data were collected on age and sex. From the Danish National Patient Register, data were collected on ICD-10 diagnosis codes (acute and chronic) registered for the first acute hospital admission in 2012; on length of stay (LOS), based on data from the first acute hospital admission in 2012; on ICD-10 disease categories, which were categorized according to 17 of the 21 ICD-10 chapters (ICD-10 chapters XV “Pregnancy, childbirth and the puerperium,” XVI “Certain conditions originating in the perinatal period,” XVII “Congenital malformations, deformations and chromosomal abnormalities,” and XIX “Injury, poisoning and certain other consequences of external causes,” were not included because of the lack of relevance); and on acute hospitalization within 6 months prior to the index admission to reflect patients’ general health.
The study was approved by the Danish Data Protection Agency (FSEID-00000882). No approval from the National Committee on Health Research Ethics was needed because only registries were used.
Statistical analysis
Data are presented as numbers and percentages or as medians with a corresponding interquartile range (IQR). To examine for differences between patients with 2 + PDs and the other groups, we used a multinomial logistic regression model for the following variables: age (adjusted for sex), sex (adjusted for age), ICD-10 disease categories (adjusted for age and sex), and acute hospitalization within 6 months (adjusted for age and sex). All analyses were carried out to estimate odds ratios (ORs) with 99% confidence intervals (CIs). The Kruskal–Wallis test was used to test for differences between patients with 2 + PDs and the other groups regarding LOS. A multinomial logistic regression model (adjusted for age and sex) was also used to examine whether PDs were associated with transitions between different departments during hospitalization.
A Cox proportional hazards model was used to test for the difference between patients with 2 + PDs and the other groups in time to readmission (within 7 days and at 90 days after discharge) and to test for the difference in time to death (during hospitalization, within 7 days after admission and at 90 days after discharge); the analyses were adjusted for age, sex, and acute hospitalization within the last 6 months. A cumulative incidence function was used to plot time to death, and a cumulative incidence function with death as competing event was used to plot time to readmission for the PDs.
The statistical analyses were conducted using the SAS 9.3 software package for Windows. Plots were created in R version 3.1.0. The level of significance was set at 0.01 to account for multiple testing, and all statistical tests were two-tailed.
Results
In 2012, a total of 4649 patients were admitted acutely to the medical unit at the ED at Amager and Hvidovre Hospital, and their characteristics are shown in Table 1. Of these patients, 904 (19.4%) had 2 + PDs, 1795 (38.6%) had one PD, and 1950 (41.9%) patients did not have a PD. Moreover, in comparing patients with 2 + PDs with the other groups, multinomial regression models showed that patients with the type 2 diabetes PD (OR 0.98, CI99% 0.96–1.00) were younger than patients with 2 + PDs and patients with the dementia PD were older than patients with 2 + PDs (OR 1.06, CI99% 1.04–1.08). There were more men in the 2 + PD group than among patients with the COPD PD (OR 1.57, CI99% 1.16–2.13) and more women in the 2 + PD group than among patients with the cardiovascular PD (OR 1.80, CI99% 1.27–2.55). Overall, the LOS was short, with a median between one and 2 days for all groups except the cancer PD, which had a median LOS of 4 days. Patients with 2 + PD had a higher LOS than patients with no PD (p = 0.001) and patients with the cardiovascular PD (p = 0.008), but a shorter LOS than patients with the cancer PD (p = 0.001). The risk of being registered with three or more ICD-10 disease categories was significantly higher for patients with one or 2 + PDs compared with patients not having a PD. Patients with 2 + PDs had a higher risk of having been acutely hospitalized within the last 6 months prior to the index admission than patients with no PD (OR 3.60, CI99% 2.88–4.52), type 2 diabetes PD (OR 2.23, CI99% 1.57–3.17), COPD PD (OR 1.78, CI99% 1.32–2.41), cardiovascular PD (OR 2.64, CI99% 1.82–3.38), musculoskeletal PD (OR 1.99, CI99% 1.16–3.43) and dementia PD (OR 1.46, CI99% 1.02–2.08).
Table 1.
Characteristics of the study population
| Variable | Total | No PD | Program diseases | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 2 diabetes | COPD | Cardiovascular | Musculoskeletal | Dementia | Cancer | 2 + PDs | ||||||||||||
| N | 4649 | 1950 | 352 | 484 | 314 | 116 | 292 | 237 | 904 | |||||||||
| Age, median (IQR) | 78.7 | (71; 85) | 78.5 | (71; 86) | 76.5 | (70; 84) | 77.6 | (72; 84) | 78.3 | (73; 86) | 81.7 | (75; 86) | 84.3 | (78; 89) | 77.4 | (71; 85) | 78.7 | (73; 84) |
| Female | 2653 | (57.1) | 1164 | (59.7) | 180 | (51.1) | 313 | (64.7) | 130 | (41.4) | 73 | (62.9) | 178 | (61.0) | 119 | (50.2) | 496 | (54.9) |
| Diagnoses per persona, median (IQR) | 2 | (1; 2) | 1 | (1; 2) | 2 | (1; 3) | 2 | (1; 3) | 1 | (1; 2) | 1 | (1; 2) | 2 | (1; 3) | 2 | (1; 2) | 2 | (1; 3) |
| LOS#, median (IQR) | 2 | (1; 6) | 1 | (1; 5) | 2 | (1; 6) | 2 | (1; 7) | 2 | (1; 5) | 1 | (0; 6) | 2 | (1; 5) | 4 | (1; 10) | 2 | (1; 7) |
| ICD-10 disease categories with 10-year history | ||||||||||||||||||
| 0–1 | 533 | (11.9) | 457 | (23.4) | 11 | (3.1) | 48 | (9.9) | 21 | (6.7) | 7 | (6.0) | 4 | (1.4) | 5 | (2.1) | 0 | (0) |
| 2–4 | 2039 | (43.9) | 1062 | (54.5) | 174 | (49.4) | 229 | (47.3) | 162 | (51.6) | 50 | (43.1) | 87 | (29.8) | 106 | (44.7) | 169 | (18.7) |
| 5+ | 2057 | (44.3) | 431 | (22.1) | 167 | (47.4) | 207 | (42.8) | 131 | (41.7) | 59 | (50.9) | 201 | (68.8) | 126 | (53.2) | 735 | (81.3) |
| Acute hospitalization 6 month prior to index admission | 1426 | (30.7) | 394 | (20.2) | 101 | (28.7) | 161 | (33.3) | 83 | (26.4) | 37 | (31.9) | 117 | (40.1) | 102 | (43.0) | 431 | (47.7) |
Results are presented as numbers and percentages unless otherwise specified
PD program disease, N number, IQR interquartile range, COPD chronic obstructive pulmonary disease, LOS length of stay
aData are based on the first hospitalization in 2012. ICD-10 disease categories = number of chapters in the updated International Classification of Diseases 10th edition
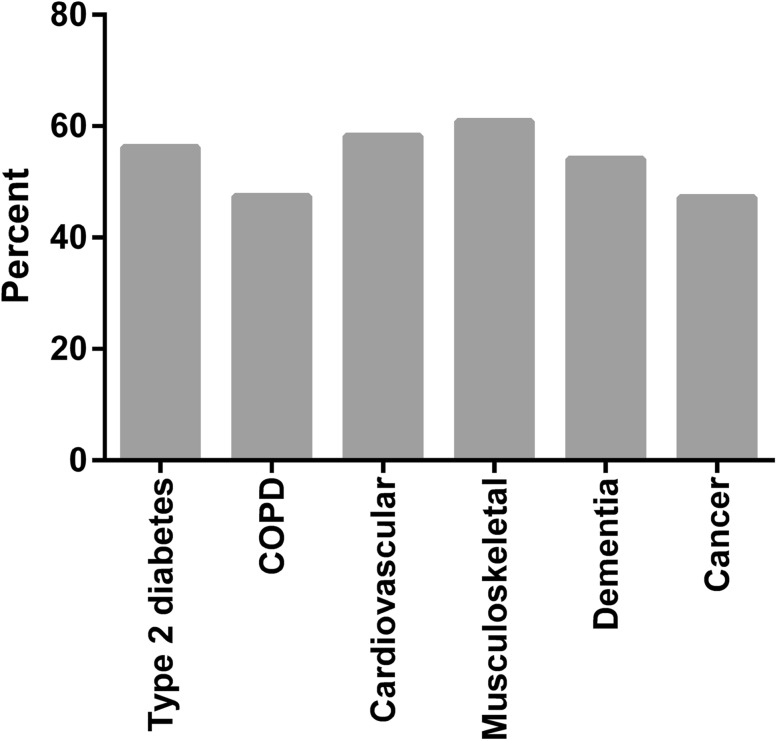
There were 47 different combinations of overlap of PDs with frequencies between 0.1 and 13.4% among the 904 patients with 2 + PDs. Figure 1 shows the proportion of patients having 2 + PDs within each of the PDs. The highest proportion was found for the musculoskeletal PD, with 60.9% of the 297 patients having 2 + PDs, and the lowest proportion was found for the cancer PD, with 47.2% of 449 patients having 2 + PDs. The four most prevalent pairs of PDs among the 904 patients who had more than one PD are shown in Table 2. The most prevalent pair was the type 2 diabetes with the cardiovascular PD, with 203 (22.5%) patients, and 40.4% of those patients had an additional PD.
Fig. 1.
Pecentage of patients having two or more program diseases among patients with at least one program disease. Number of patients with the program disease: diabetes type 2 (N = 804), COPD (N = 920), cardiovascular (N = 751), Musculoskeletal (N = 297), dementia (N = 636) and cancer (N = 449). COPD chronic obstructive pulmonary disease
Table 2.
The four most prevalent pairs of program diseases among patients with more than one program disease (N = 904)
| The four most prevalent pairs of program diseases | Patients with one or more additional PD | ||||
|---|---|---|---|---|---|
| N | % | N | % | ||
| 1 | Type 2 diabetes + cardiovascular | 203 | 22.5 | 82 | 40.4 |
| 2 | COPD + cardiovascular | 164 | 18.1 | 82 | 50.0 |
| 3 | Type 2 diabetes + COPD | 150 | 16.6 | 89 | 59.3 |
| 4 | COPD + dementia | 137 | 15.1 | 68 | 49.6 |
COPD chronic obstructive pulmonary disease
A total of 2511 (54.0%) patients were discharged directly from the medical unit at the ED, 1733 (37.3%) patients experienced one transition from the medical unit at the ED to a specialized medical department, and 405 (8.7%) patients experienced 2 + transitions between departments (Table 3). Patients belonging to the COPD PD group had a higher risk of being transferred two or more times during hospitalization than patients with 2 + PD (OR 1.99, CI99% 1.19–3.33).
Table 3.
Transitions during hospitalization, mortality and readmission for the population
| Variable | Total | No PD | Program diseases | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 2 diabetes | COPD | Cardiovascular | Musculoskeletal | Dementia | Cancer | 2 + PDs | ||||||||||||
| N | 4649 | 1950 | 352 | 484 | 314 | 116 | 292 | 237 | 904 | |||||||||
| Transitions during hospitalization | ||||||||||||||||||
| 0 | 2511 | (54.0) | 1109 | (56.9) | 200 | (56.8) | 240 | (49.6) | 150 | (47.8) | 72 | (62.1) | 160 | (54.8) | 103 | (43.5) | 477 | (52.8) |
| 1 | 1733 | (37.3) | 665 | (34.1) | 126 | (35.8) | 185 | (38.2) | 135 | (43.0) | 39 | (33.6) | 106 | (36.3) | 110 | (46.4) | 367 | (40.6) |
| 2+ | 405 | (8.7) | 176 | (9.0) | 26 | (7.4) | 59 | (12.3) | 29 | (9.2) | 5 | (4.3) | 26 | (8.9) | 24 | (10.1) | 60 | (6.64) |
| Died during hospitalization | 310 | (6.7) | 87 | (4.5) | 18 | (5.1) | 35 | (7.2) | 13 | (4.1) | 3 | (2.6) | 18 | (6.2) | 51 | (21.5) | 85 | (9.4) |
| Mortality | ||||||||||||||||||
| Within 7 days from admission | 188 | (4.0) | 57 | (2.9) | 10 | (2.8) | 21 | (4.3) | 7 | (2.2) | 0 | (0) | 17 | (5.8) | 27 | (11.4) | 49 | (5.4) |
| Within 90 days from dischargea | 499 | (11.5) | 151 | (8.1) | 33 | (9.9) | 40 | (8.9) | 28 | (9.3) | 10 | (8.9) | 55 | (20.1) | 59 | (31.7) | 123 | (15.0) |
| Readmissionsa | ||||||||||||||||||
| Within 7 days from discharge | 423 | (9.7) | 143 | (7.7) | 35 | (10.5) | 51 | (11.4) | 37 | (12.3) | 11 | (9.7) | 36 | (13.1) | 14 | (7.5) | 96 | (11.7) |
| Within 90 days from discharge | 1568 | (36.1) | 536 | (28.8) | 127 | (38.0) | 196 | (43.7) | 107 | (35.5) | 39 | (34.5) | 104 | (38.0) | 77 | (41.4) | 382 | (46.6) |
Results are presented as numbers and percentages unless otherwise specified
PD program disease, N number, COPD chronic obstructive pulmonary disease
aData are based on the patients who survived to be discharged (total N = 4339)
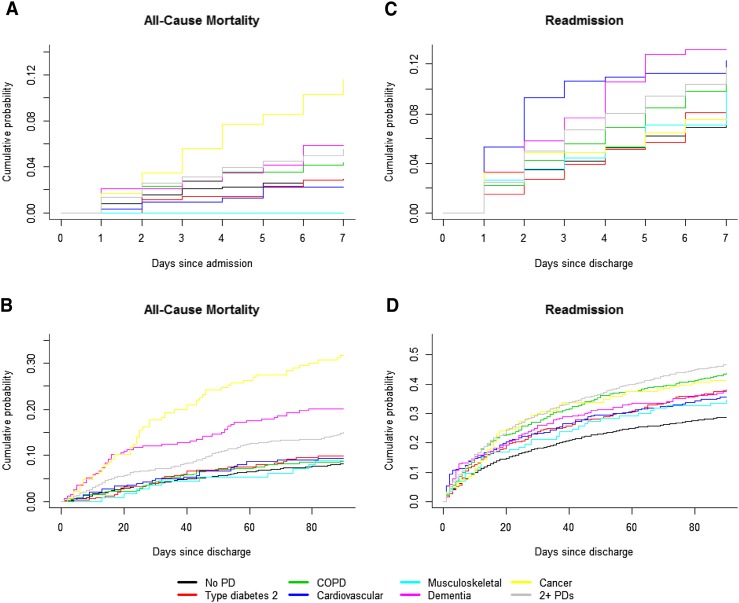
In total, 310 (6.7%) patients died during hospitalization (Table 3). When comparing patients with 2 + PDs with the other groups, patients with no PD had a lower risk of dying (hazard ratio (HR) 0.64, CI99% 0.43–0.95), and patients with the cancer PD had a higher risk (HR 1.76, CI99% 1.11–2.78). The all-cause mortality within 7 days from admission was higher only for patients with the cancer PD (HR 2.29, CI99% 1.23–4.24) compared to patients with 2 + PDs (Fig. 2a).
Fig. 2.
Cumulative incidence plot of time to event for all-cause mortality within 7 days of admission (a) and within 90 days from discharge (b) and readmission within seven (c) and 90 days from discharge (d) according to program disease. PD program disease, COPD chronic obstructive pulmonary disease
Of the 4339 patients who survived the hospitalization, 499 (11.5%) died within 90 days after discharge (Table 3). The difference between the PDs is depicted in Fig. 2b. When comparing patients with 2 + PDs with the other groups, patients with no PD (HR 0.62 CI99% 0.45–0.86) and patients with the COPD PD (HR 0.62, CI99% 0.39–1.00) had a lower risk of dying, and patients with the cancer PD had a higher risk (HR 2.51, CI99% 1.67–3.78).
In total, 423 out of 4339 (9.7%) patients were readmitted within 7 days from discharge of the patients who survived the hospitalization (Table 3). The adjusted Cox regression model showed no difference between patients with 2 + PD and any of the other groups. The cumulative incidence was 11.7% for patients with 2 + PDs and 7.7% for patients with no PD (Fig. 2c).
Overall, 1568 out of 4339 (36.1%) patients who survived the hospitalization were readmitted within 90 days of discharge of the patients (Table 3). The cumulative probability was 46.6% for patients with 2 + PD and 28.8% for patients with no PD. When comparing patients with 2 + PDs with the other groups, patients with no PD (HR 0.53, CI99% 0.45–0.63), type 2 diabetes PD (HR 0.74, CI99% 0.57–0.96) and cardiovascular PD (HR 0.71, CI99% 0.53–0.94) all had a lower risk of readmission (Fig. 2d).
Discussion
We have identified no other study that has questioned the single disease focus when treating older patients by exploring the prevalence and overlap of PDs in a large population of older medical acutely hospitalized patients. The major findings of this study are that (a) 19.4% of the patients had 2 + PDs with overlaps appearing in 47 different combinations at a mostly low prevalence, the most prevalent combination being type 2 diabetes and the cardiovascular PD. However, 40.4% of the patients with this combination had at least one more PD; (b) in total, 54.0% were discharged directly from the ED. Patients with 2 + PDs had a higher risk of both dying during hospitalization and dying after hospitalization than patients without a PD. Patients with 2 + PDs had a higher risk of readmission within 90 than patients with no PD, type 2 diabetes PD and cardiovascular PD. No differences were seen for readmission within 7 days; (c) patients with the cancer PD had the lowest proportion of patients with 2 + PDs, the highest LOS, and higher risks of dying or being readmitted within seven and 90 days.
In line with previous studies (Fortin et al. 2005; Marengoni et al. 2009; van den Bussche et al. 2011; Marengoni et al. 2011; Kirchberger et al. 2012), we found a high prevalence of patients with more than one PD. Having two or more PDs was frequent, with overlaps appearing in many combinations with a mostly low prevalence. This result is in agreement with other studies demonstrating a similar prevalence of combinations of chronic conditions despite a more extended list of chronic diseases (Marengoni et al. 2009; Kirchberger et al. 2012). The problem with overlapping PDs is the complex treatment and self-care follow-up regimes for hypothetical patients following more than one clinical guideline, as illustrated by Boyd et al. (Boyd et al. 2005) and Hughes et al. (2013). A recent study has shown that one chronic disease can adversely affect the management of another chronic disease and that the physician together with the patient must weight the pros and cons of several registered treatments (Søndergaard et al. 2015). These results highlight the question of whether or not DMPs should be constructed based on single diseases. Furthermore, polypharmacy and drug interactions are a major concern when following two or more DMPs (Boyd et al. 2005; Hughes et al. 2013). Polypharmacy may be necessary but is associated with severe adverse events (Koper et al. 2013). In addition, the use of potentially inappropriate medications is common among older persons and associated with low functional capacity and low health-related quality of life (Jensen et al. 2014). The difficulties in developing DMPs relevant to patients with multimorbidity lie in part in the complexity of the different recommendations for the medical treatment for the different chronic diseases (Fabbri et al. 2012). Resolving these difficulties requires knowledge about which chronic diseases occur together so that the recommendations can take into account possible drug interactions. Several studies have examined patterns of multimorbidity and found between three and five such patterns (Marengoni et al. 2009; Schäfer et al. 2010; García-Olmos et al. 2012; Kirchberger et al. 2012; Prados-Torres et al. 2012; Freund et al. 2012). Knowledge and understanding of disease patterns occurring in multimorbidity can provide information that may support existing DMPs and new DMPs for complex medical patients.
In this study, the most common combination among patients with 2 + PDs was the type 2 diabetes with the cardiovascular PD, with a prevalence of 22.5%. The association of type 2 diabetes and cardiovascular disease is well known (Marks and Raskin 2000); thus, it would be reasonable to suggest a DMP for patients who have both conditions. Although we found that 40.4% of these patients had at least one additional PD, this result indicates that multimorbidity may consist of many disease combinations in different patterns, supporting other international research (Prados-Torres et al. 2014). To develop DMPs only for the most prevalent pairs of PDs is therefore not a total solution.
Patients with 2 + PDs had a high mortality risk and a high risk of being acutely hospitalized 6 months prior to the index admission and of being readmitted within 90 days from discharge. Several studies have found that healthcare costs rise as patients develop more than one chronic disease (Nagl et al. 2012; Foguet-Boreu et al. 2014; Moffat and Mercer 2015; Palladino et al. 2016). Fragmented care based on treating single diseases in isolation can lead to duplication of treatment (Barnett et al. 2012; Salisbury 2012) and hence higher costs. Patients with multimorbidity do differ; however, a study from The Netherlands showed that the majority of patients with multimorbidity did not have a higher level of healthcare costs than patients with only on chronic disease, but that a small group of patients with multimorbidity had a very high level of costs (Hopman et al. 2015). These patients were older, female, had low income and suffered from more chronic diseases (Hopman et al. 2015).
Patients with the cancer PD had the highest median LOS, high mortality and readmission rates within 7 and 90 days, and the lowest proportion of patients with 2 + PDs compared to patients belonging to the other PDs, resulting in a low degree of complexity. This result indicates an advantage in keeping a single disease perspective for older patients with cancer.
We found that 41.9% of the patients did not have a PD and were therefore not eligible to enter a DMP. In this group, 88.1% had been hospitalized or examined in outpatient clinics with diseases in two or more ICD-10 disease categories, possibly reflecting the burden and complexity of the patient’ conditions and the degree of multimorbidity. Furthermore, we found that patients without a PD had a cumulative probability of 28.8% of being readmitted within 90 days, which was significantly lower than for patients with a PD, though still relatively high. Despite this complexity, patients without a PD had a LOS of only 1 day, risking fragmented care. Hence, focusing on a few chronical diseases does not solve the challenges for the older medical patients. A solution could be to use measures of frailty, which is found to be associated with an increased risk of disability, hospitalization and long-term care (Clegg et al. 2013), in combination with disease patterns as an indicator of the need for care management. Frailty in combination with disease patterns could help hospitals communicate to the primary care sector about which patients are in need of structured care to prevent hospitalizations and readmissions.
A strength of this study is its population-based nature, made possible by the Danish CPR number system, allowing linkage at the individual level across nationwide and local registries. We used a 10-year prevalence to define whether a patient belonged to a PD, as recommended by Schram et al. (2008). Furthermore, this study covered patients both living at home and in care institutions.
Our study also has limitations. First, it is likely that the registration of secondary diagnoses is focused on those relevant for the specific hospital admission and thus does not necessarily reflect all secondary diagnoses for the patient. Furthermore, the diagnoses are based on routine discharge registration and it cannot be excluded that physicians differ with regard to coding quality possibly introducing a risk of miscoding and undercoding. However, a recent study by Thygesen et al. (2011) found that the positive predictive value of ICD-10 codes used to assess the Charlson comorbidity index score was 98% in the Danish National Patient Register. By including ICD-10 codes from both hospitalizations and outpatient visits from 2002 to 2012, we have tried to increase the validity of the diagnosis codes. Second, the method used for data collection in this study was register based. Schram et al. (2008) found that the setting characteristics have an important influence on the outcome of multimorbidity, with multimorbidity being more prevalent in a general practice setting than in a hospital setting. Hence, the prevalence of multimorbidity may be underestimated in this study. Third, the inclusion of only one hospital may have reduced the generalizability of the results, though Amager and Hvidovre Hospital covers 10 different municipalities with different socioeconomic levels, and we therefore believe that the results are reasonably representative.
In conclusion, we found that overlaps of PDs defined by the DMPs appeared in many combinations with mostly low prevalence among acutely hospitalized older medical patients. Patients with 2 + PD had the highest risk of readmission; however, patients without a PD still had a cumulative risk of 28.8% for readmission within 90 days. Hence, patients without a PD as well as patients with 2 + PD are complex groups that could stand to benefit from a more holistic approach in designing DMPs. However, for patients with cancer, keeping a single disease perspective may be advantageous.
Acknowledgements
The authors would like to acknowledge Thomas Kallemose, Henrik Hedegaard Klausen, Mette Merete Pedersen and Hanne Gilkes at Optimed, Clinical Research Centre, Amager Hvidovre Hospital, University of Copenhagen, for their assistance with this project.
Complinace with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Responsible editor: D. J. H. Deeg.
Contributor Information
Helle Gybel Juul-Larsen, Phone: +45 3862 1590, Email: helle.juul-larsen@regionh.dk.
Janne Petersen, Phone: +45 3862 3332, Email: janne.petersen.01@regionh.dk.
Ditte Maria Sivertsen, Phone: +45 3862 1590, Email: ditte.maria.sivertsen@regionh.dk.
Ove Andersen, Phone: +45 3862 3335, Email: ove.andersen@regionh.dk.
References
- Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet Lond Engl. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- Bernabeu-Wittel M, Alonso-Coello P, Rico-Blázquez M, et al. Development of clinical practice guidelines for patients with comorbidity and multiple diseases. Rev Clín Esp. 2014;214:328–335. doi: 10.1016/j.rce.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Boult C, Wieland GD. Comprehensive primary care for older patients with multiple chronic conditions: “Nobody rushes you through”. J Am Med Assoc. 2010;304:1936–1943. doi: 10.1001/jama.2010.1623. [DOI] [PubMed] [Google Scholar]
- Boyd CM, Fortin M. Future of multimorbidity research: How should understanding of multimorbidity inform health system design? Public Health Rev. 2011;33(2):451–474. [Google Scholar]
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. J Am Med Assoc. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- Boyd CM, Boult C, Shadmi E, et al. Guided care for multimorbid older adults. The Gerontologist. 2007;47:697–704. doi: 10.1093/geront/47.5.697. [DOI] [PubMed] [Google Scholar]
- Capital Region, Denmark (2009a) [Forløbsprogram for Diabetes Type 2. In English: Disease Management Program for Type 2 Diabetes]. In Danish. https://www.regionh.dk/til-fagfolk/Sundhed/kronisk-sygdom/PublishingImages/Sider/Forloebsprogram-for-type-2-diabetes/Forl%C3%B8bsprogrammet%20for%20type%202-diabetes.pdf
- Capital Region, Denmark (2009b) [Forløbsprogram for KOL. In English: Disease Management Program for COPD]. In Danish. https://www.regionh.dk/til-fagfolk/Sundhed/kronisk-sygdom/PublishingImages/Sider/Forloebsprogram-for-KOL/Forloebsprogram_KOL_LR.pdf
- Capital Region, Denmark (2011) [Forløbsprogram for hjerte-kar sygdomme - Fokus på rehabilitering ved åreforkalkningssygdom i hjertet og hjertesvigt. In English: Disease Management Program for Cardio-Vascular Disease]. In Danish. https://www.regionh.dk/til-fagfolk/Sundhed/kronisk-sygdom/PublishingImages/Sider/Forloebsprogram-for-hjerte-kar/Forloebsprogram_hjertekar_m_grafik.pdf
- Capital Region, Denmark (2012a) [Forløbsprogram for Demens. In English: Disease Management Program for Dementia]. In Danish. https://www.regionh.dk/til-fagfolk/Sundhed/kronisk-sygdom/PublishingImages/Sider/Forloebsprogram-for-demens/Forloebsprogram_Demens_2012.pdf
- Capital Region, Denmark (2012b) [Forløbsprogram for Lænderyglidelser. In English: Disease Management Program for Musculoskeletal Diseases]. In Danish. https://www.regionh.dk/til-fagfolk/Sundhed/kronisk-sygdom/PublishingImages/Sider/Forloebsprogram-for-laende-ryg/Forl%C3%B8bsprogrammet%20for%20l%C3%A6nde-ryg.pdf
- Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L, Kloseck M, Crilly R, et al. Underrepresentation of individuals 80 years of age and older in chronic disease clinical practice guidelines. Can Fam Physician. 2011;57:e263–e269. [PMC free article] [PubMed] [Google Scholar]
- Danish Health and Medicines Authority (2012a) [Forløbsprogrammer for kronisk sygdom - den generiske model. In English: Disease Management Program for Chronic Disease - a generic model] In Danish. https://sundhedsstyrelsen.dk/da/sundhed/folkesygdomme/kronisk-sygdom/forloebsprogrammer.Kbh
- Danish Health and Medicines Authority (2012b) [Forløbsprogram for rehabilitering og palliation i forbindelse med kræft - del afsamlet forløbsprogram for kræft. In English: Disease Management Program for Cancer] In Danish. https://sundhedsstyrelsen.dk/da/sundhed/folkesygdomme/kronisk-sygdom/forloebsprogrammer.Kbh
- Denton FT, Spencer BG. Chronic health conditions: changing prevalence in an aging population and some implications for the delivery of health care services. Can J Aging Rev Can Vieil. 2010;29:11–21. doi: 10.1017/S0714980809990390. [DOI] [PubMed] [Google Scholar]
- Fabbri LM, Boyd C, Boschetto P, et al. How to integrate multiple comorbidities in guideline development: article 10 in integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proc Am Thorac Soc. 2012;9:274–281. doi: 10.1513/pats.201208-063ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foguet-Boreu Q, Violan C, Roso-Llorach A, et al. Impact of multimorbidity: acute morbidity, area of residency and use of health services across the life span in a region of south Europe. BMC Fam Pract. 2014;15:55. doi: 10.1186/1471-2296-15-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Bravo G, Hudon C, et al. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3:223–228. doi: 10.1370/afm.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Dionne J, Pinho G, et al. Randomized controlled trials: do they have external validity for patients with multiple comorbidities? Ann Fam Med. 2006;4:104–108. doi: 10.1370/afm.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Stewart M, Poitras M-E, et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10:142–151. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund T, Kunz CU, Ose D, et al. Patterns of multimorbidity in primary care patients at high risk of future hospitalization. Popul Health Manag. 2012;15:119–124. doi: 10.1089/pop.2011.0026. [DOI] [PubMed] [Google Scholar]
- García-Olmos L, Salvador CH, Alberquilla Á, et al. Comorbidity patterns in patients with chronic diseases in general practice. PLoS ONE. 2012;7:e32141. doi: 10.1371/journal.pone.0032141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopman P, Heins MJ, Rijken M, Schellevis FG. Health care utilization of patients with multiple chronic diseases in The Netherlands: differences and underlying factors. Eur J Intern Med. 2015;26:190–196. doi: 10.1016/j.ejim.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Hughes LD, McMurdo MET, Guthrie B. Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing. 2013;42:62–69. doi: 10.1093/ageing/afs100. [DOI] [PubMed] [Google Scholar]
- Jensen LD, Andersen O, Hallin M, Petersen J. Potentially inappropriate medication related to weakness in older acute medical patients. Int J Clin Pharm. 2014;36:570–580. doi: 10.1007/s11096-014-9940-y. [DOI] [PubMed] [Google Scholar]
- Kirchberger I, Meisinger C, Heier M, et al. Patterns of multimorbidity in the aged population. Results from the KORA-Age study. PLoS ONE. 2012;7:e30556. doi: 10.1371/journal.pone.0030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koper D, Kamenski G, Flamm M, et al. Frequency of medication errors in primary care patients with polypharmacy. Fam Pract. 2013;30:313–319. doi: 10.1093/fampra/cms070. [DOI] [PubMed] [Google Scholar]
- Lugtenberg M, Burgers JS, Clancy C, et al. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PLoS ONE. 2011;6:e25987. doi: 10.1371/journal.pone.0025987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- Marengoni A, Rizzuto D, Wang H-X, et al. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57:225–230. doi: 10.1111/j.1532-5415.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Marks JB, Raskin P. Cardiovascular risk in diabetes: a brief review. J Diabetes Complicat. 2000;14:108–115. doi: 10.1016/S1056-8727(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Moffat K, Mercer SW. Challenges of managing people with multimorbidity in today’s healthcare systems. BMC Fam Pract. 2015;16:129. doi: 10.1186/s12875-015-0344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutasingwa DR, Ge H, Upshur REG. How applicable are clinical practice guidelines to elderly patients with comorbidities? Can Fam Physician Médecin Fam Can. 2011;57:e253–e262. [PMC free article] [PubMed] [Google Scholar]
- Nagl A, Witte J, Hodek JM, Greiner W. Relationship between multimorbidity and direct healthcare costs in an advanced elderly population. Results of the PRISCUS trial. Z Für Gerontol Geriatr. 2012;45:146–154. doi: 10.1007/s00391-011-0266-2. [DOI] [PubMed] [Google Scholar]
- Palladino R, Tayu Lee J, Ashworth M, et al. Associations between multimorbidity, healthcare utilisation and health status: evidence from 16 European countries. Age Ageing. 2016 doi: 10.1093/ageing/afw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- Prados-Torres A, Poblador-Plou B, Calderón-Larrañaga A, et al. Multimorbidity patterns in primary care: interactions among chronic diseases using factor analysis. PLoS ONE. 2012;7:e32190. doi: 10.1371/journal.pone.0032190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, et al. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67:254–266. doi: 10.1016/j.jclinepi.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet. 2012;380:7–9. doi: 10.1016/S0140-6736(12)60482-6. [DOI] [PubMed] [Google Scholar]
- Schäfer I, von Leitner E-C, Schön G, et al. Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS ONE. 2010;5:e15941. doi: 10.1371/journal.pone.0015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram MT, Frijters D, van de Lisdonk EH, et al. Setting and registry characteristics affect the prevalence and nature of multimorbidity in the elderly. J Clin Epidemiol. 2008;61:1104–1112. doi: 10.1016/j.jclinepi.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Søndergaard E, Willadsen TG, Guassora AD, et al. Problems and challenges in relation to the treatment of patients with multimorbidity: general practitioners’ views and attitudes. Scand J Prim Health Care. 2015;33:121–126. doi: 10.3109/02813432.2015.1041828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starfield B. New paradigms for quality in primary care. Br J Gen Pract. 2001;51:303–309. [PMC free article] [PubMed] [Google Scholar]
- Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116:179–185. doi: 10.1016/j.amjmed.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Uhlig K, Leff B, Kent D, et al. A framework for crafting clinical practice guidelines that are relevant to the care and management of people with multimorbidity. J Gen Intern Med. 2014;29:670–679. doi: 10.1007/s11606-013-2659-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker M, Buntinx F, Metsemakers JF, et al. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol. 1998;51:367–375. doi: 10.1016/S0895-4356(97)00306-5. [DOI] [PubMed] [Google Scholar]
- van den Bussche H, Koller D, Kolonko T, et al. Which chronic diseases and disease combinations are specific to multimorbidity in the elderly? Results of a claims data based cross-sectional study in Germany. BMC Public Health. 2011;11:101. doi: 10.1186/1471-2458-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitry AI, Zhang Y. Quality of Australian clinical guidelines and relevance to the care of older people with multiple comorbid conditions. Med J Aust. 2008;189:360–365. doi: 10.5694/j.1326-5377.2008.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Weiss CO, Varadhan R, Puhan MA, et al. Multimorbidity and evidence generation. J Gen Intern Med. 2014;29:653–660. doi: 10.1007/s11606-013-2660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2014) International statistical classification of diseases and related health problems 10th revision. http://apps.who.int/classifications/icd10/browse/2015/en