Abstract
Aim: We studied the frequency of Achilles tendon xanthoma (ATX) in patients with acute coronary syndrome (ACS). Furthermore, we investigated the differences in clinical findings between ACS patients with and without ATX.
Methods: Patients with ACS (n = 335) were admitted to the coronary care unit of Nippon Medical School between July 2011 and December 2014. Informed consent for the measurement of Achilles tendon thickness (ATT) on a radiograph was obtained from 228 patients without tendon rupture. ATT of each side was measured on the radiograph in patients with ACS and in those with acromegaly (n = 18), non-familial hypercholesterolemia (non-FH; n = 96), and familial hypercholesterolemia (FH; n = 31).
Results: ATT of the right and left side in ACS patients were 6.9 ± 1.3 and 7.0 ± 1.6 (mm; mean ± SD). In acromegaly, non-FH, and FH patients, ATT of the right/left side were 6.6 ± 1.1/6.7 ± 1.1, 6.2 ± 0.9/6.6 ± 1.0, and 9.4 ± 3.3/10.0 ± 3.1, respectively. ATX (ATT ≥ 9 mm) was found in 26 (11.4%) patients with ACS. Patients with acromegaly and non-FH had no ATX, whereas all patients with FH had ATX. No differences in age and serum lipid profiles were observed between ACS patients with and without ATX. The levels of body mass index and glycated hemoglobin of ACS patients with ATX were significantly greater than those in ACS patients without ATX (26.8 ± 4.0 vs. 23.9 ± 3.3, p < 0.05, and 6.9 ± 1.4% 6.3 ± 1.3%, p < 0.05, respectively).
Conclusion: This is the first report in which the frequency of ACS patients with ATX was 11.4%. The serum lipid profiles of ACS patients with ATX were similar to those without ATX. In the future, ACS patients with ATX will be diagnosed as having FH.
Keywords: Acute coronary syndrome, Achilles tendon thickness, Achilles tendon xanthoma
Introduction
Achilles tendon xanthoma (ATX) must be essentially considered for the diagnosis of familial hypercholesterolemia (FH). For the diagnosis of ATX, Achilles tendon thickness (ATT) on the radiograph is typically measured1–3) and the criterion of xanthoma in Japan is a thickness ≥ 9 mm4). Xanthoma is observed in 100% of homozygotes and 87% of heterozygotes5). FH is a critically high-risk disease that predisposes individuals to premature coronary artery disease (CAD)6, 7); therefore, its early diagnosis is desired. The frequency of the heterozygous genotypes is reportedly 1/5007, 8) to 1/2009, 10) in the general population. The coexistence of ATX and hypercholesterolemia is considered to strongly suggest FH. Meanwhile, the frequency of FH in patients with acute myocardial infarction is recently reported through the scoring of clinical findings for diagnosis of FH; however, ATT was not measured11).
We investigated the frequency of ATX in patients with ACS. In addition, we compared the lipid profile and clinical findings between patients with and without ATX to characterize the differences between them.
Subjects and Methods
Of the patients with ACS (n = 335) who were consecutively admitted to the coronary care unit of the Nippon Medical School from July 2011 to December 2014, 16 patients died before they could be subjected to our study, 37 were discharged before examination of the radiograph, and 52 did not provide informed consent as shown in Fig. 1. Furthermore, 230 patients (181 men, 49 women) provided informed consent for the study of ATT. ATT was measured on the radiograph by the methods recommended in the Guidelines for the Management of Familial Hypercholesterolemia by the Japan Atherosclerosis Society (JAS)4, 5). With regard to the control, ATT of patients with acromegaly (n = 22), non-FH (n = 122), and FH (n = 35) under treatment in our outpatient clinic were compared with ATT of ACS patients. The diagnosis of FH in our outpatient clinic was made according to the JAS diagnostic criteria4, 5). The radiographs were previously examined for the diagnosis of heel-pad thickness in acromegaly and for ATX in outpatients with hypercholesterolemia. The two clinicians (A and B) independently measured ATT on radiographs, and the difference of diagnosis of ATX was studied to check the examiner effect.
Fig. 1.
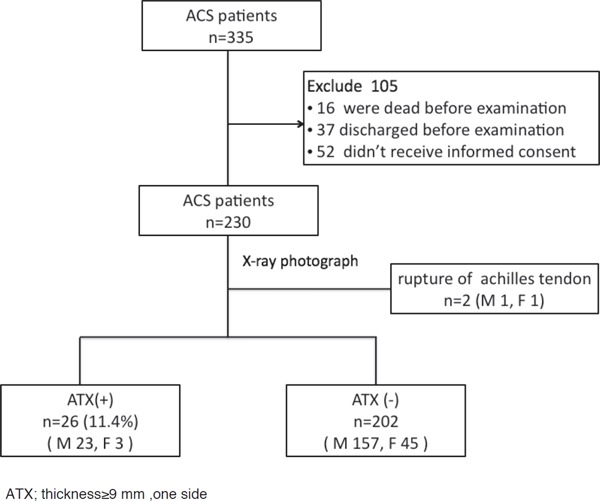
Flowchart of patient enrollment
ACS; Acute coronary syndrome, ATT; Achilles tendon thickness, ATX; Achilles tendon xanthoma, thickness ≥ 9 mm of one side.
The venous blood was prepared at the time of admission, so the patients' conditions, fasting or post-prandial, were unclear. The laboratory examinations were performed using automatic methods in our hospital's laboratory. This study was approved by the ethics committee of Nippon Medical School.
Statistical Analysis
The statistical differences between the two groups were calculated using the Wilcoxon rank sum test and Pearson's chi-square test. P-values less than 0.05 were considered to be significant. Data were processed using JMP 9 statistical software (SAS Institute) and are shown as mean ± SD.
Results
There were two cases (one man aged 85 years and one woman aged 73 years) of a unilateral thickened Achilles tendon (20.5 mm and 16.7 mm, respectively), with a normal tendon thickness of the contralateral side (6.5 mm and 7.2 mm, respectively). Each patient had a history of tendon rupture. Therefore, these two cases were omitted from the analysis, and 228 subjects were studied.
The two examiners, A and B, measured ATT of patients with ACS on radiograph to confirm ATX. The measurement value of ATT was significantly correlated between the clinician A and B, as shown in Fig. 2. One case was mismatched between the examiner A and B for diagnosis of ATX. This case was included to patients with ATX because this patient was young (aged 36 years) and had hypercholesterolemia (250 mg/dl of serum TC), and one examiner diagnosed ATT ≥ 9.0 mm.
Fig. 2.
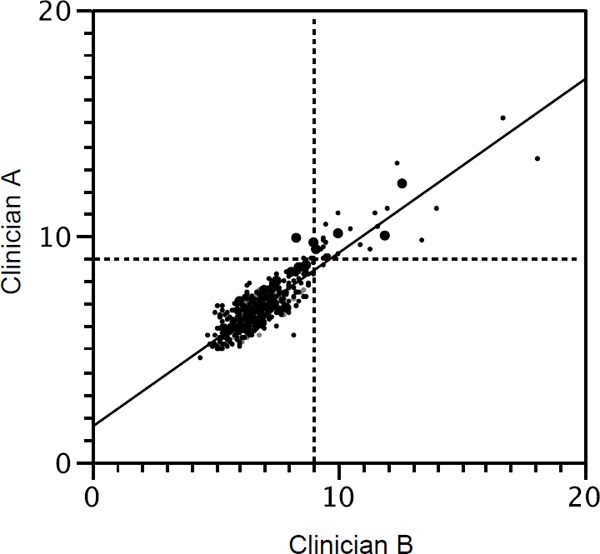
Correlation of measurement of Achilles tendon thickness (mm) between Clinician A and B
Y = 1.54 + 0.78X, R2 = 0.826, ICC(2,1) = 0.886: ICC: intra-class correlation coefficients
ATT of the right and left side in ACS patients were 6.9 ± 1.3 and 7.0 ± 1.6 mm. In acromegaly, non-FH, and FH patients, ATT of the right/left side were 6.6 ± 1.1/6.7 ± 1.1, 6.2 ± 0.9/6.6 ± 1.0, and 9.4 ± 3.3/10.0 ± 3.1, respectively. ATX (ATT ≥ 9 mm) was found in 26 (11.4%) of the patients with ACS. Patients with acromegaly and non-FH had no ATX, whereas all patients with FH had ATX.
ATT and lipid profiles are shown in Table 1. No differences in age and serum lipid profiles were observed between ACS patients with and without ATX. The serum cholesterol levels of ACS patients with ATX were not significantly higher than those without ATX and were much lower than FH outpatients before treatment (294 ± 58 mg/dl).
Table 1. Serum lipid profile of patients with acute coronary syndrome (ACS).
| Group | total | with ATX | without ATX | p value |
|---|---|---|---|---|
| n | 228 | 26 | 202 | |
| ATT-right (mm) | 6.9 ± 1.3 | 9.2 ± 1.7 | 6.6 ± 0.9 | |
| -left (mm) | 7.0 ± 1.6 | 9.9 ± 2.8 | 6.6 ± 0.9 | |
| Age (y) | 67 ± 12 | 65 ± 14 | 67 ± 12 | 0.65 |
| TC (mg/dl) | 179 ± 45 | 191 ± 54 | 178 ± 44 | 0.38 |
| HDL-C (mg/dl) | 45 ± 13 | 43 ± 11 | 45 ± 13 | 0.40 |
| TG (mg/dl) | 115 ± 81 | 132 ± 73 | 113 ± 83 | 0.09 |
| non HDL-C (mg/dl) | 134 ± 44 | 149 ± 53 | 133 ± 42 | 0.20 |
Data were shown as mean ± SD.
ATX = Achilles tendon xanthoma, ATT = Achilles tendon thickness, FH = familial hypercholesterolemia, TC= total cholesterol, TG = triglyceride, HDL-C = high-density lipoprotein cholesterol,
*with ATX vs without ATX; p < 0.05 by by Wilcoxon runk sum test
The clinical findings of ACS patients are shown in Table 2. The mean age of the first onset of ACS was slightly lower in the patients with ATX than those without ATX. The frequency of a family history of coronary heart disease (CHD) in patients with ATX (42.3%) was insignificantly higher than those without ATX (33.1%). No differences were observed in blood pressure, plasma glucose levels, smoking habits, statin pre-treatment, or ACS treatment method. Body mass index and hemoglobin A1c levels were significantly greater in patients with ATX than in those without ATX. The number of statin treatments did not differ between the two groups.
Table 2. Clinical character.
| Group (n) | with ATX (26) | without ATX (202) | p value |
|---|---|---|---|
| Gender (men/women) | 23/3 | 157/45 | |
| Age of the first onset of ACS (y) | 62 ± 15 | 65 ± 13 | 0.35 |
| SBP (mmHg) | 136 ± 26 | 138 ± 26 | 0.65 |
| DBP (mmHg) | 77 ± 17 | 79 ± 17 | 0.37 |
| BMI | 26.1 ± 4.0 | 24.0 ± 3.5 | < 0.01* |
| Plasma glucose (mg/dl) | 189 ± 99 | 160 ± 71 | 0.34 |
| HbA1c (%) | 7.1 ± 1.8 | 6.3 ± 1.3 | < 0.01* |
| Hypertension (%) | 19 (73.1) | 146 (72.2) | 0.84 |
| Family history of CHD (%) | 11 (42.3) | 67 (33.1) | 0.38 |
| Smoking (none/past/current) | 6/9/11 | 55/66/81 | 0.91 |
| Pre-treatment with statin (%) | 10 (38.5) | 55 (27.2) | 0.43 |
| ACS (STEMI/NSTEMI/recent MI/UA) | 15/2/2/7 | 126/24/15/37 |
Data were shown as mean ± SD.
by Wilcoxon runk sum test
ATX = Achilles tendon xanthoma, STEMI = ST-segment elevation myocardial infarction, NSTEMI = non–ST-segment elevation myocardial infarction, recent MI = recent myocardial infarction, UA = Unstable angina
The frequency of ATX was studied according to the age of the first onset of ACS. In younger patients (n = 49), including 38 men (< 55 years old) and 11 women (< 65 years old), the frequency of ATX was 18.4%. In older patients (n = 179), including 142 men (≥ 55 years old) and 37 women (≥ 65 years old), the frequency of ATX was 9.5%. The frequency was higher in the younger patients than in the older patients but not significant (p = 0.08 by Pearson's chi-square test).
Discussion
This is the first report focusing on the incidence of ATX in patients with new-onset ACS, which was 11.4%. Pang, et al.11) reported that the frequency of FH in patients with early onset coronary arterial diseases was 14.3%. They used the Dutch Lipid Clinic Network Criteria (DLCNC)10, 12) for the diagnosis of FH. They did not measure ATT, and the frequency of ATX was not discussed.
In the report by Pang, et al.11), the serum cholesterol concentration of patients diagnosed as FH was high and the point of DLCNC was increased. Moreover, the serum total cholesterol (TC) level is an important item in the diagnosis of FH as per the JAS recommendation and other Asian countries13). In this study, the mean TC level in ACS patients with ATX was slightly higher than those without ATX, but the difference was not significant. TC levels in ACS patients were much lower than those of FH patients in our outclinic setting. This result was contrary to our expectation.
LDL-cholesterol (LDL-C) level reportedly decreased after the onset of ACS and remained low for several weeks14). The inflammatory factors would relate to the decrease of LDL-C15). However, no reports to date have shown a decrease in LDL-C after ACS in FH. If ACS patients with ATX had FH in this study, the decrease in LDL-C would be too large to consider LDL-C level of FH. From this viewpoint, changes in lipids profile should be sequentially monitored after the onset of ACS in patients with FH.
The frequency of a family history of CHD (Table 2) was higher in ACS patients with ATX (42.3%) than in those without ATX (33.1%); however, the difference was small and insignificant. The family history of hypercholesterolemia must be essentially considered for diagnosis of FH. In this study, the family history of hypercholesterolemia was not clarified. The difference of age at the first onset of ACS (3 years) was small and insignificant between the ATX and non-ATX groups. These data were not likely the characteristics of FH.
In conclusion, the frequency of ATX in patients with ACS was 11.4%. TC levels of patients with bot ACS and ATX were unexpectedly low. In the future, the ACS patients with ATX will be diagnosed as having FH.
Acknowledgement
We greatly thank the staff members of the coronary care unit, Nippon Medical School, Tokyo. This study was partially supported by the Japanese Ministry of Health, Labour and Welfare.
Conflict of Interest
None.
References
- 1). Mabuchi H, Ito S, Haba T, Ueda K, Ueda R, Tatami R, Kametani T, Koizumi J, Ohta M, Miyamoto S, Takeda R, Takegoshi T: Discrimination of familial hypercholesterolemia and secondary hypercholesterolemia by Achilles' tendon thickness. Atherosclerosis 1977; 28: 61-68 [DOI] [PubMed] [Google Scholar]
- 2). Blankenhorn DH, Meyers HI: Radiographic determination of Achilles tendon xanthoma size. Metabolism 1969; 18: 882-886 [DOI] [PubMed] [Google Scholar]
- 3). Gattereau A, Davignon J, Levesque HP: Roentgenological evaluation of Achilles-tendon xanthomatosis. Lancet 1971; 7726: 705-706 [DOI] [PubMed] [Google Scholar]
- 4). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K: Committee Report 9: Familial Hypercholesterolemia: Executive Summary of the Japan Atheroslcerosis Society (JAS) for the Diagnosis and Preventionn of Atherosclerotic Cardiovascular Diseases in Japan-2012 Version. J Atheroscler Thromb 2014; 21: 6-10 [DOI] [PubMed] [Google Scholar]
- 5). Bujo H, Takahashi K, Saito Y, Maruyama T, Yamashita S, Matsuzawa Y, Ishibashi S, Shionoiri F, Yamada N, Kita T, Research Committeon Primary Hyperlipidemia of the Ministry of Health, Labour, and Welfare of Japan : Clinical features of familial hypercholesterolemia in Japan in a database from 1996–1998 by the research committee of the ministry of health, labour and welfare of Japan. J Atherosclr Thromb 2004; 11: 146-151 [DOI] [PubMed] [Google Scholar]
- 6). Mabuchi H, Koizumi J, Shimizu M, Takeda R: Development of coronary heart disease in familial hypercholesterolemia. Circulation, 1989; 79: 225-232 [DOI] [PubMed] [Google Scholar]
- 7). Goldstein JL, Hazzard WR, Schrott HG, Bierman EL, Motulsky AG: Hyperlipidemia in coronary heart disease. I. Lipid levels in 500 survivors of myocardial infarction. J Clin Invest. 1973; 52: 1533-1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG: Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest 1973; 52: 1544-1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Mabuchi H, Nohara A, Noguchi T, Kobayashi J, Kawashiri MA, Tada H, Nakanishi C, Mori M, Yamagishi M, Inazu A, Koizumi J: Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan.” Atherosclerosis 2011; 214: 404-407 [DOI] [PubMed] [Google Scholar]
- 10). Hopkins PN, Toth PP, Ballantyne CM, Rader DJ: Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 2011; 5 (Suppl): S9-17 [DOI] [PubMed] [Google Scholar]
- 11). Pang J, Poulter EB, Bell DA, Bates TR, Jefferson VL, Hillis GS, Schultz CJ, Watts GF: Frequency of familial hypercholesterolemia in patients with early-onset coronary artery disease admitted to a coronary care unit. J Clin Lipidol 2015; 9: 703-708 [DOI] [PubMed] [Google Scholar]
- 12). Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab 2012; 97: 3956-3964 [DOI] [PubMed] [Google Scholar]
- 13). Zhou M, Zhao D. Familial Hypercholesterolemia in Asian Populations. J Atheroscler Thromb. 2016; 23: 539-249 [DOI] [PubMed] [Google Scholar]
- 14). National Institutes of Health: Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adults Treatment Panel III), Final Report. NIH Publication No. 02-5215, September 2002
- 15). Shrivastava AK, Singh HV, Raizada A, Singh SK: Serial measurement of lipid profile and inflammatory markers in patients with acute myocardial infarction. Excli J 2015; 14: 517-526 [DOI] [PMC free article] [PubMed] [Google Scholar]


