INTRODUCTION
Osteoarthritis is the most common form of joint disease affecting the ageing population and is characterized by a high rate of comorbid conditions. It has been shown that in patients with knee OA, the number of comorbidities were associated with a limitation of activities [1], and more pain [2], thus further increasing the OA burden.
Among other comorbidities, diabetes has been proposed as a possible independent contributor to OA incidence and severity. Several putative pathogenetic factors such as the accumulation of advanced glycation end products (AGEs), low-grade systemic inflammation, oxidative stress, and impaired neuromuscular control may link diabetes with OA [3]. An epidemiological association between diabetes and OA has been confirmed in a recent meta-analysis of 49 studies [4]. However, there is little data of whether diabetes can influence OA incidence or accelerate OA progression. One study demonstrated a cross-sectional association between diabetes and severe knee pain and impaired knee function, and an increased prospective risk of arthroplasty in diabetic OA patients [5]. These findings cannot be considered conclusive, as there is uncertainty regarding the value of joint arthroplasty as an outcome that accurately reflects OA-related pain, limited function, and structural deterioration [6, 7]. Moreover, the use of joint replacement as an outcome can only be justified on a subset of patients with more advanced OA. In another study diabetes was only a predictor of joint space reduction in men with established knee OA [8]. Thus, it is still necessary to determine the independent effects of diabetes on OA-specific structural outcomes. Oral anti-diabetic drugs and insulin may also theoretically have an impact on OA progression and incidence [9, 10]. As these drugs are taken by the vast majority of diabetes patients nowadays [11] it seems neither clinically relevant nor feasible to separate the effects of diabetes per se on OA from those of anti-diabetic medications.
Therefore, we performed this study to test the hypothesis of whether there are prospective effects of medication-treated diabetes on knee OA incidence and progression in participants included in the Osteoarthritis Initiative cohort.
METHODS
Patients
For the current study, we used longitudinal data obtained from the Osteoarthritis Initiative (OAI), which is publically available at http://oai.epi-ucsf.org.
The Osteoarthritis Initiative cohort consists of a progression subcohort (patients with symptomatic tibiofemoral knee OA, n= 1390), an incidence subcohort (subjects with increased risk of OA, n = 3284) and a reference control subcohort (n=122). In this analysis we used the longitudinal data from both the progression and incidence subcohorts. The main inclusion criteria were the following: age between 45–79 years for both subcohorts, symptomatic tibiofemoral knee OA for the progression subcohort, and the presence of established or putative risk factors for incident knee OA for the incidence subcohort. The OAI subjects were recruited and enrolled between February 2004 and May 2006 at four recruitment centres in the USA. This study received ethical approval from each recruitment centre. All participants provided written informed consent. The prespecified sample size was 5000 women and men (4000 in the incidence subcohort, 800 in the progression subcohort). The sample size was expected to provide adequate numbers of knees with incident and worsening OA-related structural and clinical changes to achieve the primary aims of the OAI study.
The information about previously diagnosed diabetes was collected from the self-reported Charlson Comorbidity Index. A validity of treated diabetes self-report has been demonstrated in several studies [12, 13]. To further enhance the validity of the exposure in the current analysis, we defined diabetes status based both on self-reporting of treated diabetes and documented use of prescription oral anti-diabetic drugs and/or insulin 30 days prior to baseline. All currently used (past 30 days) prescription medications were captured using the medication inventory method wherein the participant brings in all the medications they are currently taking, and the brand name, generic name or active ingredients are recorded and matched to an entry in an online medication dictionary [14]. Participants with no information about diabetes, reported diabetes treated with diet/non-treated diabetes or who developed diabetes during the follow-up period were excluded from the analysis, as well as those participants who did not have at least two knee X-rays upon follow-up.
The initial Incidence and Progression OAI subcohort assignments were based primarily upon KL readings at the OAI clinical centers, but as assignment to Incidence and Progression Subcohorts may not precisely reflect a participant’s knee OA status, further central assessments could provide different grading results. Therefore, to study the effects of diabetes on the incidence of knee OA we selected participants who had both knees with either centrally confirmed KL<2 or KL2 without JSN from both Cohorts (incidence sample). In the selected participants all evaluable knees were analyzed. We analyzed all knees having baseline JSN<3 from both subcohorts. For the assessment of diabetes influence on knee OA progression we analyzed all knees having baseline JSN<3 from both subcohorts thus minimizing the risk of collider bias.
Clinical Measures
Height was measured in millimetres using a calibrated wall-mounted stadiometer. The measurement was performed twice in light clothing, without shoes, and during inspiration. Body weight was measured in kilograms with a calibrated standard balance beam scale. The measurement was performed twice in light clothing without shoes, heavy jewellery or wallets. BMI was calculated based on weight (in kg) divided by height (in cm) squared. We used the self-reported Western Ontario McMaster Osteoarthritis Index (WOMAC) (5 point Likert scale) for addressing pain and function to evaluate the severity of OA symptoms [15]. The possible range for pain was 0–20, for physical function - 0–68. Higher WOMAC scores represent more severe pain or reduction in function.
Smoking history and education status were assessed using self-administered questionnaire. Prior knee surgery, family history of knee replacement, and Physical Activity Scale for the Elderly (PASE) were evaluated using interview.
Radiographic assessment
Posteroanterior weight bearing knee radiographs were performed annually using a Synaflexer frame (Synarc, San Francisco, California, USA) allowing fixed standardised and reproducible knee position. X-ray interpretation was made centrally at Boston University by three readers. In case of a disagreement about the presence of radiographic OA (Kellgren and Lawrence grade 2 or greater) or disease worsening (defined either as an increase in Kellgren and Lawrence grade or as an increase in joint space narrowing grade), the reading was adjudicated by a panel of three readers. A consensus reading was achieved when at least two of the three readers agreed.
Case definitions
The cases of incident radiographic OA were defined as the new onset of a combination of joint space narrowing and KL grade 2 as suggested by Felson et al [16]. We defined the cases of knee OA progression as worsening in JSN score or a new knee replacement. The endpoints were assessed annually with a follow-up duration of 48 months.
Statistical analysis
Analyses were performed on the incidence and progression samples and further stratified by the gender. Continuous variables are presented as mean (standard deviation, SE), and categorical variables as number (percentage). Baseline differences were assessed using Student’s t-test for continuous and chi-squared test for categorical variables. Although most of the continuous variables were not normally distributed, we used mean (SE) and Student’s t-test as the preferred statistics even in the setting of non-normally distributed data [17].
We used logistic regression models to evaluate the relationship between the outcomes and medication-treated diabetes at study entry. Generalized estimating equations (GEE) were used to adjust for the correlation between knees. The models were adjusted for body mass index (BMI), age, race, gender, smoking history, education status, history of prior knee surgery, family history of knee replacement, Physical Activity Scale for the Elderly (PASE), and subcohort assignment. These potential confounders were selected based on literature data and clinical plausibility. The adjustment for the subcohort assignment was carried out as the inclusion criteria for both subcohorts were different. We did not adjust for baseline outcome variables as this approach has been shown to introduce bias [18]. In the progression sample we performed an additional adjustment for baseline KL score. The use of GEE allows one not to use imputation methods for the analysis as the participants with missing data are not excluded from the analysis [19].
Sensitivity analyses
The sensitivity analyses were performed with additional stratification by BMI subgroups (<25 kg/m2, 25–30 kg/m2, and >30 kg/m2), race (white, non-white) and using all self-reported diabetes (both treated and non-treated) as predictor.
GEE analyses were performed with the GEE package for R [20]. All analyses were made using R software, version 3.3.0.
RESULTS
Figure 1 shows a flow chart of study participants. We included 1863 participants, 1749 (93.88%) of whom were non-diabetic and 114 (6.12%) diabetic, in the incidence sample. There were 3725 analysed knees, 3498 (93.88%) non-diabetic and 228 (6.12%) diabetic. The progression sample consisted of 3443 participants, 3191 (92.68%) of whom were not diabetic, and 252 (7.32%) were diabetic. In the progression sample we analysed 3594 knees, 3335 (92.8%) of them were non-diabetic and 259 (7.2%) diabetic. The follow up time was four years.
Figure 1.
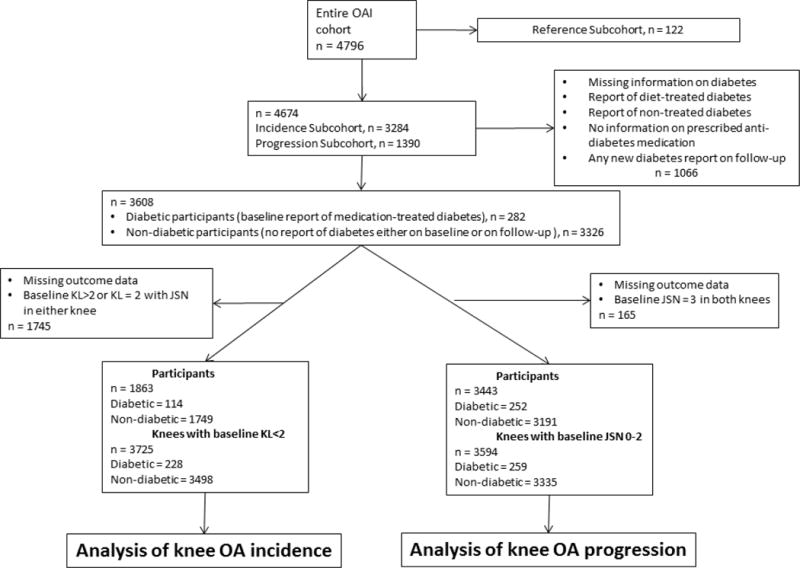
Flowchart of participants selected for the analyses.
The baseline characteristics of the studied groups are given on Table 1. At baseline, participants with diabetes from the incidence sample were older (by a mean of 2.95 years), had a higher BMI (by a mean of 3.73 kg/m2), were more likely to be non-white than white, had less frequent family history of knee replacement, were less educated, and had lower levels of physical activity. Diabetic participants had more knee pain and had impaired knee function as reflected by increased WOMAC pain and WOMAC function scores. Diabetic participants had higher rates of knee KL 1–2 rates grades on both knees.
Table 1.
Baseline characteristics of participants with and without medication-treated diabetes
| Participants with KL <2/KL = 2 without JSN at baseline n = 1863 n of knees = 3725 |
Participants with baseline JSN 0–2 n = 3443 n of knees = 3594 |
|||||
|---|---|---|---|---|---|---|
| Participants without diabetes | Participants with diabetes | p value | Participants without diabetes | Participants with diabetes | p value | |
| n | 1749 | 114 | 3191 | 252 | ||
| n of knees | 3498 | 228 | 3335 | 259 | ||
| Age (years) | 59.42 (8.79) | 62.37 (9.06) | < 0.001 | 61.08 (9.1) | 63.62 (8.99) | < 0.001 |
| Sex | 0.019 | 0.15 | ||||
| Female | 687 (39.28) | 58 (50.88) | 1314 (41.18) | 116 (46.03) | ||
| Male | 1062 (60.72) | 56 (49.12) | 1877 (58.82) | 136 (53.97) | ||
| BMI, kg/m2 | 27.35 (4.41) | 31.08 (4.41) | < 0.001 | 28.16 (4.59) | 31.96 (4.64) | < 0.001 |
| Race | < 0.001 | < 0.001 | ||||
| Other non-white | 15 (0.86) | 4 (3.51) | 35 (1.1) | 8 (3.17) | ||
| White or Caucasian | 1521 (87.11) | 65 (57.02) | 2712 (85.07) | 145 (57.54) | ||
| Black or African American | 196 (11.23) | 42 (36.84) | 420 (13.17) | 94 (37.3) | ||
| Asian | 14 (0.8) | 3 (2.63) | 21 (0.66) | 5 (1.98) | ||
| History of knee surgery | 215 (12.31) | 15 (13.16) | 0.9 | 707 (22.17) | 62 (24.7) | 0.4 |
| Family history of knee OA | 301 (17.43) | 7 (6.19) | 0.003 | 532 (16.81) | 25 (10.08) | 0.0075 |
| PASE | 170.56 (81.52) | 146.49 (82.01) | 0.002 | 164.94 (81.56) | 137.23 (74.85) | < 0.001 |
| Education | < 0.001 | < 0.001 | ||||
| Less than high school graduate | 29 (1.66) | 11 (9.65) | 72 (2.26) | 22 (8.73) | ||
| High school graduate | 191 (10.92) | 16 (14.04) | 363 (11.38) | 53 (21.03) | ||
| Some college | 355 (20.3) | 39 (34.21) | 675 (21.15) | 86 (34.13) | ||
| College graduate | 400 (22.87) | 20 (17.54) | 710 (22.25) | 40 (15.87) | ||
| Some graduate school | 153 (8.75) | 10 (8.77) | 271 (8.49) | 17 (6.75) | ||
| Graduate degree | 621 (35.51) | 18 (15.79) | 1100 (34.47) | 34 (13.49) | ||
| Smoking history | 0.28 | 0.07 | ||||
| Never | 955 (54.6) | 56 (49.12) | 1719 (53.87) | 125 (49.6) | ||
| Current | 97 (5.54) | 10 (8.77) | 174 (5.45) | 23 (9.13) | ||
| Former | 682 (38.99) | 47 (41.23) | 1275 (39.95) | 103 (40.87) | ||
| Current, but never regular | 5 (0.29) | 1 (0.88) | 5 (0.16) | 1 (0.4) | ||
| WOMAC subscales | ||||||
| WOMAC Pain, left knee | 1.57 (2.6) | 3.06 (4.24) | < 0.001 | 2.04 (3.08) | 3.55 (4.32) | < 0.001 |
| WOMAC Pain, right knee | 1.73 (2.5) | 3.05 (3.61) | < 0.001 | 2.15 (2.89) | 3.6 (3.87) | < 0.001 |
| WOMAC Function, left knee | 5.45 (8.76) | 9.42 (12.49) | < 0.001 | 7.17 (10.27) | 11.53 (13.4) | < 0.001 |
| WOMAC Function, right knee | 5.27 (7.91) | 9.87 (11.44) | < 0.001 | 6.82 (9.3) | 12.14 (12.39) | < 0.001 |
| KL, knees (%) | 0.0174 | 0.006 | ||||
| 0 | 2176 (62.21) | 120 (52.63) | 1229 (36.85) | 79 (30.5) | ||
| 1 | 843 (24.1) | 75 (32.89) | 633 (18.98) | 49 (18.92) | ||
| 2 | 477 (13.64) | 33 (14.47) | 976 (29.27) | 72 (27.8) | ||
| 3 | 0 | 0 | 497 (14.9) | 59 (22.78) | ||
| 4 | 0 | 0 | 0 (0) | 0 (0) | ||
| JSN, medial compartment, knees (%) | 0.2 | < 0.001 | ||||
| 0 | 3200 (91.48) | 201 (88.16) | 2149 (64.44) | 143 (55.21) | ||
| 1 | 296 (8.46) | 27 (11.84) | 811 (24.32) | 65 (25.1) | ||
| 2 | 0 (0) | 0 (0) | 375 (11.24) | 51(19.69) | ||
| 3 | 0 (0) | 0 (0) | ||||
| JSN, lateral compartment, knees (%) | 0.8 | 0.68 | ||||
| 0 | 3462 (98.97) | 225 (98.68) | 3068 (91.99) | 235 (90.73) | ||
| 1 | 34 (0.97) | 3 (1.32) | 142 (4.26) | 14 (5.41) | ||
| 2 | 0 (0) | 0 (0) | 125 (3.75) | 10 (3.86) | ||
| 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
Data are presented as the mean (SD) or n (%). BMI, Body Mass Index; KL, Kellgren and Lawrence grade, PASE, Physical Activity Scale for the Elderly; WOMAC, Western Ontario and McMaster Universities OA index; KL, Kellgren and Lawrence scale (grades 0–4), JSN, joint space narrowing (grades 0–3). P values were calculated using a two-sample t-test or Pearson’s chi-squared test. Possible ranges for WOMAC pain score are 0–20, possible ranges for WOMAC function score are 0–68.
There were similar baseline differences between diabetic and non-diabetic participants from the progression sample. Diabetics were older by a mean 2.54 and more obese with a mean BMI higher by 3.8 kg/m2. Unlike the incidence sample, no differences were found in gender and diabetics had higher rates of knees with JSN grades 2 and 3.
Table 2 shows the frequencies of the prescribed anti-diabetic drugs in the incidence and progression samples. The most commonly prescribed drug was metformin (more than half of the diabetic participants) followed by sulfonylureas, thiazolidinediones, insulins, meglitinides, and miglitol.
Table 2.
Prescribed anti-diabetic medications in incidence and progression samples at baseline.
| Incidence sample n = 114 |
Progression sample n = 252 |
|
|---|---|---|
| Metformin | 62 (54.39) | 163 (64.68) |
| Sulfonylureas | ||
| Glimepiride | 13 (11.4) | 28 (11.11) |
| Glipizide | 20 (17.54) | 50 (19.84) |
| Glyburide | 18 (15.79) | 44 (17.46) |
| Insulins | ||
| Insulin | 2 (1.75) | 4 (1.59) |
| Insulin Aspart | 0 (0) | 1 (0.4) |
| Insulin Glargine | 8 (7.02) | 14 (5.55) |
| Insulin Human | 8 (7.02) | 15 (5.95) |
| Insulin Isophane | 5 (4.39) | 10 (3.97) |
| Insulin Lispro | 4 (3.51) | 8 (3.17) |
| Insulin Protamine Lispro | 0 (0) | 1 (0.4) |
| Insulin Zinc | 0 (0) | 1 (0.4) |
| Alpha-glucosidase inhibitors | ||
| Miglitol | 1 (0.88) | 1 (0.4) |
| Meglitinides | ||
| Nateglinide | 1 (0.88) | 1 (0.4) |
| Repaglinide | 2 (1.75) | 6 (2.38) |
| Thiazolidinediones | ||
| Rosiglitazone | 21 (18.42) | 39 (15.48) |
| Pioglitazone | 16 (14.03) | 33 (13.1) |
The table shows number of participants (%)
The frequencies of outcomes are presented on table 3. The rates of incident OA approximated 5% while the progression rates were close to 20%. Knee joint replacement accounted for almost 15% of the progression cases.
Table 3.
The number (%) of joints with incidence or radiographic progression of knee OA
| Without treated diabetes | With treated diabetes | Total | |
|---|---|---|---|
| N of knees in incidence sample | 3498 | 228 | 3725 |
| Knee OA incidence | 181 (5.17) | 11 (4.8) | 192 (5.15) |
| N of knees in progression sample | 3335 | 259 | 3594 |
| Increase in JSN grade | 598 (17.9) | 41 (15.8) | 639 (17.8) |
| Knee replacement | 85 (2.55) | 6 (2.31) | 91 (2.53) |
| Progression of knee OA | 650 (19.5) | 46 (17.76) | 696 (19.4) |
Table 4 shows the results of unadjusted and adjusted GEE analyses of the association between medication-treated diabetes and knee OA incidence and progression.
Table 4.
Medication-treated diabetes as a predictor of knee OA incidence and radiographic progression.
| Unadjusted models | Final models* | Final models adjusted for baseline KL score | ||||
|---|---|---|---|---|---|---|
| Clinical and radiographic outcomes | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value |
| Total sample | ||||||
| Incident knee OA | 0,93 (0.45–1.91) | 0.84 | 0.53 (0.23–1.25) | 0.15 | – | – |
| Progression of knee OA | 0.9 (0.64–1.27) | 0.54 | 0.64 (0.44–0.94) | 0.02 | 0.66 (0.44 – 0.98) | 0.04 |
| Males | ||||||
| Incident knee OA | 1.15 (0.43–3.06) | 0.77 | 0.59 (0.18–1.97) | 0.39 | – | – |
| Progression of knee OA | 0.83 (0.46–1.48) | 0.52 | 0.59 (0.31–1.13) | 0.11 | 0.63 (0.31–1.25) | 0.19 |
| Females | ||||||
| Incident knee OA | 0.79 (0.27–2.3) | 0.66 | 0.5 (0.14–1.81) | 0.29 | – | – |
| Progression of knee OA | 0.97 (0.63–1.49) | 0.89 | 0.68 (0.42–1.09) | 0.11 | 0.68 (0.41–1.12) | 0.13 |
Statistically significant associations are shown in italics.
Adjusted for body mass index (BMI), age, race, gender, OAI subcohort assignment, smoking history, education status, history of prior knee surgery, family history for knee OA, Physical Activity Scale for the Elderly (PASE) at baseline
Diabetes was not associated with knee OA incidence in both crude and final models. There was no significant association of diabetes and knee OA progression in non-adjusted models. However, after adjustment for multiple covariates diabetes became associated with decreased risk of knee OA progression. This association remained significant after additional adjustment for baseline KL scores. In gender subgroups there were no significant associations between medication-treated diabetes and knee OA incidence and progression in both adjusted and non-adjusted models.
Sensitivity analyses
The numbers of participants in the sensitivity analyses are presented in Supplementary tables S1–S2. Only a small percentage (about 10%) of diabetes patients had normal weight. The amount of participants with all self-reported diabetes was approximately 20% higher than those participants with medication-treated diabetes. The results of the sensitivity analyses are shown in Supplementary tables S3–S4. In the sensitivity analyses there was no effect of either medication-treated or all self-reported diabetes on the incidence of knee OA. Medication treated diabetes was associated with a reduced risk of knee OA progression in the overweight participants in adjusted models and in obese participants in both crude and adjusted models. There was an association of all self-reported diabetes with knee OA progression in obese patients in both non-adjusted and final models.
DISCUSSION
In this study, we found that in an OAI cohort, medication-treated diabetes had no effect on knee OA incidence but was independently associated with decreased progression of knee OA.
The strengths of this analysis are that it is based on a large population with a well-defined cohort that incorporated participants with both early and advanced knee OA. The population-based design increases the generalizability of the study findings, and reduces the risk of selection bias. Another strength of this study is the use of standardised and reproducible procedures for the knee radiograph acquisition and extensive adjudication process to determine KL and JSN grades. In addition, the use of recently proposed definition of incident knee OA [16] probably reduced the risk of misclassification of incident knee OA cases.
Our study also has several limitations. The most important one is that we did not differentiate between the effects of diabetes and anti-diabetic medications. The information on diabetes came from participant self-reporting and the information about prescribed anti-diabetic medications. However, the accuracy of self-report of treated diabetes has been shown to be sufficient for use in epidemiological studies [12, 13]. We also did not differentiate between type 1 and type 2 diabetes and we could not assess the duration of diabetes and the level of diabetes control.
The results contradict findings by Schett who showed that type 2 diabetes predicted the development of severe OA defined as arthroplasty in an analysis of prospective follow up of the population-based Bruneck cohort [5]. As the authors point out, one explanation of their findings may be the different decision-making for arthroplasty in diabetic versus nondiabetic subjects. Theoretically the difference can also be explained by the characteristics of the studied cohorts. In the Bruneck cohort, 44.9% of participants at baseline had signs of polyneuropathy indicating complicated diabetes, and their median glycated haemoglobin was 7.2% [5], which can be seen as poor diabetes control in accordance with current standards [21]. The Bruneck study participants were recruited in 1990; a considerable time before the results of the pivotal UKPDS study were published, which led to changes in the diabetes treatment paradigm towards more stringent glycaemic control [22]. Although the current study had no information on glycated haemoglobin levels, it can be speculated that in the pre-UKPDS era the less aggressively treated diabetes had a different impact on OA severity as opposed to the diabetes treated after the introduction of new treatment guidelines.
In contrast with findings of Eymard et al. who showed joint space reduction in diabetic men with established knee OA [8] we observed an independent association between treated diabetes and decreased knee OA progression. In comparison with the data by Eymard et al. our study has a larger sample size and larger proportion of subjects with diabetes. The characteristics of diabetic patients in the study by Eymard et al. and in this analysis differ in several ways. First, we analysed a sample of participants with medication-treated diabetes as opposed to Eymard et al. who did not specify what proportion of their patients had medication-treated diabetes or were only treated with lifestyle modifications. Based on general population data [23], it may be suggested that up to 14% of patients were not treated with drugs. Thus, the differences in the medication status of the diabetes patients could significantly influence the results. Apart from their glucose lowering action, oral anti-diabetic drugs and insulin have been shown to have pleiotropic properties including anti-inflammatory [24, 25] and cartilage-protective effects [9, 10]. For example, the most commonly used anti-diabetic drug metformin reduces inflammation, decreases the number of Th17 cells and increases the number of Treg cells in a mice model of collagen-induced arthritis [26]. Therefore, it could be hypothesized that medications used to treat diabetes attenuate the deleterious effects of diabetes on the knee OA incidence and may even lead to decreased progression of knee OA.
In addition, the participants from the study by Eymard et al. were Caucasian while in our study a significant proportion (36–37% in the diabetes groups) were African-American. Whether ethnic differences in diabetes genetics and pathophysiology can explain the contradictory results to be tested in future studies. The ethnic composition of our study may also limit the external validity of our findings.
Our findings are in line with recently published results of Singapore Chinese Health Study, a prospective cohort of 63257 Chinese men and women, which showed an inverse association of diabetes and the risk of total knee replacement thus suggesting a protective effect of diabetes and/or anti-diabetic medications on the knee OA [27]. Our results also concur with the findings of studies from South Korea and Sweden, showing no association between metabolic syndrome and radiographic knee OA [28, 29].
The finding of decreased knee OA progression in patients with medication-treated diabetes only after adjustment for multiple covariates may indicate that in crude analysis this effect was masked by stronger associated risk factors such as obesity.
A lack of diabetes effect on knee OA incidence is supported with the results of the sensitivity analysis, with no significant relationship shown. The findings of protective effect of diabetes on knee OA progression only in the overweight and obese subgroups may be due to the smaller sample sizes of the group of diabetic patients with normal weight. Another explanation may be different treatment approaches used in overweight/obese vs. normal weight diabetic patients. A lack of diabetes effect on knee OA progression in gender and race subgroups may be explained by the reduction of the statistical power resulting from the stratification.
Our findings are far from being conclusive but they indicate that, in general, treated diabetes does not have an impact on OA incidence. It should be pointed out that the diabetic group in this study probably represents a population of patients with different type of diabetes, different levels of diabetes control, and different diabetes treatment regimens. Recently, diabetes appeared as a highly heterogeneous disease with diverse genetic background resulting in various phenotypes [30]. Therefore, there still may be an opportunity that in subgroups of patients with particular phenotype of diabetes, poor diabetes control or taking particular anti-diabetic drugs there is a meaningful link between diabetes and OA incidence.
At this time, the clinical significance of our findings is uncertain. Further investigation of cartilage protective and anti-inflammatory effects of anti-diabetic drugs may probably lead to the discovery of new approaches to OA treatment.
In conclusion, in OAI participants, medication-treated diabetes was not associated with the incidence of knee OA but independently reduced knee OA progression. The underlying mechanisms of these findings need to be further investigated.
Supplementary Material
Acknowledgments
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
We would like to acknowledge Jonathan McFarland for editing the manuscript.
Funding: the analysis presented in this paper had no funding
FUNDING
None
Footnotes
Compliance with ethical standards
Conflict of interest: all authors declare that they have no conflict of interest
CONTRIBUTIONS
All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data (2) drafting the article or revising it critically for important intellectual content (3) final approval of the version to be submitted. Ivan Shirinsky takes responsibility for the integrity of the work as a whole, from inception to finished article.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
References
- 1.Kadam UT, Croft PR. Clinical comorbidity in osteoarthritis: associations with physical function in older patients in family practice. Journal of Rheumatology. 2007;34:1899–1904. [PubMed] [Google Scholar]
- 2.Zullig LL, Bosworth HB, Jeffreys AS, Corsino L, Coffman CJ, Oddone EZ, Yancy WS, Jr, Allen KD. The association of comorbid conditions with patient-reported outcomes in Veterans with hip and knee osteoarthritis. Clinical Rheumatology. 2014 doi: 10.1007/s10067-014-2707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Annals of the Rheumatic Diseases. 2011;70:1354–1356. doi: 10.1136/ard.2010.146399. [DOI] [PubMed] [Google Scholar]
- 4.Louati K, Vidal C, Berenbaum F, Sellam J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open. 2015;1:e000077. doi: 10.1136/rmdopen-2015-000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schett G, Kleyer A, Perricone C, Sahinbegovic E, Iagnocco A, Zwerina J, Lorenzini R, Aschenbrenner F, Berenbaum F, D’Agostino MA, Willeit J, Kiechl S. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care. 2013;36:403–409. doi: 10.2337/dc12-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altman RD, Abadie E, Avouac B, Bouvenot G, Branco J, Bruyere O, Calvo G, Devogelaer JP, Dreiser RL, Herrero-Beaumont G, Kahan A, Kreutz G, Laslop A, Lemmel EM, Menkes CJ, Pavelka K, Van De Putte L, Vanhaelst L, Reginster JY. Total joint replacement of hip or knee as an outcome measure for structure modifying trials in osteoarthritis. Osteoarthritis and Cartilage. 2005;13:13–19. doi: 10.1016/j.joca.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Gossec L, Paternotte S, Maillefert JF, Combescure C, Conaghan PG, Davis AM, Gunther K-P, Hawker G, Hochberg M, Katz JN. The role of pain and functional impairment in the decision to recommend total joint replacement in hip and knee osteoarthritis: an international cross-sectional study of 1909 patients. Report of the OARSI-OMERACT Task Force on total joint replacement. Osteoarthritis and Cartilage. 2011;19:147–154. doi: 10.1016/j.joca.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eymard F, Parsons C, Edwards MH, Petit-Dop F, Reginster JY, Bruyere O, Richette P, Cooper C, Chevalier X. Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthritis and Cartilage. 2015;23:851–859. doi: 10.1016/j.joca.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi T, Notoya K, Naito T, Unno S, Nakamura A, Martel-Pelletier J, Pelletier JP. Pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, reduces the progression of experimental osteoarthritis in guinea pigs. Arthritis Rheum. 2005;52:479–487. doi: 10.1002/art.20792. [DOI] [PubMed] [Google Scholar]
- 10.Cai L, Okumu FW, Cleland JL, Beresini M, Hogue D, Lin Z, Filvaroff EH. A slow release formulation of insulin as a treatment for osteoarthritis. Osteoarthritis and Cartilage. 2002;10:692–706. doi: 10.1053/joca.2002.0813. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. 2012 [Google Scholar]
- 12.Margolis KL, Lihong Q, Brzyski R, Bonds DE, Howard BV, Kempainen S, Simin L, Robinson JG, Safford MM, Tinker LT, Phillips LS. Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5:240–247. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider AL, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the atherosclerosis risk in communities study. American Journal of Epidemiology. 2012;176:738–743. doi: 10.1093/aje/kws156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. European Journal of Epidemiology. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 15.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 16.Felson DT, Niu J, Guermazi A, Sack B, Aliabadi P. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Annals of the Rheumatic Diseases. 2011;70:1884–1886. doi: 10.1136/ard.2011.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lydersen S. Statistical review: frequently given comments. Annals of the Rheumatic Diseases. 2015;74:323–325. doi: 10.1136/annrheumdis-2014-206186. [DOI] [PubMed] [Google Scholar]
- 18.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. American Journal of Epidemiology. 2005;162:267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 19.Twisk J, de Vente W. Attrition in longitudinal studies. How to deal with missing data. Journal of Clinical Epidemiology. 2002;55:329–337. doi: 10.1016/s0895-4356(01)00476-0. [DOI] [PubMed] [Google Scholar]
- 20.Carey VJ. Generalized Estimation Equation Solver. R package version. 2015;4:13–19. [Google Scholar]
- 21.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Home PD. Impact of the UKPDS–an overview. Diabetic Medicine. 2008;25(Suppl 2):2–8. doi: 10.1111/j.1464-5491.2008.02501.x. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 24.Cameron AR, Morrison VL, Levin D, Mohan M, Forteath C, Beall C, McNeilly AD, Balfour DJ, Savinko T, Wong AK, Viollet B, Sakamoto K, Fagerholm SC, Foretz M, Lang CC, Rena G. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circulation Research. 2016;119:652–665. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heliovaara MK, Herz M, Teppo AM, Leinonen E, Ebeling P. Pioglitazone has anti-inflammatory effects in patients with Type 2 diabetes. Journal of Endocrinological Investigation. 2007;30:292–297. doi: 10.1007/BF03346296. [DOI] [PubMed] [Google Scholar]
- 26.Son HJ, Lee J, Lee SY, Kim EK, Park MJ, Kim KW, Park SH, Cho ML. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators of Inflammation. 2014;2014:973986. doi: 10.1155/2014/973986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung YY, Allen JC, Ang LW, Yuan JM, Koh WP. Diabetes mellitus and the risk of total knee replacement among Chinese in Singapore, the Singapore Chinese Health Study. Sci Rep. 2017;7:40671. doi: 10.1038/srep40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin D. Association between metabolic syndrome, radiographic knee osteoarthritis, and intensity of knee pain: results of a national survey. Journal of Clinical Endocrinology and Metabolism. 2014;99:3177–3183. doi: 10.1210/jc.2014-1043. [DOI] [PubMed] [Google Scholar]
- 29.Engstrom G, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Lohmander LS. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis and Cartilage. 2009;17:168–173. doi: 10.1016/j.joca.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet. 2014;383:1084–1094. doi: 10.1016/S0140-6736(13)62219-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


