Abstract
Hypercholesterolemia, particularly an increase in low-density lipoprotein cholesterol (LDL-C) levels, contributes substantially to the development of coronary artery disease and the risk for cardiovascular events. As the first-line pharmacotherapy, statins have been shown to reduce both LDL-C levels and cardiovascular events. However, despite intensive statin therapy, a sizable proportion of statin-treated patients are unable to achieve the recommended target LDL-C levels, and not all patients can avoid future cardiovascular events. Proprotein convertase subtilisin/kexin type 9 (PCSK9) plays a key role in cholesterol homeostasis by enhancing the degradation of hepatic low-density lipoprotein receptor (LDLR). Owing to its importance in lipid metabolism, PCSK9 has emerged as a novel pharmacological target for lowering LDL-C levels. In this review, the potential role of circulating PCSK9 as a new biomarker of lipid metabolism is described. Next, previous studies evaluating the effects of lipid-modifying pharmacological agents, particularly statins, on circulating PCSK9 concentrations are summarized. Statins decrease hepatic intracellular cholesterol, resulting in increased LDLRs as well as increased PCSK9 protein. There is a clear dose-response effect of statin treatment on PCSK9 level, as increasing doses of statins also increase the level of circulating PCSK9. Finally, the available therapeutic strategies to inhibit PCSK9 are present. Monoclonal antibodies against PCSK9, in combination with statins, are one of the most promising and novel approaches to achieve further reduction of LDL-C levels and reduce the risk of cardiovascular events.
Keywords: Familial hypercholesterolemia (FH), Low-density lipoprotein cholesterol (LDL-C), Low-density lipoprotein receptor (LDLR), Proprotein convertase subtilisin/kexin type 9 (PCSK9), Statin
Introduction
Hypercholesterolemia, particularly an increase in low-density lipoprotein cholesterol (LDL-C) levels, contributes substantially to the development of coronary artery disease (CAD) and the risk for cardiovascular events. Statins represent the first-line pharmacotherapy for hypercholesterolemia, having been shown to reduce both LDL-C levels and cardiovascular events1). However, a considerable number of statintreated patients do not achieve the recommended target LDL-C levels, despite intensive statin therapy, and some go on to develop cardiovascular events and atherosclerosis progression2).
Proprotein convertase subtilisin/kexin type 9 (PCSK9), also known as neural apoptosis-regulated convertase 1 (NARC-1), was first described in 20033). The human PCSK9 gene is located on the small arm of chromosome 1p32 and contains 12 exons that encode 692 amino acids4). PCSK9 is mainly secreted by the liver, but it is also highly expressed in the intestine and kidney. PCSK9 comprises a signal peptide (1–30 amino acids), a prodomain (amino acids 31–152), a catalytic domain (amino acids 153–421) and a C-terminal domain (amino acids 422–692) (Fig. 1). ProPCSK9 is a protein of 75 kDa, and following autocatalytic cleavage in the endoplasmic reticulum, the prodomain is separated from the 62 kDa mature PCSK9. Mature PCSK9 is secreted together with the prodomain, thus forming a prosegment-PCSK9 complex that forces the PCSK9 catalytic domain into an inactive conformation4). PCSK9 enhances the endosomal and lysosomal degradation of hepatic low-density lipoprotein receptor (LDLR), resulting in increased serum LDL-C concentrations5, 6). Thus, PCSK9 is a key regulator of serum LDL-C levels7). Genetic variants of PCSK9 affect circulating PCSK9 concentrations8) as well as plasma LDL-C levels9). Furthermore, gain-of-function mutations in PCSK9 result in familial hypercholesterolemia (FH), a genetic disease characterized by greatly increased levels of LDL-C10, 11), whereas loss-of-function mutations of PCSK9 are associated with significantly decreased serum LDL-C levels12) and an approximately 80–90% reduction in cardiovascular disease13). To date, several ELISA-based methods have been developed to measure circulating PCSK9 concentrations14–20).
Fig. 1.
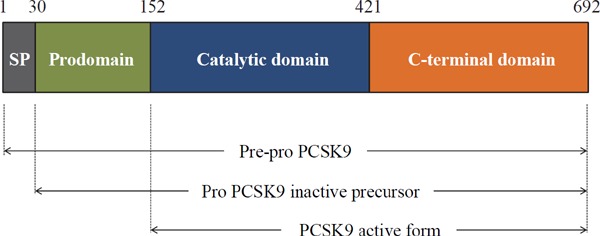
PCSK9 protein structure
PCSK9 comprises a signal peptide (1–30 amino acids), a prodomain (amino acids 31–152), a catalytic domain (amino acids 153–421) and a C-terminal domain (amino acids 422–692). The molecular weight of proPCSK9 is 75 kDa, and the mature form is 62 kDa. Following autocatalytic cleavage in the endoplasmic reticulum, the prodomain is separated from the 62 kDa mature PCSK9 protein and both are secreted following the formation of a prosegment-PCSK9 complex.
SP, signal peptide; PCSK9, proprotein convertase subtilisin/kexin type 9.
In this review, the potential role of PCSK9 as a new biomarker of lipid metabolism is described. Next, previous studies evaluating the effects of lipid-modifying pharmacological agents, particularly statins, on circulating PCSK9 concentrations are summarized, and finally, data are presented on PCSK9 inhibition as a novel approach to the treatment of hypercholesterolemia.
PCSK9 Concentrations and LDL-C Levels
Several groups have reported a correlation between circulating PCSK9 concentration and LDL-C level14–16, 21, 22). Furthermore, plasma PCSK9 concentration has been shown to positively correlate with the LDL-apolipoprotein (apo) B100 fractional catabolic rate, suggesting that PCSK9 is a marker of LDL catabolism23). However, the correlation between PCSK9 and LDL-C level has shown to be less significant than expected, with several factors potentially associated with this observation. First, the serum PCSK9 level does not reflect total hepatic PCSK9 secretion, as the high levels of PCSK9 are cleared from circulation by binding to hepatic LDLRs. However, the mechanism by which PCSK9 is cleared from the circulation is not fully understood, as Cameron et al. have reported that plasma PCSK9 is cleared by an LDLR-independent mechanism24). Second, circulating PCSK9 is present not only in its free form, but is also as a complex with apoB-containing lipoproteins25). Furthermore, PCSK9 directly increases hepatic production of apoB-containing lipoproteins7). Third, among several ELISAs that have been developed to measure PCSK9 concentration14–20), it remains unclear which form of PCSK9 is detected by each assay, except for two reports of the detection of both mature and furin-cleaved PCSK914, 17). Moreover, PCSK9 concentrations varied widely between different assays (80–4000 ng/mL). Finally, PCSK9 concentrations are reduced with fasting (up to 58% lower following 36 h of fasting26, 27). Despite wide fluctuations in plasma PCSK9 concentrations over the course of a day, however, little diurnal variation in plasma LDL-C levels has been reported. Thus, additional factors may contribute to the relationship between circulating PCSK9 and LDL-C levels.
Lipid-Modifying Pharmacological Agents and PCSK9 Concentrations
Statins
Statins, HMG-CoA reductase inhibitors, are the most commonly prescribed class of LDL-C-lowering drugs, although they have several limitations. One limitation is that a linear dose-dependent LDL-C lowering effect is not seen with statin use28), and another is termed the “stain escape phenomenon,” whereby LDL-C levels are reported to increase following prolonged statin therapy29). Statins decrease hepatic intracellular cholesterol resulting in increased nuclear translocation of sterol-regulatory element binding protein-2 (SREBP-2), which activates LDLRs as well as PCSK9 gene expression30–32) and increases circulating PCSK9 levels14–16, 33) (Fig. 2). As might be expected, both PCSK9 and LDLR levels are increased by statin therapy34). The increased PCSK9 binds to LDLR and directs it toward lysosomal degradation rather than to regular recycling4, 35), and thus has the potential to limit the efficacy of statin-induced LDL-C reduction30, 36). These observations explain the “rule of 6%” for statins, whereby each doubling of the statin dose results only in an approximately 6% additional reduction in LDL-C level. Given this limitation of statin therapy, PCSK9 inhibition represents a logical strategy to enhance statin-induced LDL-C reduction34).
Fig. 2.
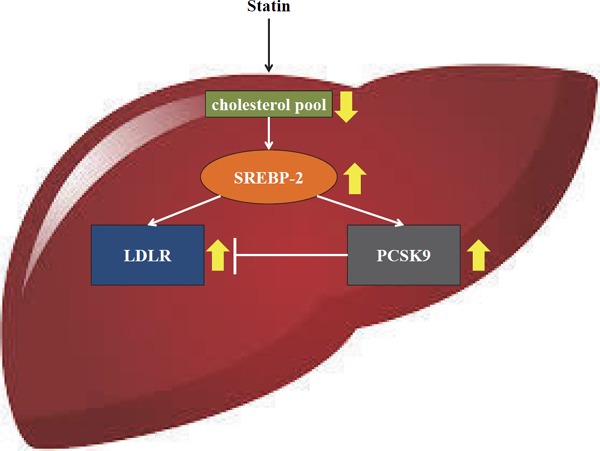
SREBP-2-mediated co-expression of LDLR and PCSK9
Statin-induced reduction of the cholesterol pool in hepatocyte activates SREBP-2-medicated LDLR expression, thereby increasing hepatic LDL-C uptake. Concurrently, SREBP-2 up-regulates the expression of PCSK9 and enhances hepatic LDLR degradation. Thus, SREBP-2-mediated co-expression of LDLR and PCSK9 prevent excessive cholesterol uptake in hepatocytes in order to preserve cholesterol homeostasis.
SREBP-2, sterol-regulatory element binding protein-2; LDLR, low-density lipoprotein receptor; PCSK9, proprotein convertase subtilisin/kexin type 9.
The effect of statin therapy on circulating PCSK9 concentration is summarized in Table 1. Mayne et al. reported that 6 weeks of treatment with 10 mg of atorvastatin significantly increased plasma PCSK9 by 7.4%37). Costet et al. also reported that 10 mg of atorvastatin treatment increased PCSK9 by 14% at 6 weeks38). Guo et al. reported that atorvastatin 10 mg slightly increased serum PCSK9 by 5–7%, although the effect was not significant, while atorvastatin 20 mg significantly increased serum PCSK9 by 35% at 8 weeks39). Careskey et al. reported that 16 weeks of treatment with 40 mg of atorvastatin reduced LDL-C levels by 42%, with a significant 34% increase in serum PCSK9 levels15). Welder et al. reported that 80 mg of atorvastatin increased PCSK9 levels by 47% and decreased LDL-C levels by 55%33). Finally, Khera et al. reported that 16 weeks of treatment with 10 mg and 80 mg atorvastatin increased circulating PCSK9 levels by 19% and 27%, respectively40). These results suggest a clear dose-response effect of atorvastatin on PCSK9 levels, with higher doses of atorvastatin causing a greater increase in circulating PCSK9 concentrations. However, the effects of other types of statins on circulating PCSK9 levels have been less evaluated. Lakoski et al. demonstrated that 10 mg of simvastatin for 6 weeks did not increase circulating PSCK9 levels21), while Berthold et al. reported that 40 mg of simvastatin treatment for 14 days increased circulating PCSK9 concentrations by 68%41). In addition, Khera et al. reported that 16 weeks of treatment with simvastatin 20 mg increased circulating PCSK9 levels by 13% (not statistically significant), whereas simvastatin 80 mg significantly increased PCSK9 levels by 41% in the same period40). Awan et al. reported that 20 mg of rosuvastatin increased PCSK9 by 35% in women and 28% in men after 1 year of treatment22), while Raal et al. reported that 40 mg of rosuvastatin for 4 weeks increased PCSK9 levels by 37% in heterozygous FH42). Recently, we reported that 4 mg of pitavastatin and 20 mg of pravastatin significantly increased serum PCSK9 levels by 31% and 34%, respectively43). A recent metaanalysis demonstrated that a greater PCSK9 elevation was observed with lipophilic statins (atorvastatin, simvastatin, pitavastatin, and fluvastatin) compared with hydrophilic statins (rosuvastatin and pravastatin)44). Taken together, each type of statins appears to cause an increase in plasma PCSK9 concentration, although the extent of the increase in circulating PCSK9 may be dependent on the dose and the liphophilic or hydrophilic natures of the statin.
Table 1. Effect of statins on circulating PCSK9 levels.
| Types of statin | Dose (mg/day) | Duration of treatment | % change in PCSK9 | Author (reference) |
|---|---|---|---|---|
| Atorvastatin | 10 | 1 day | 13% | Guo (39) |
| 10 | 1 day | 24% | Costet (38) | |
| 10 | 4 weeks | 5.70% | Guo (39) | |
| 10 | 6 weeks | 7.40% | Mayne (37) | |
| 10 | 6 weeks | 14% | Costet (38) | |
| 10 | 8 weeks | 7.20% | Guo (39) | |
| 10 | 16 weeks | 19% | Khera (40) | |
| 20 | 4 weeks | 30% | Guo (39) | |
| 20 | 8 weeks | 35% | Guo (39) | |
| 20 | 8 weeks | 39% | Guo (98) | |
| 40 | 12 weeks | 38% | Tremblay (99) | |
| 40 | 16 weeks | 34% | Careskey (15) | |
| 80 | 1 day | 27% | Guo (39) | |
| 80 | 4 weeks | 37% | Raal (42) | |
| 80 | 16 weeks | 27% | Khera (40) | |
| 80 | 16 weeks | 47% | Welder (33) | |
| Simvastatin | 10 | 6 weeks | no change | Lakoski (21) |
| 20 | 16 weeks | 13% | Khera (40) | |
| 40 | 14 days | 68% | Berthold (41) | |
| 80 | 16 weeks | 41% | Khera (40) | |
| Rosuvastatin | 20 | 1 year | 28–35% | Awan (22) |
| 40 | 4 weeks | 37% | Raal (42) | |
| Pitavastatin | 4 | 8 months | 31% | Nozue (43) |
| Pravastatin | 20 | 8 months | 34% | Nozue (43) |
PCSK9; proprotein convertase subtilisin/kexin type 9.
It is important to understand the rapidity with which statins increase PCSK9 levels, or whether this effect is sustained over time. Welder et al. reported that serum PCSK9 levels increased by 47% after 4 weeks of 80 mg atorvastatin, and this increase was sustained at 8, 12, and 16 weeks. They concluded that high-dose atorvastatin caused a rapid, sustained increase in serum PCSK933). Guo et al. also reported that atorvastatin 10 mg showed a tendency to increase PCSK9 levels but this effect was not statistically significant, although atorvastatin 20 mg significantly increased serum PCSK9 by 30% at 4 weeks and by 35% at 8 weeks39). They further investigated the rapidity of atorvastatin treatment on PCSK9 level, and found that serum PCSK9 rapidly increased by 13% following 24 h treatment with atorvastatin 10 mg and by 27% with atorvastatin 80 mg39). Thus, they concluded that the short-term impact of low-dose atorvastatin on PCSK9 level was time and dose dependent, with a rapid increase in PCSK9 level observed within 24 h. This indicates that, although atorvastatin may up-regulate both the LDLR and PCSK9 genes by activating SREBP-2, the PCSK9 gene response to atorvastatin might be faster or more sensitive – or more dose-dependent – than the LDLR gene. By comparison, Okada et al. reported that plasma PCSK9 levels were significantly increased at 12 weeks of statin therapy, although this increase was not sustained and was lower at 52 weeks45). Therefore, increases in serum PCSK9 levels caused by statin therapy appear to differ over short-, middle-, and long-term periods. Statins likely increase the expression and secretion of the PCSK9 protein to such an extent that circulating PCSK9 levels exceed LDLR binding capacity, resulting in increased PCSK9 protein being detected in the serum at an early stage of treatment46). However, the increased PCSK9 protein subsequently binds to LDLR and this complex is cleared from the circulation. Thus, circulating PCSK9 may reach a plateau concentration in the long term.
More importantly, statin treatment completely abolishes the correlation between PCSK9 and plasma LDL-C level, restricting its putative usefulness as a biomarker of lipid metabolism in routine clinical practice33, 47). It is therefore relevant to consider whether baseline circulating PCSK9 levels can predict the efficacy of statins on LDL-C reduction. At least three studies have addressed this question, leading to discrepant results14, 21, 33). First, Dubuc et al. observed a significant positive correlation between baseline plasma PCSK9 level and the percentage reduction in LDL-C after statin therapy14). Welder et al. reported a modest relationship between baseline PCSK9 level and change in LDL-C, with a relatively higher baseline PCSK9 level tending to be associated with a numerically greater decrease in LDL-C; however, this correlation did not achieve statistical significance33). Finally, another study failed to show a significant relationship between baseline PCSK9 level and response to LDL-lowering therapy21). Additional randomized studies with higher doses and other types of statins are therefore needed to explicitly address this question.
Another question is whether the statin-induced increase in PCSK9 correlates with the magnitude of statin-induced reduction in LDL-C level. Awan et al. observed a significant relationship between LDL-C reduction and increased PCSK9 concentration in response to statin therapy22). Berthold et al. also reported that increased PCSK9 was inversely correlated with the percentage change in LDL-C concentration41). Welder et al. showed a trend toward an inverse correlation between percentage change in PCSK9 level and in LDL-C level, although this trend did not achieve statistical significance33). However, Careskey et al. observed no significant relationship between increased PCSK9 level and reduced LDL-C after 4 months of treatment with 40 mg atorvastatin15). Lakoski et al. also reported that changes in plasma PCSK9 level did not correlate with the magnitude of the LDL-C lowering response achieved with 10 mg of simvastatin21). Consistent with these reports, we previously showed that the percentage change in PCSK9 level did not correlate with the LDL-C reduction observed after statins therapy48). Furthermore, although the reduction in LDL-C was greater with pitavastatin than with pravastatin, the level of increase in PCSK9 did not differ between these statins48). Therefore, it should be noted that the serum PCSK9 level might not reflect the total statin-induced increase in hepatic PCSK9 secretion, as higher levels of PCSK9 bind to hepatic LDLRs and are subsequently removed from the circulation. This likely explains why statininduced increases in serum PCSK9 levels might not reflect the full extent of the modulation of hepatic synthesis and secretion of the PCSK9 protein by statins.
Ezetimibe
Another commonly prescribed LDL-C-lowering drug is ezetimibe, a cholesterol absorption inhibitor that potently inhibits the absorption of biliary and dietary cholesterol by binding the cholesterol transport protein Niemann-Pick C1-Like 149). Ezetimibe lowers hepatic cholesterol levels by decreasing the amount of cholesterol supplied to the liver through intestinal uptake, leading to increased hepatic LDLR expression, which results in increased LDL uptake from the plasma and thus decreased circulating LDL-C levels49, 50). LDL-C reductions of approximately 21% and 23% were achieved by ezetimibe monotherapy and combination therapy with statins, respectively51). Compared with statins, fewer studies have been reported on the effect of ezetimibe (alone or combined with statins) on PCSK9 levels. In a pre-clinical study, ezetimibe therapy alone for 7 months significantly increased plasma PCSK9 levels by 37% in dyslipidemic monkeys52). Davignon et al. showed that ezetimibe added to statin therapy further increased PCSK9 levels beyond the increases observed with a statin alone53). Dubuc et al. also reported that treatment with ezetimibe in combination with statins was associated with a significant increase in plasma PCSK9 levels compared with statin therapy alone14). Berthold et al. investigated the effect of ezetimibe and simvastatin, alone and in combination, on circulating PCSK9 concentrations in healthy men. They found that ezetimibe treatment alone for 2 weeks increased plasma PCSK9 concentrations by 10%, although this increase was not significant41). Furthermore, when ezetimibe was added to 40 mg of simvastatin, an incremental increase in PCSK9 concentration was not observed. Lakoski et al. reported 6 weeks of ezetimibe monotherapy had no significant effect on PCSK9 concentration, although LDL-C levels were lowered by 20%21). Miyoshi et al. also reported that PCSK9 concentrations were unchanged following ezetimibe monotherapy or in combination with strong statins for 24 weeks54). Thus, although there are some discrepancies regarding the effect of ezetimibe on circulating PCSK9 levels, a recent metaanalysis reported that there was no significant elevation in plasma PCSK9 levels with statin/ezetimibe combination therapy compared with statin monotherapy44). There are a number of potential explanations for these discrepancies. First, these differences may be related to the study period, as increases in serum PCSK9 levels caused by statin therapy differ according to treatment duration. Indeed, Okada et al. reported that ezetimibe as add-on to stain therapy had no effect on plasma PCSK9 levels at 12 weeks, but that PCSK9 was significantly increased at 52 weeks45). Second, statin treatment maximally increases circulating PCSK9 to a plateau concentration, after which no further increase is possible by further lowering of LDL-C using ezetimibe. Third, statins upregulate peroxisome proliferator-activated receptor (PPAR)-α, β, γ, and δ, which are involved in the regulation of PCSK9 expression in the liver55, 56). Finally, ezetimibe might be unable to increase PCSK9 concentrations because of its weak LDL-C-lowering effect.
Fibrates
Activators of PPAR-α, fibrates are the next most commonly prescribed class of lipid-lowering drugs. Following its activation by fibrates, PPAR-α alters the transcription of multiple target genes that play a key role in lipid metabolism. Fibrates thus reduce plasma levels of triglycerides by approximately 30–50% and increase high-density lipoprotein cholesterol levels by 5–15%. Unlike statin treatment, the effect of fibrate treatment on LDL-C and PCSK9 is less clear. Kourimate et al. demonstrated that fibrate treatment reduced PCSK9 mRNA levels and PCSK9 protein expression in hepatocytes57). Post-hoc analysis of a subgroup of the FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) study indicated that 6-week treatment with 200 mg of fenofibrate moderately decreased plasma PCSK9 concentrations by 8.5% in patients with type 2 diabetes19). Chan et al. also reported that 12-week treatment with fenofibrate 145 mg decreased serum PCSK9 concentrations by 13% in patients with type 2 diabetes who were treated with statins58). However, in contrast to these results, Mayne et al. reported that 24-week treatment with fibrates increased serum PCSK9 levels by 17%37), while Costet et al. reported that 6-week treatment with fenofibrate 160 mg increased PCSK9 by 26%38). Troutt et al. reported that fenofibrate (200 mg for 12 weeks) significantly increased circulating PCSK9 levels by 25%59). The precise reasons for these discrepancies in the effect of fibrate on circulating PCSK9 concentrations and the mechanisms of fibrate-induced changes in PCSK9 remain unclear. Noguchi et al. compared the effects of bezafibrate (400 mg), a pan-agonist for PPAR-α, β, and δ with fenofibrate (200 mg), a more selective ligand for PPAR-α, on plasma PCSK9 concentrations60). They reported that plasma PCSK9 concentrations at 8 weeks were significantly increased by 39.7% for bezafibrate and 66.8% for fenofibrate. Thus, the effect of fibrate on circulating PCSK9 may differ according to type of fibrate. Additional studies are therefore required to better understand the mechanism by which fibrate induces changes in PCSK9 concentrations.
Nicotinic Acid (Niacin)
Nicotinic acid (niacin) has been used clinically as an LDL-C lowering drug for more than 50 years, although the mechanism behind its LDL-C lowering effect remains unclear. Khera et al. reported that one year of treatment with a combination of simvastatin 20 mg and niacin decreased PCSK9 levels by 13%, suggesting that niacin offsets the statin-mediated increase in PCSK940). Furthermore, niacin decreased PCSK9 levels by 17% in patients treated with both atorvastatin and fenofibrate. This reduction was significantly positively correlated with LDL-C reduction (r = 0.62, p = 0.006). Thus, the LDL-C reduction observed with niacin therapy may be due in part to a reduction in PCSK9.
Inhibitors of PCSK9
Several therapeutic approaches to inhibit PCSK9 are summarized in Table 2. These approaches include blocking the binding of PCSK9 to LDLR using antibodies, adnectins, or mimetic peptides; inhibiting PCSK9 expression with CRISPR/Cas9 genome-editing technology, antisense oligonucleotides (ASOs), or small interfering RNA (siRNA); and interfering with PCSK9 secretion from the endoplasmic reticulum.
Table 2. A new approach to PCSK9-targeting drugs.
| Mechanisms | Representative agents |
|---|---|
| Blocking the combination of PCSK9 and LDLR | Mimetic peptides |
| Adnectins | |
| Monoclonal antibodies (alirocumab, evolocumab, bococizumab) | |
| Inhibiting PCSK9 expression | CRISPR/Cas9 technology |
| Small molecule (berberine, oleanoic acid) | |
| Antisense oligonucleotides | |
| Small interfering RNA | |
| Interfering with PCSK9 secretion | Sortilin |
| Sec24a | |
PCSK9; proprotein convertase subtilisin/kexin type 9, LDLR; low-density lipoprotein receptor.
Monoclonal Antibodies
Monoclonal antibodies to PCSK9 are the most common method of PCSK9 inhibition since this approach was first described in 200961). These antibodies bind to a specific region of PCSK9 to inhibit the interaction between PCSK9 and LDLR. The first neutralizing anti-PCSK9 antibody was shown to increase LDLR expression in hepatocytes and reduce LDL-C concentrations by 30%61). Several other monoclonal antibodies have since been described, with dose-dependent reductions of 20–50% in LDL-C levels62, 63). Many clinical trials of monoclonal antibodies against PCSK9, including evolocumab and alirocumab, have been reported64–73). In phase II studies lasting 8–12 weeks, alirocumab lowered LDL-C levels by 40–70% when added to statin therapy64–66). Evolocumab has been shown to reduce LDL-C levels by approximately 60% in phase III trials67–71). Thus, significant reductions in LDL-C levels have been reported for each PCSK9 antibody. Furthermore, the effects of PCSK9 antibodies on LDL-C levels are not affected by age, gender, body mass index, LDL-C concentration, background statin intensity, or dose of statins64–73). Thus, PCSK9 antibody therapy represents one of the most promising and novel approaches to reducing LDL-C levels. However, unlike evolocumab and alirocumab, which are fully humanized monoclonal antibodies, bococizumab is a humanized monoclonal antibody with 3% murine sequence remaining in the antigenbinding complementarity-determining regions74). Bococizumab lowered LDL-C by 54.2% at 12 weeks, but this reduction was attenuated to 40.4% at 52 weeks. After 1 year of bococizumab treatment, 48% of patients had detectable antidrug antibodies, and a titer-dependent attenuation in LDL-C reduction was noted. Thus, the development of bococizumab was discontinued in November 2016.
It remains questionable whether the reduction of LDL-C using PCSK9 antibodies might reduce the risk of cardiovascular events. Sabatine et al. reported that evolocumab reduced LDL-C levels by 61%, and the rate of cardiovascular events following 1 year of treatment was reduced to 0.95% from 2.18% in the control group (hazard ratio, 0.47; p = 0.003)75). In a posthoc analysis of the ODYSSEY LONG TERM (Longterm Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy) trial, the rate of major adverse cardiovascular events (death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) was lower with alirocumab than with placebo (hazard ratio, 0.52; p = 0.02)76). A systematic review and meta-analysis of 24 randomized control trials showed that use of PCSK9 antibodies was associated with a lower rate of all-cause mortality (odds ratio, 0.45; 95% confidence interval [CI], 0.23–0.86; p = 0.015) and myocardial infarction (odds ratio, 0.49; 95% CI, 0.26–0.93; p = 0.03) and no statistically significant reduction in cardiovascular mortality (odds ratio, 0.50; 95% CI, 0.23–1.10; p = 0.084) compared with no anti-PCSK9 treatment77). Furthermore, the more recent randomized, double-blind, placebo-controlled FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) trial demonstrated that evolocumab as add-on to statin therapy decreased LDL-C levels from 92 mg/dL to 30 mg/dL (−59%). Relative to placebo, evolocumab treatment significantly reduced the risk of the primary composite endpoint such as cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization (hazard ratio, 0.85; 95% CI, 0.79–0.92; p < 0.001)78). However, this reduction in events was less than would be expected for such a significant reduction in LDL-C, and the reasons for this disconnect between LDL-C lowering effect and cardiovascular events remain unclear.
The next question is whether elevated circulating PCSK9 and inhibition of PCSK9 affect the progression/regression of atherosclerosis. A significant correlation between circulating PCSK9 and carotid intimamedia wall thickness has been reported79). In addition, Li et al. reported that plasma PCSK9 levels were positively associated with platelet count in patients with CAD, suggesting a potential link between PCSK9 and platelets that may underlie the mechanisms involved in atherosclerosis80). Furthermore, Cheng et al. found that PCSK9 concentrations were linearly associated with a higher necrotic core fraction, as evaluated by intravascular ultrasound with virtual histology within coronary plaques81). Thus, the PCSK9 protein appears to be a key modulating factor in atherosclerosis82). However, Zhu et al. reported that there was no significant relationship between PCSK9 concentration and cardiovascular events. They concluded that although PCSK9 is an important therapeutic target for reducing LDL-C, it is unlikely to present a biomarker of atherosclerotic risk83). A recent meta-analysis also reported that circulating PCSK9 concentration was not significantly associated with the risk of cardiovascular events84). To date, no trial has evaluated whether LDL-C lowering with a PCSK9 antibody has favorable effects on coronary atherosclerosis, and no data have assessed whether the very low LDL-C levels achieved with a PCSK9 antibody lead to an incremental benefit in reducing disease progression compared with statins alone. The GLAGOV (Global Assessment of Plaque Regression with a PCSK9 Antibody as Measured by Intravascular Ultrasound) trial was designed to evaluate the effects of evolocumab on the progression of atherosclerosis using intravascular ultrasound85). This trial demonstrated that 420 mg of evolocumab in addition to statins for 76 weeks reduced LDL-C levels from 92.6 mg/dL to 36.6 mg/dL (−56.3%), and significantly decreased the percent atheroma volume by 0.95% compared with placebo86). Thus, a PCSK9 antibody in combination with statin therapy had a favorable effect on progression of coronary artery plaques. However, no trial to date has examined whether PCSK9 inhibitors can stabilize coronary artery plaques, as reported with statins.
It has been reported that PCSK9 antibodies reduce lipoprotein (Lp)(a) levels66–68, 77, 87). However, the mechanisms of this effect are poorly understood. Serum Lp(a) levels in FH with LDLR mutations have been shown to be elevated, suggesting that Lp(a) is catabolized via the LDLR pathway88). In addition, serum Lp(a) was elevated in patients with FH as a result of PCSK9 gain-of-function mutations to the same extent as that caused by LDLR mutations in FH89). This suggests that LDLR plays an important role in Lp(a) catabolism. However, statins, whose main mechanism of action involves the up-regulation of LDLR, are incapable of reducing Lp(a) levels90). Furthermore, apoB in Lp(a) does not interact with LDLR, suggesting that LDLR does not play a role in Lp(a) kinetics91). Recently, we have reported that serum PCSK9 levels were positively correlated with serum levels of Lp(a), small, dense LDL, and oxidized LDL in patients with CAD48). Thus, the interaction between serum PCSK9 and apoB-containing lipoproteins plays a role in atherosclerosis7). Although there are no reports about the effects of PCSK9 antibodies on small, dense LDL or oxidized LDL, our findings suggest that PCSK9 antibodies might reduce Lp(a) to the same extent that they reduce small, dense LDL and oxidized LDL levels. Indeed, only one study to data has reported that PCSK9 antibodies reduced LDL-C levels and particle numbers92). These effects of PCSK9 antibodies on apoB-containing atherogenic lipoproteins might be responsible for the associated reduction in risk for cardiovascular events.
Antisense Oligonucleotides (ASO) and Small Interfering RNA (siRNA)
ASO and siRNA induce PCSK9 mRNA degradation. Thus, both strategies ultimately result in the silencing of the gene. Graham et al. demonstrated that an ASO for PCSK9 reduced total cholesterol by 53% in mice fed a high-fat diet, while also causing a two-fold increase in hepatic LDLR protein levels93). Similarly, Gupta et al. reported that an ASO lowered PCSK9 mRNA expression by 60% in mice and increased hepatic LDLR protein expression almost threefold94). Furthermore, Yamamoto et al. reported that the strong inhibition of PCSK9 by twice weekly ASO administration for 6 weeks reduced LDL-C levels by 43% in atherogenic diet-fed mice95). Administration of siRNA against PCSK9 mRNA in rats reduced LDL-C concentrations by 30%96), while in monkeys, LDL-C was reduced by 56–70% and the effects lasted for a week96). Fitzgerald et al. reported the results of a phase I trial which investigated the safety and efficacy of an administration of siRNA in healthy volunteers. siRNA produced a 70% reduction in circulating PCSK9 protein levels and a 40% reduction in LDL-C from baseline97). This study was the first to demonstrate the use of an siRNA therapy to modulate a clinically validated endpoint in humans. These results support the further assessment of siRNA therapy in patients with hypercholesterolemia, including those treated with statins. However, relative to antibody-based therapies, siRNA represents a novel treatment strategy and their longterm safety remains unknown. Therefore, further trials are necessary to evaluate the efficacy and safety of siRNA for reducing LDL-C levels and risk of cardiovascular events.
Conclusions
PCSK9 has been identified as a key regulator of serum cholesterol levels and represent a novel pharmacological target for hypercholesterolemia. The major classes of commonly prescribed lipid-lowering agents, particularly statins, clearly increase circulating PCSK9 levels and which likely diminishes the effect of these drugs on the reduction of LDL-C concentrations. Thus, PCSK9 inhibitors, particularly monoclonal antibodies against PCSK9, in combination with statins, are one of the most promising and effective approaches to achieving very low LDL-C levels and reducing the risk of cardiovascular events.
Disclosure
None.
Conflict of Interest
None.
References
- 1). Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists' (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet, 2005; 366: 1267-1278 [DOI] [PubMed] [Google Scholar]
- 2). Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A, Nozato T, Miyake S, Takeyama Y, Morino Y, Yamauchi T, Muramatsu T, Hibi K, Terashima M, Michishita I, TRUTH Investigators Comparison of arterial remodeling and changes in plaque composition between patients with progression versus regression of coronary atherosclerosis during statin therapy (from the TRUTH study). Am J Cardiol, 2012; 109: 1247-1253 [DOI] [PubMed] [Google Scholar]
- 3). Naureckiene S, Ma L, Sreekumar K, Purandare U, Lo CF, Huang Y, Chiang LW, Grenier JM, Ozenberger BA, Jacobsen JS, Kennedy JD, DiStefano PS, Wood A, Bingham B. Functional characterization of Narc 1, a novel proteinase related to proteinase K. Arch Biochem Biophys, 2003; 420: 55-67 [DOI] [PubMed] [Google Scholar]
- 4). Seidah NG, Awan Z, Chrétien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res, 2014; 114: 1022-1036 [DOI] [PubMed] [Google Scholar]
- 5). Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res, 2012; 53: 2515-2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, Cohen JC, Hobbs HH. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem, 2007; 282: 18602-18612 [DOI] [PubMed] [Google Scholar]
- 7). Shapiro MD, Fazio S. PCSK9 and Atherosclerosis - Lipids and Beyond. J Atheroscler Thromb, 2017; 24: 462-472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Chernogubova E, Strawbridge R, Mahdessian H, Mälarstig A, Krapivner S, Gigante B, Hellénius ML, de Faire U, Franco-Cereceda A, Syvänen AC, Troutt JS, Konrad RJ, Eriksson P, Hamsten A, van 't Hooft FM. Common and low-frequency genetic variants in the PCSK9 locus influence circulating PCSK9 levels. Arterioscler Thromb Vasc Biol, 2012; 32: 1526-1534 [DOI] [PubMed] [Google Scholar]
- 9). Chen SN, Ballantyne CM, Gotto AM, Jr., Tan Y, Willerson JT, Marian AJ. A common PCSK9 haplotype, encompassing the E670G coding single nucleotide polymorphism, is a novel genetic marker for plasma low-density lipoprotein cholesterol levels and severity of coronary atherosclerosis. J Am Coll Cardiol, 2005; 45: 1611-1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derré A, Villéger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet, 2003; 34: 154-156 [DOI] [PubMed] [Google Scholar]
- 11). Mabuchi H. Half a Century Tales of Familial Hypercholesterolemia (FH) in Japan. J Atheroscler Thromb, 2017; 24: 189-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet, 2005; 37: 161-165 [DOI] [PubMed] [Google Scholar]
- 13). Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med, 2006; 354: 1264-1272 [DOI] [PubMed] [Google Scholar]
- 14). Dubuc G, Tremblay M, Paré G, Jacques H, Hamelin J, Benjannet S, Boulet L, Genest J, Bernier L, Seidah NG, Davignon J. A new method for measurement of total plasma PCSK9: clinical applications. J Lipid Res, 2010; 51: 140-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Careskey HE, Davis RA, Alborn WE, Troutt JS, Cao G, Konrad RJ. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res, 2008; 49: 394-398 [DOI] [PubMed] [Google Scholar]
- 16). Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab, 2009, 94: 2537-2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Hori M, Ishihara M, Yuasa Y, Makino H, Yanagi K, Tamanaha T, Kishimoto I, Kujiraoka T, Hattori H, Harada-Shiba M. Removal of plasma mature and furincleaved proprotein convertase subtilisin/kexin 9 by lowdensity lipoprotein-apheresis in familial hypercholesterolemia: development and application of a new assay for PCSK9. J Clin Endocrinol Metab, 2015; 100: E41-49 [DOI] [PubMed] [Google Scholar]
- 18). Mayne J, Raymond A, Chaplin A, Cousins M, Kaefer N, Gyamera-Acheampong C, Seidah NG, Mbikay M, Chrétien M, Ooi TC. Plasma PCSK9 levels correlate with cholesterol in men but not in women. Biochem Biophys Res Commun, 2007; 361: 451-456 [DOI] [PubMed] [Google Scholar]
- 19). Lambert G, Ancellin N, Charlton F, Comas D, Pilot J, Keech A, Patel S, Sullivan DR, Cohn JS, Rye KA, Barter PJ. Plasma PCSK9 concentrations correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clin Chem, 2008; 54: 1038-1045 [DOI] [PubMed] [Google Scholar]
- 20). Cariou B, Le Bras M, Langhi C, Le May C, Guyomarc'h-Delasalle B, Krempf M, Costet P. Association between plasma PCSK9 and gamma-glutamyl transferase levels in diabetic patients. Atherosclerosis, 2010; 211: 700-702 [DOI] [PubMed] [Google Scholar]
- 21). Lakoski SG, Xu F, Vega GL, Grundy SM, Chandalia M, Lam C, Lowe RS, Stepanavage ME, Musliner TA, Cohen JC, Hobbs HH. Indices of cholesterol metabolism and relative responsiveness to ezetimibe and simvastatin. J Clin Endocrinol Metab, 2010; 95: 800-809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Awan Z, Seidah NG, MacFadyen JG, Benjannet S, Chasman DI, Ridker PM, Genest J. Rosuvastatin, proprotein convertase subtilisin/kexin type 9 concentrations, and LDL cholesterol response: the JUPITER trial. Clin Chem, 2012; 58: 183-189 [DOI] [PubMed] [Google Scholar]
- 23). Chan DC, Lambert G, Barrett PH, Rye KA, Ooi EM, Watts GF. Plasma proprotein convertase subtilisin/kexin type 9: a marker of LDL apolipoprotein B-100 catabolism? Clin Chem, 2009; 55: 2049-2052 [DOI] [PubMed] [Google Scholar]
- 24). Cameron J, Bogsrud MP, Tveten K, Strøm TB, Holven K, Berge KE, Leren TP. Serum levels of proprotein convertase subtilisin/kexin type 9 in subjects with familial hypercholesterolemia indicate that proprotein convertase subtilisin/kexin type 9 is cleared from plasma by low-density lipoprotein receptor-independent pathways. Transl Res, 2012; 160: 125-130 [DOI] [PubMed] [Google Scholar]
- 25). Kosenko T, Golder M, Leblond G, Weng W, Lagace TA. Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation. J Biol Chem, 2013, 288: 8279-8288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Persson L, Cao G, Ståhle L, Sjöberg BG, Troutt JS, Konrad RJ, Gälman C, Wallén H, Eriksson M, Hafström I, Lind S, Dahlin M, Amark P, Angelin B, Rudling M. Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler Thromb Vasc Biol, 2010; 30: 2666-2672 [DOI] [PubMed] [Google Scholar]
- 27). Browning JD, Horton JD. Fasting reduces plasma proprotein convertase, subtilisin/kexin type 9 and cholesterol biosynthesis in humans. J Lipid Res, 2010; 51: 3359-3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Knopp RH. Drug treatment of lipid disorders. N Engl J Med, 1999; 341: 498-511 [DOI] [PubMed] [Google Scholar]
- 29). Rubinstein A, Weintraub M. Escape phenomenon of low-density lipoprotein cholesterol during lovastatin treatment. Am J Cardiol, 1995; 76: 184-186 [DOI] [PubMed] [Google Scholar]
- 30). Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, Prat A. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol, 2004; 24: 1454-1459 [DOI] [PubMed] [Google Scholar]
- 31). Dong B, Wu M, Li H, Kraemer FB, Adeli K, Seidah NG, Park SW, Liu J. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J Lipid Res, 2010; 51: 1486-1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Berge KE, Ose L, Leren TP. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler Thromb Vasc Biol, 2006; 26: 1094-1100 [DOI] [PubMed] [Google Scholar]
- 33). Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G, Konrad RJ. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res, 2010; 51: 2714-2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Mabuchi H, Nohara A. Therapy: PCSK9 inhibitors for treating familial hypercholesterolaemia. Nat Rev Endocrinol, 2015; 11: 8-9 [DOI] [PubMed] [Google Scholar]
- 35). Lambert G, Charlton F, Rye KA, Piper DE. Molecular basis of PCSK9 function. Atherosclerosis, 2009; 203: 1-7 [DOI] [PubMed] [Google Scholar]
- 36). Jeong HJ, Lee HS, Kim KS, Kim YK, Yoon D, Park SW. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J Lipid Res, 2008; 49: 399-409 [DOI] [PubMed] [Google Scholar]
- 37). Mayne J, Dewpura T, Raymond A, Cousins M, Chaplin A, Lahey KA, Lahaye SA, Mbikay M, Ooi TC, Chrétien M. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis, 2008; 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Costet P, Hoffmann MM, Cariou B, Guyomarc'h Delasalle B, Konrad T, Winkler K. Plasma PCSK9 is increased by fenofibrate and atorvastatin in a non-additive fashion in diabetic patients. Atherosclerosis, 2010; 212: 246-251 [DOI] [PubMed] [Google Scholar]
- 39). Guo YL, Liu J, Xu RX, Zhu CG, Wu NQ, Jiang LX, Li JJ. Short-term impact of low-dose atorvastatin on serum proprotein convertase subtilisin/kexin type 9. Clin Drug Investig, 2013; 33: 877-883 [DOI] [PubMed] [Google Scholar]
- 40). Khera AV, Qamar A, Reilly MP, Dunbar RL, Rader DJ. Effects of niacin, statin, and fenofibrate on circulating proprotein convertase subtilisin/kexin type 9 levels in patients with dyslipidemia. Am J Cardiol, 2015; 115: 178-182 [DOI] [PubMed] [Google Scholar]
- 41). Berthold HK, Seidah NG, Benjannet S, Gouni-Berthold I. Evidence from a randomized trial that simvastatin, but not ezetimibe, upregulates circulating PCSK9 levels. PLoS One, 2013; 8: e60095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Raal F, Panz V, Immelman A, Pilcher G. Elevated PCSK9 levels in untreated patients with heterozygous or homozygous familial hypercholesterolemia and the response to high-dose statin therapy. J Am Heart Assoc, 2013; 2: e000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Nozue T, Hattori H, Ogawa K, Kujiraoka T, Iwasaki T, Michishita I. Effects of Statin Therapy on Plasma Proprotein Convertase Subtilisin/kexin Type 9 and Sortilin Levels in Statin-Naive Patients with Coronary Artery Disease. J Atheroscler Thromb, 2016; 23: 848-856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Sahebkar A, Simental-Mendía LE, Guerrero-Romero F, Golledge J, Watts GF. Effect of statin therapy on plasma proprotein convertase subtilisin kexin 9 (PCSK9) concentrations: a systematic review and meta-analysis of clinical trials. Diabetes Obes Metab, 2015; 17: 1042-1055 [DOI] [PubMed] [Google Scholar]
- 45). Okada K, Iwahashi N, Endo T, Himeno H, Fukui K, Kobayashi S, Shimizu M, Iwasawa Y, Morita Y, Wada A, Shigemasa T, Mochida Y, Shimizu T, Sawada R, Uchino K, Umemura S, Kimura K. Long-term effects of ezetimibe-plus-statin therapy on low-density lipoprotein cholesterol levels as compared with double-dose statin therapy in patients with coronary artery disease. Atherosclerosis, 2012; 224: 454-456 [DOI] [PubMed] [Google Scholar]
- 46). Konrad RJ, Troutt JS, Cao G. Effects of currently prescribed LDL-C-lowering drugs on PCSK9 and implications for the next generation of LDL-C-lowering agents. Lipids Health Dis, 2011; 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Cariou B, Ouguerram K, Zaïr Y, Guerois R, Langhi C, Kourimate S, Benoit I, Le May C, Gayet C, Belabbas K, Dufernez F, Chétiveaux M, Tarugi P, Krempf M, Benlian P, Costet P. PCSK9 dominant negative mutant results in increased LDL catabolic rate and familial hypobetalipoproteinemia. Arterioscler Thromb Vasc Biol, 2009; 29: 2191-2197 [DOI] [PubMed] [Google Scholar]
- 48). Nozue T, Hattori H, Ogawa K, Kujiraoka T, Iwasaki T, Hirano T, Michishita I. Correlation between serum levels of proprotein convertase subtilisin/kexin type 9 (PCSK9) and atherogenic lipoproteins in patients with coronary artery disease. Lipids Health Dis, 2016; 15: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science, 2004; 303: 1201-1204 [DOI] [PubMed] [Google Scholar]
- 50). Weinglass AB, Kohler M, Schulte U, Liu J, Nketiah EO, Thomas A, Schmalhofer W, Williams B, Bildl W, McMasters DR, Dai K, Beers L, McCann ME, Kaczorowski GJ, Garcia ML. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc Natl Acad Sci USA, 2008; 105: 11140-11145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Nozue T, Michishita I, Mizuguchi Effects of ezetimibe on remnant-like particle cholesterol, lipoprotein (a), and oxidized low-density lipoprotein in patients with dyslipidemia. J Atheroscler Thromb, 2010; 17: 37-4420075599 [Google Scholar]
- 52). Hentze H, Jensen KK, Chia SM, Johns DG, Shaw RJ, Davis HR, Jr, Shih SJ, Wong KK. Inverse relationship between LDL cholesterol and PCSK9 plasma levels in dyslipidemic cynomolgus monkeys: effects of LDL lowering by ezetimibe in the absence of statins. Atherosclerosis, 2013; 231: 84-90 [DOI] [PubMed] [Google Scholar]
- 53). Davinnon J, Dubuc G. Statins and ezetimibe modulate plasma proprotein convertase subtilisin kexin-9 (PCSK9) levels. Trans Am Clin Climatol Assoc, 2009; 120: 163-173 [PMC free article] [PubMed] [Google Scholar]
- 54). Miyoshi T, Nakamura K, Doi M, Ito H. Impact of Ezetimibe Alone or in Addition to a Statin on Plasma PCSK9 Concentrations in Patients with Type 2 Diabetes and Hypercholesterolemia: A Pilot Study. Am J Cardiovasc Drugs, 2015; 15: 213-219 [DOI] [PubMed] [Google Scholar]
- 55). Han L, Li M, Liu Y, Han C, Ye P. Atorvastatin may delay cardiac aging by upregulating peroxisome proliferatoractivated receptors in rats. Pharmacology, 2012; 89: 74-82 [DOI] [PubMed] [Google Scholar]
- 56). Sanderson LM, Boekschoten MV, Desvergne B, Müller M, Kersten S. Transcriptional profiling reveals divergent roles of PPARalpha and PPARbeta/delta in regulation of gene expression in mouse liver. Physiol Genomics, 2010; 41: 42-52 [DOI] [PubMed] [Google Scholar]
- 57). Kourimate S, Le May C, Langhi C, Jarnoux AL, Ouguerram K, Zaïr Y, Nguyen P, Krempf M, Cariou B, Costet P. Dual mechanisms for the fibrate-mediated repression of proprotein convertase subtilisin/kexin type 9. J Biol Chem, 2008; 283: 9666-9673 [DOI] [PubMed] [Google Scholar]
- 58). Chan DC, Hamilton SJ, Rye KA, Chew GT, Jenkins AJ, Lambert G, Watts GF. Fenofibrate concomitantly decreases serum proprotein convertase subtilisin/kexin type 9 and very-low-density lipoprotein particle concentrations in statin-treated type 2 diabetic patients. Diabetes Obesity and Metab, 2010; 12: 752-756 [DOI] [PubMed] [Google Scholar]
- 59). Troutt JS, Alborn WE, Cao G, Konrad RJ. Fenofibrate treatment increases human serum proprotein convertase subtilisin kexin type 9 levels. J Lipid Res, 2010; 51: 345-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Noguchi T, Kobayashi J, Yagi K, Nohara A, Yamaaki N, Sugihara M, Ito N, Oka R, Kawashiri MA, Tada H, Takata M, Inazu A, Yamagishi M, Mabuchi H. Comparison of effects of bezafibrate and fenofibrate on circulating proprotein convertase subtilisin/kexin type 9 and adipocytokine levels in dyslipidemic subjects with impaired glucose tolerance or type 2 diabetes mellitus: results from a crossover study. Atherosclerosis, 2011; 217: 165-170 [DOI] [PubMed] [Google Scholar]
- 61). Chan JC, Piper DE, Cao Q, Liu D, King C, Wang W, Tang J, Liu Q, Higbee J, Xia Z, Di Y, Shetterly S, Arimura Z, Salomonis H, Romanow WG, Thibault ST, Zhang R, Cao P, Yang XP, Yu T, Lu M, Retter MW, Kwon G, Henne K, Pan O, Tsai MM, Fuchslocher B, Yang E, Zhou L, Lee KJ, Daris M, Sheng J, Wang Y, Shen WD, Yeh WC, Emery M, Walker NP, Shan B, Schwarz M, Jackson SM. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci USA, 2009; 106: 9820-9825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62). Zhang L, McCabe T, Condra JH, Ni YG, Peterson LB, Wang W, Strack AM, Wang F, Pandit S, Hammond H, Wood D, Lewis D, Rosa R, Mendoza V, Cumiskey AM, Johns DG, Hansen BC, Shen X, Geoghagen N, Jensen K, Zhu L, Wietecha K, Wisniewski D, Huang L, Zhao JZ, Ernst R, Hampton R, Haytko P, Ansbro F, Chilewski S, Chin J, Mitnaul LJ, Pellacani A, Sparrow CP, An Z, Strohl W, Hubbard B, Plump AS, Blom D, Sitlani A. An anti-PCSK9 antibody reduces LDL-cholesterol on top of a statin and suppresses hepatocyte SREBP-regulated genes. Int J Biol Sci, 2012; 8: 310-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). Ni YG, Di Marco S, Condra JH, Peterson LB, Wang W, Wang F, Pandit S, Hammond HA, Rosa R, Cummings RT, Wood DD, Liu X, Bottomley MJ, Shen X, Cubbon RM, Wang SP, Johns DG, Volpari C, Hamuro L, Chin J, Huang L, Zhao JZ, Vitelli S, Haytko P, Wisniewski D, Mitnaul LJ, Sparrow CP, Hubbard B, Carfí A, Sitlani A. A PCSK9-binding antibody that structurally mimics the EGF(A) domain of LDL-receptor reduces LDL cholesterol in vivo. J Lipid Res, 2011; 52: 78-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet, 2012; 380: 29-36 [DOI] [PubMed] [Google Scholar]
- 65). McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol, 2012; 59: 2344-2353 [DOI] [PubMed] [Google Scholar]
- 66). Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 2012; 367: 1891-1900 [DOI] [PubMed] [Google Scholar]
- 67). Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA, DESCARTES Investigators A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med, 2014; 370: 1809-1819 [DOI] [PubMed] [Google Scholar]
- 68). Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, Wasserman SM, Weiss R, LAPLACE-2 Investigators Effect of evolocumab or ezetimibe added to moderate-or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA, 2014; 311: 1870-1882 [DOI] [PubMed] [Google Scholar]
- 69). Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, Kim JB, Scott R, Wasserman SM, Bays H, MENDEL-2 Investigators Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol, 2014; 63: 2531-2540 [DOI] [PubMed] [Google Scholar]
- 70). Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, Bruckert E, Cho L, Dent R, Knusel B, Xue A, Scott R, Wasserman SM, Rocco M, GAUSS-2 Investigators Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol, 2014; 63: 2541-2548 [DOI] [PubMed] [Google Scholar]
- 71). Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni-Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D, RUTHERFORD-2 Investigators PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet, 2015; 385: 331-340 [DOI] [PubMed] [Google Scholar]
- 72). Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, Liu T, Mohanavelu S, Hoffman EB, McDonald ST, Abrahamsen TE, Wasserman SM, Scott R, Sabatine MS, LAPLACE-TIMI 57 Investigators Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet, 2012; 380: 2007-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73). Dias CS, Shaywitz AJ, Wasserman SM, Smith BP, Gao B, Stolman DS, Crispino CP, Smirnakis KV, Emery MG, Colbert A, Gibbs JP, Retter MW, Cooke BP, Uy ST, Matson M, Stein EA. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol, 2012; 60: 1888-1898 [DOI] [PubMed] [Google Scholar]
- 74). Ridker PM, Tardif JC, Amarenco P, Duggan W, Glynn RJ, Jukema JW, Kastelein JJP, Kim AM, Koenig W, Nissen S, Revkin J, Rose LM, Santos RD, Schwartz PF, Shear CL, Yunis C, SPIRE Investigators Lipid-Reduction Variability and Antidrug-Antibody Formation with Bococizumab. N Engl J Med, 2017; 376: 1517-1526 [DOI] [PubMed] [Google Scholar]
- 75). Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA, Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med, 2015; 372: 1500-1509 [DOI] [PubMed] [Google Scholar]
- 76). Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ, ODYSSEY LONG TERM Investigators Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med, 2015; 372: 1489-1499 [DOI] [PubMed] [Google Scholar]
- 77). Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, Brockmeyer M, Kandzari DE, Kubica JM, D'Agostino RB, Sr, Kubica J, Volpe M, Agewall S, Kereiakes DJ, Kelm M. Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Antibodies in Adults With Hypercholesterolemia: A Systematic Review and Metaanalysis. Ann Intern Med, 2015; 163: 40-51 [DOI] [PubMed] [Google Scholar]
- 78). Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR, FOURIER Steering Committee and Investigators Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med, 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]
- 79). Huijgen R, Fouchier SW, Denoun M, Hutten BA, Vissers MN, Lambert G, Kastelein JJ. Plasma levels of PCSK9 and phenotypic variability in familial hypercholesterolemia. J Lipid Res, 2012; 53: 979-983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80). Li S, Zhu CG, Guo YL, Xu RX, Zhang Y, Sun J, Li JJ. The relationship between the plasma PCSK9 levels and platelet indices in patients with stable coronary artery disease. J Atheroscler Thromb, 2015; 22: 76-84 [DOI] [PubMed] [Google Scholar]
- 81). Cheng JM, Oemrawsingh RM, Garcia-Garcia HM, Boersma E, van Geuns RJ, Serruys PW, Kardys I, Akkerhuis KM. PCSK9 in relation to coronary plaque inflammation: Results of the ATHEROREMO-IVUS study. Atherosclerosis, 2016; 248: 117-122 [DOI] [PubMed] [Google Scholar]
- 82). Li S, Li JJ. PCSK9: A key factor modulating atherosclerosis. J Atheroscler Thromb, 2015; 22: 221-230 [DOI] [PubMed] [Google Scholar]
- 83). Zhu YM, Anderson TJ, Sikdar K, Fung M, McQueen MJ, Lonn EM, Verma S. Association of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) With Cardiovascular Risk in Primary Prevention. Arterioscler Thromb Vasc Biol, 2015; 35: 2254-2259 [DOI] [PubMed] [Google Scholar]
- 84). Xiao Y, Peng C, Huang W, Zhang J, Gao Y, Kim JH, Yeoh EK, Su X. Circulating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Concentration and Risk of Cardiovascular Events - Systematic Review and Meta-Analysis of Prospective Studies. Circ J, 2017. April 11. 10.1253/circj.CJ-16-1142 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 85). Puri R, Nissen SE, Somaratne R, Cho L, Kastelein JJ, Ballantyne CM, Koenig W, Anderson TJ, Yang J, Kassahun H, Wasserman SM, Scott R, Borgman M, Nicholls SJ. Impact of PCSK9 inhibition on coronary atheroma progression: Rationale and design of Global Assessment of Plaque Regression with a PCSK9 Antibody as Measured by Intravascular Ultrasound (GLAGOV). Am Heart J, 2016; 176: 83-92 [DOI] [PubMed] [Google Scholar]
- 86). Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA, 2016; 316: 2373-2384 [DOI] [PubMed] [Google Scholar]
- 87). Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Langslet G, Bays H, Blom D, Eriksson M, Dent R, Wasserman SM, Huang F, Xue A, Albizem M, Scott R, Stein EA. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol, 2014; 63: 1278-1288 [DOI] [PubMed] [Google Scholar]
- 88). Kraft HG, Lingenhel A, Raal FJ, Hohenegger M, Utermann G. Lipoprotein(a) in homozygous familial hypercholesterolemia. Arterioscler Thromb Vasc Biol, 2000; 20: 522-528 [DOI] [PubMed] [Google Scholar]
- 89). Tada H, Kawashiri MA, Yoshida T, Teramoto R, Nohara A, Konno T, Inazu A, Mabuchi H, Yamagishi M, Hayashi K. Lipoprotein(a) in familial hypercholesterolemia with proprotein convertase subtilisin/kexin type 9 (PCSK9) gain-of-function mutations. Circ J, 2016; 80: 512-518 [DOI] [PubMed] [Google Scholar]
- 90). Tziomalos K, Athyros VG, Wierzbicki AS, Mikhailidis DP. Lipoprotein a: where are we now? Curr Opin Cardiol, 2009; 24: 351-357 [DOI] [PubMed] [Google Scholar]
- 91). Lamon-Fava S, Diffenderfer MR, Marcovina SM. Lipoprotein(a) metabolism. Curr Opin Lipidol, 2014; 25: 189-193 [DOI] [PubMed] [Google Scholar]
- 92). Koren MJ, Kereiakes D, Pourfarzib R, Winegar D, Banerjee P, Hamon S, Hanotin C, McKenney JM. Effect of PCSK9 inhibition by alirocumab on lipoprotein particle concentrations determined by nuclear magnetic resonance spectroscopy. J Am Heart Assoc, 2015; 4: e002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93). Graham MJ, Lemonidis KM, Whipple CP, Subramaniam A, Monia BP, Crooke ST, Crooke RM. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res, 2007; 48: 763-767 [DOI] [PubMed] [Google Scholar]
- 94). Gupta N, Fisker N, Asselin MC, Lindholm M, Rosenbohm C, ørum H, Elmén J, Seidah NG, Straarup EM. A locked nucleic acid antisense oligonucleotide (LNA) silences PCSK9 and enhances LDLR expression in vitro and in vivo. PLoS One, 2010; 5: e10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95). Yamamoto T, Harada-Shiba M, Nakatani M, Wada S, Yasuhara H, Narukawa K, Sasaki K, Shibata MA, Torigoe H, Yamaoka T, Imanishi T, Obika S. Cholesterol-lowering Action of BNA-based Antisense Oligonucleotides Targeting PCSK9 in Atherogenic Diet-induced Hypercholesterolemic Mice. Mol Ther Nucleic Acids, 2012; 1: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96). Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, Butler D, Charisse K, Dorkin R, Fan Y, Gamba-Vitalo C, Hadwiger P, Jayaraman M, John M, Jayaprakash KN, Maier M, Nechev L, Rajeev KG, Read T, Röhl I, Soutschek J, Tan P, Wong J, Wang G, Zimmermann T, de Fougerolles A, Vornlocher HP, Langer R, Anderson DG, Manoharan M, Koteliansky V, Horton JD, Fitzgerald K. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA, 2008; 105: 11915-11920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97). Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, Hutabarat RM, Clausen VA, Karsten V, Cehelsky J, Nochur SV, Kotelianski V, Horton J, Mant T, Chiesa J, Ritter J, Munisamy M, Vaishnaw AK, Gollob JA, Simon A. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet, 2014; 383: 60-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98). Guo YL, Xu RX, Zhu CG, Wu NQ, Cui ZP, Li JJ. Policosanol attenuates statin-induced increases in serum proprotein convertase subtilisin/kexin type 9 when combined with atorvastatin. Evid Based Complement Alternat Med, 2014; 2014: 926087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99). Tremblay AJ, Lamarche B, Lemelin V, Hoos L, Benjannet S, Seidah NG, Davis HR, Jr, Couture P. Atorvastatin increases intestinal expression of NPC1L1 in hyperlipidemic men. J Lipid Res, 2011; 52: 558-565 [DOI] [PMC free article] [PubMed] [Google Scholar]


