Abstract
Aims: Coronary collateral circulation (CCC) is crucial during an acute ischemic attack. Evidences showed that omentin-1 exhibited remarkable antiatherogenic effects and ischemia-induced revascularization. The aim of this study was to investigate the relationship between plasma omentin-1 levels and CCC in patients with ≥ 90% angiography-proven coronary occlusion.
Methods: 142 patients with ≥ 90% luminal diameter stenosis in at least one major epicardial coronary artery were recruited. Among them, 79 patients with Rentrop 0–1 grade were classified into the poor CCC group and 63 patients with Rentrop 2–3 grade were included into the good CCC group. The association between plasma omentin-1 levels and CCC status was assessed.
Results: Plasma omentin-1 level was significantly higher in patients with good CCC than those with poor CCC (566.57 ± 26.90 vs. 492.38 ± 19.70 ng/mL, p = 0.024). Besides, omentin-1 was positively correlated with total cholesterol (TC), high-density lipoprotein, and gensini score but inversely with hyperlipidemia and body mass index (all p values < 0.05). Multivariate regression analysis indicated that omentin-1 [odds ratio (OR) = 1.002, 95% confidence interval (CI): 1.000 – 1.004, p = 0.041)], TC, the number of the diseased vessels, a higher frequency of left circumflex artery and right coronary artery, chronic total occlusion, and gensini score remained as the independent predictors of good CCC.
Conclusion: Higher plasma omentin-1 level was associated with better CCC development. Our findings suggest that omentin-1 may be an alternative marker for adequate CCC in patients with ≥ 90% coronary occlusion.
Keywords: Coronary heart disease, Coronary collateral circulation, Omentin-1
Introduction
Coronary heart disease (CHD) is one of the major causes of death worldwide. In recent years, revascularization including percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) has greatly improved the treatment, symptoms, and prognosis of CHD. However, the presence of restenosis is inevitable and approximately one-fifth of the patients in whom the extent of coronary atherosclerosis is especially severe cannot be treated with revascularization simultaneously1). In this case, well-developed coronary collateral circulation (CCC) may be an alternative therapeutic strategy for these patients other than conventional revascularization.
CCC pre-exists in the normal coronary arteries that can develop mature and functional arterioles following a severe coronary arterial occlusion2), which also serves as an alternative source of blood flow to the ischemic myocardium. The well-developed CCC can lower myocardial ischemia and necrosis, reduce the infarct size, and benefit on the function of myocardium and ventricular aneurysm formation3). Studies have shown the important clinical significance of pre-existent CCC that decreased in-hospital death from anterior myocardial infarction4). Moreover, the presence of good CCC was associated with better clinical outcomes including long-term survival, major adverse cardiovascular event rates, right ventricular infarction, complete atrioventricular block, cardiogenic shock, and ventricular tachycardia/ventricular fibrillation in patients with acute and inferior ST-elevation myocardial infarction5, 6).
Fluid shear stress (FSS) is induced by coronary artery stenosis and is known as the initial factor of CCC2), but a contradictory clinical phenomenon is that patients with the similar degrees of coronary artery stenosis exhibit different degrees of CCC, and the potential mechanisms remain unclear. As known, the formation of CCC is a complex multifactorial process, monocytes7), vascular smooth muscle cells, and endothelial cells are essential for remodeling of pre-existent interconnecting arterioles into well-developed CCC2, 8, 9); cytokines such as intercellular adhesion molecule, monocyte chemoattractant protein-1 (MCP-1), and nitric oxide (NO) are crucial in controlling these cell behaviors7). Therefore, seeking and investigating vital regulators associated with CCC may show the mechanisms of formation of CCC and provide important clinical significance.
Adipose tissues not only play roles in energy storage but also function as an important active endocrine organ that can produce and secrete numerous bioactive adipokines involved in the development of cardiovascular diseases (CVDs)10). Omentin-1, also called intellectin-1, is a novel adipokine that is highly expressed in visceral fat11). It has been shown that plasma omentin-1 level was decreased in obesity and negatively correlated with body mass index (BMI), waist circumference, leptin levels, and insulin resistance12). Moreover, evidences showed that omentin-1 was inversely associated with the presence and angiographic severity of CHD in metabolic syndrome (MetS) patients13), and more important is that it markedly displays counteractive effects against atherosclerosis and ischemia-induced revascularization14–16). However, there have been no previous reports regarding the correlation between plasma omentin-1 and CCC.
Based on the above researches, we reasonably speculated that plasma omentin-1 may associate with CCC. Thus, the aim of this study was to investigate whether omentin-1 can act as a potential predictor for poor or good CCC in patients with CHD with severe coronary artery stenosis.
Methods
Study Population
A total of 142 subjects enrolled in this study were patients who underwent coronary angiography at the cardiac catheterization room of Xiangya Hospital, Central South University, Hunan Province, China and were recruited between 2014 and 2016. All patients were diagnosed with ≥ 90% luminal diameter stenosis in at least one major epicardial coronary artery. The exclusion criteria were as follows: recent history of acute coronary syndrome, PCI within the previous three months, history of CABG, decompensated heart failure or valvular heart disease, any concomitant inflammation or infectious diseases or neoplastic diseases, and severe liver and kidney dysfunction. General characteristics including history of smoking (current or former smoker), drinking (current or former drinker), hypertension, and diabetes mellitus (DM) were recorded by interviewing patients directly. BMI was calculated as weight (kg) divided by squared body height in meters (m2). Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg or use of antihypertensive treatment. DM was defined as a fasting plasma glucose ≥ 7.0 mmol/L or use of DM therapy. Hyperlipidemia was defined as serum total cholesterol (TC) ≥ 240 mg/dl, low-density lipoprotein cholesterol (LDL) ≥ 130 mg/dl, or taking any hypolipemic agents. Informed consent was obtained from all participants. The study protocol was approved by the Ethics Committee of Xiangya Hospital, Central South University, Changsha, Hunan, China.
Coronary Angiography
Coronary angiography was routinely performed for all patients using standard Judkins technique. Examination of the angiograms and evaluation of the grade of CCC were performed by two experienced interventional cardiologists. Severe coronary artery stenosis was diagnosed if there was ≥ 90% luminal diameter stenosis in at least one major epicardial coronary artery. The CCC grading was performed according to the Rentrop classification17): grade 0, no filling of any collateral vessels; grade 1, filling of side branches of the artery to be perfused by collateral vessels without visualization of epicardial segment; grade 2, partial filling of the epicardial artery by collateral vessels; and grade 3, complete filling of epicardial artery by collateral vessel. If there was more than one occluded vessel with a luminal stenosis of 90% or more, the highest Rentrop grade of collateral development was selected as collateral grade. The severity of CHD was determined according to Gensini scoring system18). The patients were divided into two groups in accordance with Rentrop grade: poor CCC (grades 0 and 1) and good CCC (grades 2 and 3).
Laboratory Analysis
Fasting venous blood samples used for complete blood count and biochemistry panel were collected from all subjects in the morning before coronary angiography. Blood samples used for omentin-1 measurement were withdrawn in an ethylene diamine tetraacetic acid (EDTA) containing anticoagulative tube when performing the coronary angiography. Immediately, the blood samples were centrifuged with 3,000 rpm for 10 min at 4°C to prepare the hemocytes and plasma samples. All samples were subpacked in several small tubes and stored at −80°C until analysis. The quantitative measurement of plasma omentin-1 was determined using a sandwich enzyme-linked immunosorbent assay (ELISA) kit (Cat. No.: RD191100200R, BioVendor, Brno, Czech Republic) according to the manufacturer's instructions. The kit was highly sensitive and specific, as the limit of detection was 0.5 ng/mL, and the average intra-/inter-assay coefficient of variation was below 5%.
Statistical Analysis
Continuous variables were expressed as the mean ± standard deviation or mean ± standard error of the mean as statement. Comparison of parametric values was between the two groups using independent sample t test. Categorical variables were compared by the chi-square test. Correlation analysis was computed to examine the association between two variables using Spearman or Pearson correlation analysis, as appropriate. The receiver operating characteristic (ROC) curve analysis was used for the determination of optimal cutoff value of omentin-1 for the prediction of good CCC. Univariate and multivariate logistic regression analyses were performed to determine the independent predictors of good CCC. All statistical analyses were performed using the SPSS 13.0 (SPSS Inc., Chicago, IL, USA) software package. A two-tailed p value < 0.05 was considered to be statistically significant.
Results
Clinical and Laboratory Characteristics
The general characteristics of the study population were summarized in Table 1. A total of 142 subjects including 79 patients with poor CCC and 63 patients with good CCC were recruited in the study. The two groups were matched in sex and age. Most patients were male in both groups (83.5% vs. 82.5%, p = 0.874). The mean age was 62 ± 10 years in the poor CCC group and also was 62 ± 10 years in the good CCC group (p = 0.942). Furthermore, there were no significant differences between the two groups in BMI (p = 0.412), systolic blood pressure (SBP) (p = 0.775), diastolic blood pressure (DBP) (p = 0.088), heart rate (HR) (p = 0.084), fasting plasma glucose (FPG) (p = 0.422), triglyceride (TG) (p = 0.079), high-density lipoprotein (HDL) (p = 0.483), positive history of smoking (p = 0.223) and alcohol consumption (p = 0.430), hypertension (p = 0.895), DM (p = 0.806), hyperlipidemia (p = 0.099), types of CHD (p = 0.294), and previous PCI history (p = 0.098). Likewise, no significant differences were observed among the two groups in cardiovascular medications consisted of calcium channel blocker (p = 0.663), angiotensin receptor blocker/angiotensin converting enzyme inhibitor (p = 0.512), beta-blocker (p = 0.163), nitrates (p = 0.110), statins (p = 0.841), antiplatelet agents (p = no data), and anticoagulants (p = 0.159). However, patients with good CCC showed significantly higher levels in TC (4.88 ± 1.61 vs. 4.20 ± 1.21 mmol/L, p = 0.005), LDL (3.15 ± 1.26 vs. 2.67 ± 0.93 mmol/L, p = 0.009), and gensini score (145 ± 45 vs. 106 ± 37, p < 0.001) compared to those with poor CCC.
Table 1. Clinical characteristics of the study subjects.
| Poor CCC | Good CCC | p values | |
|---|---|---|---|
| (n = 79) | (n = 63) | ||
| Male (n, %) | 66 (83.5) | 52 (82.5) | 0.874 |
| Age (years) | 62 ± 10 | 62 ± 10 | 0.942 |
| BMI (kg/m2) | 24.45 ± 3.45 | 24.89 ± 2.88 | 0.412 |
| SBP (mmHg) | 132 ± 20 | 133 ± 19 | 0.775 |
| DBP (mmHg) | 75 ± 11 | 78 ± 10 | 0.088 |
| HR (bpm) | 70 ± 12 | 74 ± 14 | 0.084 |
| FPG (mmol/L) | 6.11 ± 1.89 | 5.88 ± 1.54 | 0.422 |
| TG (mmol/L) | 1.83 ± 1.19 | 2.32 ± 2.10 | 0.079 |
| TC (mmol/L) | 4.20 ± 1.21 | 4.88 ± 1.61 | 0.005 |
| HDL (mmol/L) | 1.05 ± 0.29 | 1.09 ± 0.25 | 0.483 |
| LDL (mmol/L) | 2.67 ± 0.93 | 3.15 ± 1.26 | 0.009 |
| Smoking (n, %) | 53 (67.1) | 36 (57.1) | 0.223 |
| Drinking (n, %) | 34 (43.0) | 23 (36.5) | 0.430 |
| Hypertension (n, %) | 51 (64.6) | 40 (63.5) | 0.895 |
| DM (n, %) | 31 (39.2) | 26 (41.3) | 0.806 |
| Hyperlipidemia (n, %) | 50 (63.3) | 48 (76.2) | 0.099 |
| CCB (n, %) | 31 (39.2) | 27 (42.9) | 0.663 |
| ARB/ACEI (n, %) | 55 (69.6) | 47 (74.6) | 0.512 |
| BB (n, %) | 65 (82.3) | 57 (90.5) | 0.163 |
| Nitrates (n, %) | 37 (46.8) | 38 (60.3) | 0.110 |
| Statins (n, %) | 76 (96.2) | 61 (96.8) | 0.841 |
| Antiplatelet agents (n, %) | 79 (100.0) | 63 (100.0) | ND |
| Anticoagulant (n, %) | 53 (67.1) | 49 (77.8) | 0.159 |
| Gensini score | 106 ± 37 | 145 ± 45 | < 0.001 |
| Types of CHD | |||
| Angina pectoris | 53 (67.1) | 41 (65.1) | |
| Myocardial infarction | 16 (20.2) | 18 (28.6) | 0.294 |
| Ischemic cardiomyopathy | 10 (12.7) | 4 (6.3) | |
| Previous PCI history (n, %) | 12 (15.2) | 4 (6.3) | 0.098 |
Datas were expressed as mean ± standard deviation or the number (%) of patients. CCC, coronary collateral circulation; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; FPG, fasting plasma glucose; TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; DM, diabetes mellitus; CCB, calcium channel blocker; ARB, angiotensin receptor blocker; ACEI, angiotensin converting enzyme inhibitor; BB, beta-blocker; ND, no data; CHD, coronary heart disease; PCI, percutaneous coronary intervention.
As shown in Table 2, coronary angiographic analysis indicated that coronary diseases with ≥ 90% occlusion of the left main coronary (LM), left anterior descending artery (LAD), left circumflex artery (LCX), and right coronary artery (RCA) were 1.3% vs. 1.6% (p = 0.872), 75.9% vs. 82.5% (p = 0.339), 43.0% vs. 63.5% (p = 0.015), and 48.1% vs. 81.0% (p < 0.001) in the poor CCC and good CCC groups, respectively. The distribution of the number of diseased vessels was also significantly different between the two groups (p < 0.001), as the patients with one-vessel disease, two-vessel disease, and three-vessel disease were 50.7% vs. 19.0%, 31.6% vs. 34.9%, and 17.7% vs. 46.1% in the poor CCC and good CCC groups, respectively. Furthermore, patients in the good CCC group showed higher chronic total occlusion (CTO) than those in the poor CCC group (92.1% vs. 17.7%, p < 0.001).
Table 2. Coronary angiographic findings of the patients.
| Poor CCC | Good CCC | p values | |
|---|---|---|---|
| (n = 79) | (n = 63) | ||
| Location of the disease (n, %) | |||
| LM | 1 (1.3) | 1 (1.6) | 0.872 |
| LAD | 60 (75.9) | 52 (82.5) | 0.339 |
| LCX | 34 (43.0) | 40 (63.5) | 0.015 |
| RCA | 38 (48.1) | 51 (81.0) | < 0.001 |
| Number of the diseased vessels (n, %) | |||
| One-vessel disease | 40 (50.7) | 12 (19.0) | |
| Two-vessels disease | 25 (31.6) | 22 (34.9) | < 0.001 |
| Three-vessels disease | 14 (17.7) | 29 (46.1) | |
| CTO (n, %) | 14 (17.7) | 58 (92.1) | < 0.001 |
Datas were expressed as number (%). LM, left main coronary; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; CTO, chronic total occlusion.
Associations between Plasma Omenin-1 and CCC
To ascertain the association of omentin-1 with CCC, we first performed ELISA assay to determine the plasma omentin-1 concentration in the total subjects. As shown in Fig. 1, plasma omentin-1 concentration was significantly higher in patients with good CCC than those with poor CCC (566.57 ± 26.90 vs. 492.38 ± 19.70 ng/mL, p = 0.024, Fig. 1). Meanwhile, as shown in Table 3, further correlation analyses in the total subjects indicated that plasma omentin-1 level was negatively correlated with hyperlipidemia (r = −0.171, p = 0.041) and BMI (r = −0.221, p = 0.008) but positively correlated with TC (r = 0.218, p = 0.009), HDL (r = 0.255, p = 0.002) and gensini score (r = 0.176, p = 0.036).
Fig. 1.
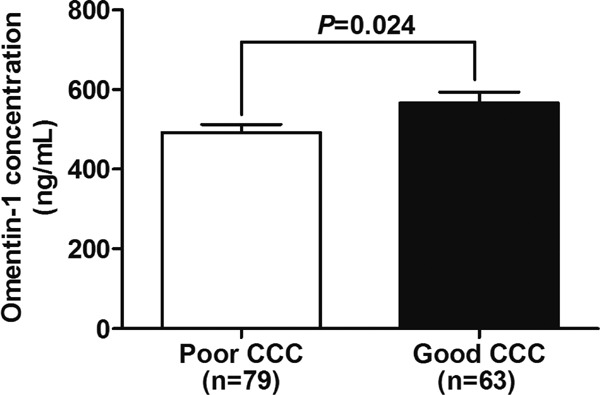
Plasma omentin-1 levels in good and poor CCC groups.
Data were expressed as mean ± standard error of the mean.
Table 3. Correlations between omentin-1 and other variables in the study subjects.
| r | p values | |
|---|---|---|
| Rentrop grade | 0.127 | 0.132 |
| Smoking | 0.073 | 0.387 |
| Hypertension | −0.080 | 0.346 |
| DM | 0.066 | 0.433 |
| Hyperlipidemia | −0.171 | 0.041 |
| BMI | −0.221 | 0.008 |
| TG | −0.053 | 0.534 |
| TC | 0.218 | 0.009 |
| HDL | 0.255 | 0.002 |
| LDL | 0.159 | 0.058 |
| Gensini score | 0.176 | 0.036 |
As shown earlier that plasma omentin-1 level was higher in patients with good CCC, so we furtherly performed logistic analyses to determine whether omentin-1 can serve as an independent predictor of good CCC development. As shown in Table 4, univariate logistic analyses demonstrated that plasma omentin-1 level [odds ratio (OR) = 1.002, 95% confidence interval (CI): 1.000–1.004, p = 0.028) was an independent predictor of good CCC, and TC (OR = 1.418, 95% CI: 1.097–1.833, p = 0.008), LDL (OR = 1.519, 95% CI: 1.093–2.112, p = 0.013), the number of the diseased vessels (OR = 2.627, 95% CI: 1.671 –4.132, p < 0.001), a higher frequency of LCX (OR = 2.302, 95% CI: 1.167–4.540, p = 0.016), RCA (OR = 4.586, 95% CI: 2.127–9.886, p < 0.001), CTO (OR = 53.857, 95% CI: 18.276–158.710, p < 0.001), and gensini score (OR = 1.023, 95% CI: 1.014–1.033, p < 0.001) were also independent predictors of good CCC. When adjusted for possible confounding factors including sex, age, smoking, BMI, TC, and HDL, we found that not only plasma omentin-1 level (OR = 1.002, 95% CI: 1.000–1.004, p = 0.041), but also TC (OR = 1.442, 95% CI: 1.094–1.901, p = 0.009), the number of the diseased vessels (OR = 2.443, 95% CI: 1.520 –3.926, p < 0.001), a higher frequency of LCX (OR = 2.166, 95% CI: 1.031–4.551, p = 0.041), RCA (OR = 4.712, 95% CI: 2.078–10.684, p < 0.001), CTO (OR = 105.359, 95% CI: 25.913–428.379, p < 0.001), and gensini score (OR = 1.024, 95% CI: 1.013–1.034, p < 0.001) were independent predictors of good CCC. Furthermore, the ROC analysis provided a cutoff value of 572.4 ng/mL for plasma omentin-1 level to predict good CCC with 43% sensitivity and 75% specificity, and the area under the ROC curve was 0.598 (95% CI: 0.505–0.692, p = 0.044, Fig. 2).
Table 4. Logistic regression analyses for the independent predictors of good CCC in the study subjects.
| Univariate |
Multivariate a |
|||
|---|---|---|---|---|
| OR (95% CI) | p values | OR (95% CI) | p values | |
| Sex | 0.931 (0.386–2.248) | 0.874 | 1.902 (0.641–5.650) | 0.247 |
| Age | 1.001 (0.967–1.037) | 0.942 | 1.007 (0.970–1.046) | 0.716 |
| Smoking | 0.654 (0.330–1.298) | 0.224 | 0.604 (0.267–1.366) | 0.226 |
| BMI | 1.045 (0.941–1.160) | 0.410 | 1.051 (0.938–1.178) | 0.394 |
| Omentin-1 | 1.002 (1.000–1.004) | 0.028 | 1.002 (1.000–1.004) | 0.041 |
| DM | 1.088 (0.554–2.137) | 0.806 | 1.064 (0.512–2.214) | 0.867 |
| Hypertension | 0.955 (0.479–1.903) | 0.895 | 0.913 (0.434–1.924) | 0.812 |
| Hyperlipidemia | 1.856 (0.887–3.884) | 0.101 | 1.343 (0.544–3.317) | 0.523 |
| TG | 1.230 (0.959–1.577) | 0.103 | 1.185 (0.879–1.598) | 0.265 |
| TC | 1.418 (1.097–1.833) | 0.008 | 1.442 (1.094–1.901) | 0.009 |
| HDL | 1.557 (0.455–5.324) | 0.481 | 1.048 (0.265–4.152) | 0.946 |
| LDL | 1.519 (1.093–2.112) | 0.013 | 0.696 (0.199–2.430) | 0.570 |
| Number of the diseased vessels | 2.627 (1.671–4.132) | < 0.001 | 2.443 (1.520–3.926) | < 0.001 |
| LCX | 2.302 (1.167–4.540) | 0.016 | 2.166 (1.031–4.551) | 0.041 |
| RCA | 4.586 (2.127–9.886) | < 0.001 | 4.712 (2.078–10.684) | < 0.001 |
| CTO | 53.857 (18.276–158.710) | < 0.001 | 105.359 (25.913–428.379) | < 0.001 |
| Gensini score | 1.023 (1.014–1.033) | < 0.001 | 1.024 (1.013–1.034) | < 0.001 |
Adjusted for sex, age, smoking, BMI, TC and HDL. In this statistical analysis, plasma omentin-1 levels, as well as age, BMI, TG, TC, HDL, LDL, number of the diseased vessels and gensini score are continuous values, others are binary variables. OR, odds ratio; CI, confidence interval; Other abbreviations are showed in Table 1 and Table 2.
Fig. 2.
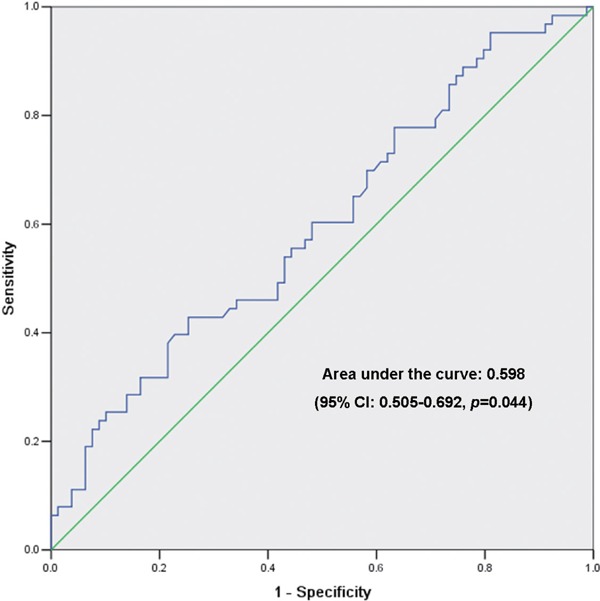
Receiver operating characteristic (ROC) curve analysis of plasma omentin-1 level for the prediction of good CCC development.
Discussion
To our knowledge, the results presented here represent the first report to demonstrate that omentin-1 was associated with good CCC development. In this study, we found that plasma omentin-1 level was significantly higher in patients with good CCC than those with poor CCC; meanwhile, logistic regression analysis and ROC curve analysis indicated that omentin-1 can serve as an independent predictor of good CCC development. With the exception of omentin-1, TC, the number of the diseased vessels, a higher frequency of LCX and RCA, CTO, and gensini score were also shown to be as an independent predictor of good CCC development.
With the extensive use of revascularization involving PCI and CABG, the treatment and prognosis of patients with CHD have been improved enormously. However, the clinical significance of adequate CCC is irreplaceable for patients who are not suitable for conventional revascularization regardless of severe coronary artery stenosis. Clinically, collateral arteries preventing myocardial ischemia during a brief occlusion are present in every third individual19). Collateral flow sufficient to prevent myocardial ischemia during coronary occlusion amounts to one-fourth –one-fifth the normal flow through the open vessel19). Moreover, well-developed CCC also in favor of the long-term clinical outcomes of patients with coronary CTO and well-developed CCC who undergo revascularization, showed that good CCC significantly lowers the incidence of cardiac death and major adverse cardiac events in the revascularization group compared to the simplex medication group as evidences20).
Considering the important clinical benefits of CCC in CHD, many studies have been established to understand the mechanism of collateral development. Numerous clinical factors were certified to be the determinants of good CCC in patients with CHD such as degree of coronary stenosis, proximal lesion location, longer duration of symptoms, longer duration of lesion occlusion, heart rate (lower), neutrophil-to-lymphocyte ratio (N/L), circulating monocyte count, and male gender7, 21–24). Among them, the degree of coronary stenosis was considered to be the strongest predictor as confirmed by several studies7). In our study, we demonstrated that the number of the diseased vessels, CTO, and gensini score not only was significant higher in patients with good CCC than in those with poor CCC, but can also serve as an independent predictor of the good CCC development. In accordance with previous studies, our results also verified that the severity of coronary stenosis was the determinant of good CCC, as it is the initiation of FSS that caused CCC development2).
Despite many advances in understanding the physiological and pathological processes of CCC, the mechanism of CCC development remains unclear, especially for the paradox that patients with the similar degree of coronary stenosis show distinct collateral vessel development. Therefore, it prompts us that some regulators may play pivotal roles in collateral vessel development out of the degree of coronary stenosis. Studies have proved that the remodeling process of mature CCC has been termed arteriogenesis25), which has been shown to be regulated by many cytokines including vascular endothelial growth factor, activin receptor-like kinase 1, MCP-1, tumor necrosis factor-alpha, and fibroblast growth factor9, 19). Except these cytokines, adipokines such as adiponectin26, 27), apelin28, 29), and secreted frizzled-related protein 530) have also been testified to be associated with collateral vessel development. Consequently, seeking and elucidating the momentous regulators involved in CCC development is worthy for clinical practice.
Omentin-1, also referred to as intelectin-1, is abundantly expressed in human visceral adipose tissue11). Studies have demonstrated that serum omentin-1 levels were associated with obesity-linked diseases and CHD and negatively correlated with the presence and severity of CHD in patients with MetS and carotid intima/media thickness in healthy men31). Given the important roles of omentin-1 in cardiovascular protective effects, we detected the plasma omentin-1 levels in patients with good and poor CCC. In our study, we firstly demonstrated that individuals with good CCC showed significant higher plasma omentin-1 levels compared with those with poor CCC. Besides, logistic regression analysis indicated that plasma omentin-1 level was an independent predictor of good CCC that was furtherly confirmed by ROC analysis. Our results indicated that plasma omentin-1 level significantly correlates with good CCC, suggesting that omentin-1 may play remarkable roles in CCC development. Nevertheless, the underlying mechanism regarding higher plasma omentin-1 levels in relation to good CCC is not fully understood. Maruyama et al.16) indicated that omentin-1 enhanced blood flow recovery and capillary density in ischemic limbs of wild-type mice in vivo, which were accompanied by increased phosphorylation of Akt and endothelial nitric oxide synthase. Meanwhile, omentin-1 also increased the capacity of tube formation and decreased apoptotic activity in cultured human umbilical vein endothelial cells16). Furthermore, as smooth muscle cell phenotype transformation is crucial for regulating CCC development8), evidences showed that omentin-1 suppressed both contractile and synthetic phenotypes (α-SMA and SMemb) with downregulation of PI3K, c-Src, Raf-1, ERK-1/2, and NF-κB, but not AKT and a-tubulin14). Besides, Takeda et al.32) showed that skewed polarization of macrophages toward an M2 phenotype supports collateral artery growth, it could be that omentin-1 regulates macrophage differentitation by inhibiting pro-inflammatory M1 phenotype and promoting pro-angiogenic M2 phenotype15). These findings are highly relevant, making the present study on the association of omentin-1 with CCC interesting and significant.
Besides, we also found that the number of the diseased vessels, a higher frequency of RCA, and CTO were independent predictor of well-developed CCC, which was consistent with the previous study28, 33). Moreover, we observed that plasma TC level also acted as an independent predictor of the good CCC development, which was different from a previous study in a Turkey population finished by Ege et al.34). Furthermore, as confirmed in previous studies12), our study showed that plasma omenin-1 level was significantly negative with BMI and positive with HDL level, but it is puzzling that positive correlation existed between plasma omenin-1 level and TC level that was inconsistent with an existing results35). In relation to these controversial results, we consider that the type of disease, sample size, inclusion criteria for patients with CHD (especially the extent of coronary stenosis), and races because of genetic diversity may make contributions. However, the detailed mechanism still needs further investigation.
There are some limitations that should be recognized in our study. First, we obtained the results from only one population with a relative small sample size. Therefore, our findings would need to be replicated with a larger sample size and another population. Second, the Rentrop scoring system was used for coronary collateral grading in our study, even though small microvascular caliber vessels may not be visualized angiographically because of modern-day digital storage media36). Finally, although we determined the remarkable correlation between plasma omentin-1 level and good CCC development, but the underlying mechanism was unclear, further studies are needed to be focus on the function of omentin-1 in CCC development.
Conclusion
In conclusion, this is the first study to demonstrate that plasma omentin-1 level is significantly higher in patients with good CCC than in those with poor CCC and can serve as an independent predictor of good CCC in patient with CHD with ≥ 90% coronary artery occlusion. Our study provides an alternative target for therapeutic strategies and medication to promote CCC development in patients with CHD. However, further studies in different populations with larger sample size are needed to confirm these findings, and additional studies are necessary to address the underlying function and mechanism.
Acknowledgments
This study was supported by the China Postdoctoral Science Foundation (2015M582350), and National Natural Science Foundation of China (No. 81570239, No. 81670453 and No. 81570461), and the National Basic Research Program of China (973 Program) (No. 2014CB542402).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1). Seiler C: The human coronary collateral circulation. Eur J Clin Invest, 2010; 40: 465-476 [DOI] [PubMed] [Google Scholar]
- 2). Schaper W: Collateral circulation: past and present. Basic Res Cardiol, 2009; 104: 5-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Karrowni W, El Accaoui RN, Chatterjee K: Coronary collateral circulation: its relevance. Catheter Cardiovasc Interv, 2013; 82: 915-928 [DOI] [PubMed] [Google Scholar]
- 4). Pérez-Castellano N, García EJ, Abeytua M, Soriano J, Serrano JA, Elízaga J, Botas J, López-Sendón JL, Delcán JL: Influence of collateral circulation on in-hospital death from anterior acute myocardial infarction. J Am Coll Cardiol, 1998; 31: 512-518 [DOI] [PubMed] [Google Scholar]
- 5). Desch S, de Waha S, Eitel I, Koch A, Gutberlet M, Schuler G, Thiele H: Effect of coronary collaterals on long-term prognosis in patients undergoing primary angioplasty for acute ST-elevation myocardial infarction. Am J Cardiol, 2010; 106: 605-611 [DOI] [PubMed] [Google Scholar]
- 6). Yaylak B, Altintas B, Ede H, Baysal E, Akyuz S, Bilge O, Sevuk U, Erdogan G, Ciftci H: Impact of Coronary Collateral Circulation on In-Hospital Death in Patients with Inferior ST Elevation Myocardial Infarction. Cardiol Res Pract, 2015; 2015: 242686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Meier P, Schirmer SH, Lansky AJ, Timmis A, Pitt B, Seiler C: The collateral circulation of the heart. BMC Med, 2013; 11: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Hutcheson R, Terry R, Chaplin J, Smith E, Musiyenko A, Russell JC, Lincoln T, Rocic P: MicroRNA-145 restores contractile vascular smooth muscle phenotype and coronary collateral growth in the metabolic syndrome. Arterioscler Thromb Vasc Biol, 2013; 33: 727-736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Simons M, Eichmann A: Molecular controls of arterial morphogenesis. Circ Res. 2015; 116: 1712-1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Li ZY, Wang P, Miao CY: Adipokines in inflammation, insulin resistance and cardiovascular disease. Clin Exp Pharmacol Physiol, 2011; 38: 888-896 [DOI] [PubMed] [Google Scholar]
- 11). Schäffler A, Neumeier M, Herfarth H, Fürst A, Schölmerich J, Büchler C: Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta, 2005; 1732: 96-102 [DOI] [PubMed] [Google Scholar]
- 12). de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman M, Fried SK, Gong DW, Shuldiner AR, Pollin TI, McLenithan JC: Omentin plasma levels and gene expression are decreased in obesity. Diabetes, 2007; 56: 1655-1661 [DOI] [PubMed] [Google Scholar]
- 13). Shang FJ, Wang JP, Liu XT, Zheng QS, Xue YS, Wang B, Zhao LY: Serum omentin-1 levels are inversely associated with the presence and severity of coronary artery disease in patients with metabolic syndrome. Biomarkers, 2011; 16: 657-662 [DOI] [PubMed] [Google Scholar]
- 14). Watanabe K, Watanabe R, Konii H, Shirai R, Sato K, Matsuyama TA, Ishibashi-Ueda H, Koba S, Kobayashi Y, Hirano T, Watanabe T: Counteractive effects of omentin-1 against atherogenesis†. Cardiovasc Res, 2016; 110: 118-128 [DOI] [PubMed] [Google Scholar]
- 15). De Jager SC, Pasterkamp G: Atheroprotective properties of human Omentin-1 in experimental atherosclerosis. Cardiovasc Res, 2016; 110: 1-3 [DOI] [PubMed] [Google Scholar]
- 16). Maruyama S, Shibata R, Kikuchi R, Izumiya Y, Rokutanda T, Araki S, Kataoka Y, Ohashi K, Daida H, Kihara S, Ogawa H, Murohara T, Ouchi N: Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism. J Biol Chem, 2012; 287: 408-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Rentrop KP, Cohen M, Blanke H, Phillips RA: Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol, 1985; 5: 587-592 [DOI] [PubMed] [Google Scholar]
- 18). Gensini GG: A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol, 1983; 51: 606. [DOI] [PubMed] [Google Scholar]
- 19). Seiler C, Stoller M, Pitt B, Meier P: The human coronary collateral circulation: development and clinical importance. Eur Heart J, 2013; 34: 2674-2682 [DOI] [PubMed] [Google Scholar]
- 20). Jang WJ, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, Kim WS, Lee YT, Gwon HC: Long-term survival benefit of revascularization compared with medical therapy in patients with coronary chronic total occlusion and well-developed collateral circulation. JACC Cardiovasc Interv, 2015; 8: 271-279 [DOI] [PubMed] [Google Scholar]
- 21). Akın F, Ayça B, Çelik Ö, Şahin C: Predictors of poor coronary collateral development in patients with stable coronary artery disease: neutrophil-to-lymphocyte ratio and platelets. Anatol J Cardiol, 2015; 15: 218-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Kocaman SA, Sahinarslan A, Akyel A, Timurkaynak T, Boyaci B, Cengel A: The association of circulating monocyte count with coronary collateral growth in patients with diabetes mellitus. Acta Diabetol, 2010; 47: 49-54 [DOI] [PubMed] [Google Scholar]
- 23). Zorkun C, Akkaya E, Zorlu A, Tandoğan I: Determinants of coronary collateral circulation in patients with coronary artery disease. Anadolu Kardiyol Derg, 2013; 13: 146-151 [DOI] [PubMed] [Google Scholar]
- 24). Kocaman SA, Arslan U, Tavil Y, Okuyan H, Abaci A, Cengel A: Increased circulating monocyte count is related to good collateral development in coronary artery disease. Atherosclerosis, 2008; 197: 753-756 [DOI] [PubMed] [Google Scholar]
- 25). Sweet DT, Chen Z, Givens CS, Owens AP, 3rd, Rojas M, Tzima E: Endothelial Shc regulates arteriogenesis through dual control of arterial specification and inflammation via the notch and nuclear factor-κ-light-chain-enhancer of activated B-cell pathways. Circ Res, 2013; 113: 32-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Mouquet F, Cuilleret F, Susen S, Sautière K, Marboeuf P, Ennezat PV, McFadden E, Pigny P, Richard F, Hennache B, Vantyghem MC, Bertrand M, Dallongeville J, Jude B, Van Belle E: Metabolic syndrome and collateral vessel formation in patients with documented occluded coronary arteries: association with hyperglycaemia, insulin-resistance, adiponectin and plasminogen activator inhibitor-1. Eur Heart J, 2009; 30: 840-849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Soydinc S, Davutoglu V, Sari I: High serum levels of adiponectin improve coronary collateral development in patients with coronary artery disease. Tohoku J Exp Med, 2007; 211: 347-352 [DOI] [PubMed] [Google Scholar]
- 28). Akboga MK, Akyel A, Sahinarslan A, Demirtas CY, Yayla C, Boyaci B, Yalcin R: Relationship between plasma apelin level and coronary collateral circulation. Atherosclerosis, 2014; 235: 289-294 [DOI] [PubMed] [Google Scholar]
- 29). Momiyama Y: Association between plasma apelin levels and coronary collateral development in patients with stable angina pectoris. Atherosclerosis, 2014; 235: 349-350 [DOI] [PubMed] [Google Scholar]
- 30). Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT, Shimizu I, Fuster JJ, Katanasaka Y, Yoshida S, Qiu Y, Yamaguchi TP, Matsushita T, Murohara T, Gokce N, Bates DO, Hamburg NM, Walsh K: An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med, 2014; 20: 1464-1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Fuster JJ, Ouchi N, Gokce N, Walsh K: Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ Res, 2016; 118: 1786-1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Takeda Y, Costa S, Delamarre E, Roncal C, Leite de Oliveira R, Squadrito ML, Finisguerra V, Deschoemaeker S, Bruyère F, Wenes M, Hamm A, Serneels J, Magat J, Bhattacharyya T, Anisimov A, Jordan BF, Alitalo K, Maxwell P, Gallez B, Zhuang ZW, Saito Y, Simons M, De Palma M, Mazzone M: Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature, 2011; 479: 122-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Fan Y, Li S, Li XL, Lin XL, Zhu CG, Xu RX, Qing P, Wu NQ, Jiang LX, Xu B, Gao RL, Li JJ: Plasma endothelin-1 level as a predictor for poor collaterals in patients with ≥ 95% coronary chronic occlusion. Thromb Res, 2016; 142: 21-25 [DOI] [PubMed] [Google Scholar]
- 34). Ege MR, Acıkgoz S, Zorlu A, Sıncer I, Guray Y, Guray U, Demirkan B, Kisacik H: Mean platelet volume: an important predictor of coronary collateral development. Platelets, 2013; 24: 200-204 [DOI] [PubMed] [Google Scholar]
- 35). Saremi A, Asghari M, Ghorbani A: Effects of aerobic training on serum omentin-1 and cardiometabolic risk factors in overweight and obese men. J Sports Sci, 2010; 28: 993-998 [DOI] [PubMed] [Google Scholar]
- 36). Traupe T, Gloekler S, de Marchi SF, Werner GS, Seiler C: Assessment of the human coronary collateral circulation. Circulation, 2010; 122: 1210-1220 [DOI] [PubMed] [Google Scholar]


