Abstract
Aim: Adiponectin (APN) is an adipocyte-derived bioactive molecule with antiatherogenic properties. We previously reported that cystatin C (CysC) abolished the anti-atherogenic effects of APN. We aimed to elucidate the clinical significance of CysC–APN complex in patients with coronary artery disease (CAD).
Methods: We enrolled 43 stable CAD male patients to examine the relationship between CysC–APN complex and coronary plaque characteristics. Serum was immunoprecipitated by the anti-APN antibody and immunoblotted by the anti-CysC antibody to demonstrate the presence of CysC–APN complexes in vivo. To confirm their binding in vitro, HEK293T cell lysates overexpressing myc-APN and FLAG-CysC were immunoprecipitated with an anti-myc or anti-FLAG antibody, followed by immunoblotting with an anti-APN or anti-CysC antibody.
Results: CysC was identified as a specific co-immunoprecipitant with APN by the anti-APN antibody in human serum. In vitro, FLAG-CysC was co-immunoprecipitated with myc-APN by the antimyc antibody and myc-APN was co-immunoprecipitated with FLAG-CysC by the anti-FLAG antibody. Among CAD patients, serum CysC–APN complex levels negatively correlated with fibrotic components of coronary plaques and positively correlated with either necrotic or lipidic plus necrotic components. Plaque burden negatively correlated with serum APN levels but not serum CysC–APN complex levels. Serum CysC levels had no association with plaque characteristics. In multivariate analysis, CysC–APN complex levels were identified as the strongest negative factor for fibrotic components and the strongest positive factor for both necrotic and lipidic plus necrotic components.
Conclusion: Measuring serum CysC–APN complex levels is helpful for evaluating coronary plaque instability in CAD patients.
Keywords: Adiponectin, Cystatin C, Atherosclerosis, Plaque, Coronary artery disease
Introduction
Coronary artery disease (CAD) is one of the leading causes of death in many countries. The global status report on noncommunicable diseases 2014 demonstrated that 17.5 million people died of CAD in 2012 and that the number of deaths due to CAD is predicted to increase to 22.2 million in 20301). Although a reduction in blood pressure and serum low-density lipoprotein-cholesterol (LDL-C) and glucose levels improves the prognosis of cardiovascular diseases2–4), patients with well-controlled these parameters still have a high risk of cardiovascular events. Acute coronary syndrome (ACS) is induced by the rupture of an atherosclerotic plaque and subsequent luminal thrombosis5), and a vulnerable plaque is characterized by its large necrotic core and fibrous cap thinning6, 7). Therefore, the accurate evaluation of a coronary plaque is important for identifying high-risk patients having a vulnerable plaque.
Intravascular ultrasound (IVUS) is a useful modality for intracoronary imaging8), and the iMap®-IVUS system (Boston Scientific, Marlborough, MA) is a developed version using a 40 MHz radiofrequency. Previous studies have validated that iMap®-IVUS can accurately evaluate lesion components both in vitro and in vivo9). This system classifies coronary plaque composition into four subtypes, fibrotic, lipidic, necrotic, and calcified, and evaluates plaque vulnerability. However, the detection of a vulnerable plaque using IVUS is invasive. A reliable and simple screening method to select candidates whose coronary arteries should be examined in detail with IVUS is urgently needed.
Visceral fat obesity is a common feature in CAD patients. We found adiponectin (APN), the most abundant adipocyte-derived secretory protein from human visceral fat tissues, and plasma APN levels negatively correlate with human visceral fat mass10). High plasma levels of APN are associated with insulin sensitivity in the healthy population11), and hypoadiponectinemia is an independent risk factor for diabetes, hypertension, and CAD12–14). Many clinical and experimental studies have revealed that APN has beneficial effects on the cardiovascular system15, 16) and that low plasma APN levels are associated with the presence of a vulnerable plaque in stable CAD male patients17). However, the mechanisms by which APN affects the incidence of cardiovascular events in stable CAD patients are still unknown.
We have demonstrated that proteins interacting with APN (e.g., platelet-derived growth factor-BB, calreticulin, cystatin C (CysC), E-selectin ligand-1, and Mac-2 binding protein) modulate APN-mediated vasculoprotective effects18–22). CysC is a cysteine protease inhibitor that is constantly produced by human cells in general and excreted into the bloodstream23). Serum CysC levels are a well-known marker of renal function and are suggested to be superior to serum creatinine levels24, 25), and a combination of CysC and creatinine is more accurate than each separately26, 27). Elevated serum CysC levels indicate an increasing risk for cardiovascular events in subjects regardless of renal dysfunction28, 29). We have previously reported that CysC reduces the clearance of plasma APN, leading to the inhibition of APN-mediated vasculoprotective effects19), and a recent report has demonstrated that serum CysC levels might be linked to carotid plaque size and instability30). However, the clinical significance of the CysC–APN complex on plaque vulnerability is still unknown.
Aim
We aimed to demonstrate the CysC–APN interaction thoroughly both in vivo and in vitro and to clarify its clinical significance on coronary plaque instability in stable CAD patients with relatively normal renal function.
Methods
Immunoprecipitation and Immunoblotting
Transfection, immunoprecipitation, and immunoblotting were performed as described previously20, 22). Briefly, 100 µg mouse anti-human APN monoclonal antibody (ANOC9121; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) or mouse control immunoglobulin G (IgG) was coupled to 5 mg tosylactivated Dynabeads® (M-280; Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. After washing, approximately 0.4 mg of these magnetic beads was used for immunoprecipitation of human serum containing 200 ng APN. For in vitro analysis, expression vectors of myc-APN and FLAG-CysC31) (kindly provided by Dr. John Hulleman) were co-transfected into HEK293T cells with Lipofectamine 2000 (Life Technologies, Carlsbad, CA), according to the manufacture's protocol. The cell lysates were used for immunoprecipitation with anti-c-myc magnetic beads (Thermo Fisher Scientific, Waltham, MA) or anti-FLAG M2 magnetic beads (Sigma-Aldrich, St. Louis, MO). After gel electrophoresis and transfer to the nitrocellulose membranes (BioRad, Hercules, CA), the membranes were sequentially incubated with a biotinylated mouse antihuman APN antibody (100 ng/mL for in vivo or 200 ng/mL for in vitro analysis) and an HRP-conjugated streptavidin (Thermo Fisher Scientific, Waltham, MA), or with an anti-human CysC monoclonal antibody (Abcam, Cambridge, UK) and an HRP-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). The secondary antibody was detected by enhanced chemiluminescence systems (Thermo Fisher Scientific, Waltham, MA). The CysC band intensity measured with Image J (National Institutes of Health, Bethesda, MD) was used to identify CysC–APN complex levels.
Subjects
The enrolled subjects in this study were 43 male patients with stable angina who underwent selective percutaneous coronary intervention and IVUS at the Department of Cardiology in Hyogo Prefectural Nishinomiya Hospital, Japan. Patients who were aged > 85 years, had renal dysfunction (serum creatinine > 1.5 mg/dL) or malignant diseases, or whose target lesions were chronic total occlusion or in-stent restenosis were excluded from this study. This study was conducted according to the Declaration of Helsinki, and was approved by the ethics committees of both Hyogo Prefectural Nishinomiya Hospital and Osaka University Graduate School of Medicine. Written informed consent was obtained from each patient.
Measurement of Serum Parameters
Fasting serum biochemical markers were measured in commercial chemical laboratories. APN levels were measured by using a total APN enzyme-linked immunosorbent assay (ELISA) kit (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) according to the manufacturer's instructions.
Evaluation of Plaques in Coronary Arteries by iMap®-IVUS
IVUS was performed with a 40 MHz catheter (AtlantisTM SR Pro Coronary Imaging Catheter, Boston Scientific, Marlborough, MA) and the evaluation of the plaque characteristics were performed as described previously32). The plaques were classified into four types according to the tissue components, and the percentage of each component in the entire plaque was calculated as “components volume (mm3)/plaque volume (mm3) × 100” and named as fibrotic, lipidic, necrotic, or calcified components, respectively.
Statistical Analysis
Statistical analysis was performed with JMP Pro version 11.2.1 (SAS Institute Inc., Cary, NC). Spearman's correlation coefficient and multiple regression analysis were used to evaluate the association between plaque and clinical characteristics. Statistical significance was defined as p < 0.05.
Results
CysC Binds to APN in vivo and in vitro
To investigate the CysC–APN complex in vivo, human serum was used for immunoprecipitation with the anti-APN antibody or control IgG. Immunoblotting with the anti-APN or anti-CysC antibody demonstrated that CysC was specifically co-immunoprecipitated with APN (Fig. 1A), indicating the presence of the CysC–APN complex in the human serum.
Fig. 1.
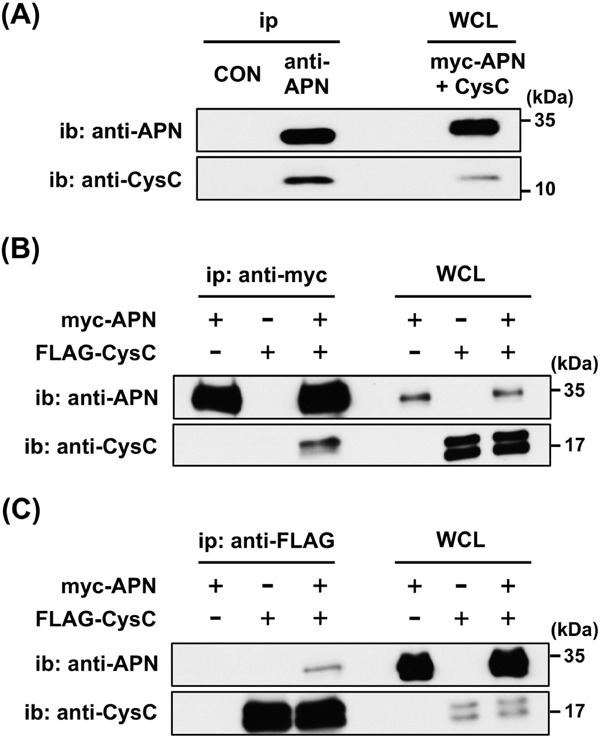
CysC–APN complex in serum and in vitro.
(A) Human serum was immunoprecipitated by the anti-APN antibody, followed by immunoblotting with either the anti-APN or anti-CysC antibody. Whole cell lysate (WCL) of HEK293T cells overexpressing both myc-tagged APN and CysC was used as a positive control. Experiments were repeated more than five times, and representative data are shown.
(B and C) Cell lysate expressing myc-APN and FLAG-CysC was used for the immunoprecipitation and immunoblotting. FLAG-CysC was co-immunoprecipitated with myc-APN by the anti-myc antibody (B), and myc-APN was coimmunoprecipitated with FLAG-CysC by the anti-FLAG antibody (C).
CON: control IgG, WCL: whole cell lysate, ip: immunoprecipitation, ib: immunoblotting. Experiments were repeated more than three times, and representative data are shown.
To confirm this CysC–APN interaction in vitro, HEK293T cell lysates overexpressing myc-APN and FLAG-CysC were immunoprecipitated with the antimyc antibodies, followed by immunoblot analysis using the anti-APN or anti-CysC antibody. FLAG-CysC was specifically co-immunoprecipitated with myc-APN by the anti-myc antibody (Fig. 1B). In addition, the same cell lysates immunoprecipitated with the anti-FLAG antibody demonstrated that myc-APN was also the specific co-immunoprecipitant with FLAG-CysC (Fig. 1C).
Patient Characteristics
The clinical characteristics of the 43 male CAD patients are listed in Table 1. All patients were diagnosed with stable angina and received medication, including statins (56%), anti-platelet agents (81%), anti-hypertensive agents (67%), and anti-diabetic agents (42%) (Table 2). Due to medical treatment, fasting blood sugar (FBS) levels were well controlled (HbA1c: 6.5% ± 0.8%). However, blood pressure and serum LDL-C levels were not sufficiently controlled (blood pressure: 133/78 mmHg, LDL-C: 108 ± 32 mg/dL). The average age and body mass index (BMI) were 69.3 ± 9.5 years and 25.1 ± 3.8 kg/m2, respectively.
Table 1. Clinical characteristics of the enrolled patients.
| mean ± SD | range | |
|---|---|---|
| Age (year) | 69.3 ± 9.5 | 37–83 |
| BMI (kg/m2) | 25.1 ± 3.8 | 19.9–34.4 |
| Systolic blood pressure (mmHg) | 133 ± 18 | 92–170 |
| Diastolic blood pressure (mmHg) | 78 ± 10 | 60–103 |
| Fasting blood sugar (mg/dL) | 111 ± 26 | 77–202 |
| IRI (µU/mL) | 8.5 ± 5.5 | 1.6–23.2 |
| HOMA–R | 2.3 ± 1.6 | 0.42–7.5 |
| HbA1c (NGSP) (%) | 6.5 ± 0.8 | 5.2–8.5 |
| Total cholesterol (mg/dL) | 175 ± 35 | 109–267 |
| Triglyceride (mg/dL) | 127 ± 61 | 50–361 |
| HDL–C (mg/dL) | 42.3 ± 9.4 | 26–68 |
| LDL–C (mg/dL) | 108 ± 32 | 50–200 |
| hsCRP (mg/dL) | 0.21 ± 0.29 | 0.01–1.16 |
| serum creatinine (mg/dL) | 0.89 ± 0.22 | 0.48–1.40 |
| eGFR (mL/min/1.73 m2) | 70.6 ± 20.9 | 38.2–127.9 |
| CysC (mg/L) | 1.14 ± 0.30 | 0.63–1.77 |
| APN (µg/mL) | 9.9 ± 5.0 | 3.5–28.1 |
Data are expressed as mean ± standard deviation (SD).
IRI: immunoreactive insulin, hsCRP: high-sensitive C-reactive protein.
Table 2. Medication of the patients.
| number (%) | |
|---|---|
| Statins | 24 (55.8%) |
| Anti-platelet agents | 35 (81.4%) |
| Anti-hypertensive agents | 29 (67.4%) |
| Anti-diabetic agents | 18 (41.9%) |
| Oral hypoglycemic agents (OHA) alone | 12 (27.9%) |
| Insulin alone | 4 (9.3%) |
| OHA + Insulin | 2 (4.7%) |
Table 3 shows the coronary plaque characteristics of the CAD patients evaluated by iMap®-IVUS. At the culprit lesion, the plaque burden and the percentage contribution of each component to the entire plaque were calculated. The average plaque burden was 64.3% ± 5.0%.
Table 3. Characteristics of coronary plaques.
| mean ± SD | range | |
|---|---|---|
| Plaque burden (%) | 64.3 ± 5.0 | 55.1–78.2 |
| Fibrotic components (%) | 54.1 ± 12.7 | 29.2–86.6 |
| Lipidic components (%) | 10.3 ± 2.2 | 4.5–14.8 |
| Necrotic components (%) | 32.1 ± 11.0 | 8.6–55.4 |
| Calcified components (%) | 4.0 ± 3.0 | 0.6–11.8 |
| Lipidic plus Necrotic components (%) | 42.4 ± 12.3 | 13.1–67.6 |
Data are expressed as mean ± SD.
CysC–APN Complex is Associated with Coronary Plaque Instability
To clarify the clinical significance of the CysC–APN complex with plaque vulnerability, serum CysC–APN complex levels in CAD patients were assessed by immunoprecipitation–immunoblot analysis. The association between plaque characteristics and serum APN, CysC, or CysC–APN complex levels are shown in Table 4. Among them, serum CysC–APN complex levels negatively correlated with fibrotic components (r = −0.384, p = 0.011) and positively correlated with necrotic (r = 0.406, p = 0.007) and lipidic plus necrotic (r = 0.417, p = 0.005) components (Fig. 2A–D). The plaque burden negatively correlated with serum APN levels (r = −0.317, p = 0.039) but not with serum CysC–APN complex levels (Fig. 2E and F). Serum CysC levels correlated with neither the plaque burden nor any component.
Table 4. Correlation between plaque characteristics and APN, CysC, or CysC–APN complex.
| APN |
CysC |
CysC–APN complex |
||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Plaque burden (%) | −0.317 | 0.039 | −0.064 | 0.682 | −0.020 | 0.899 |
| Fibrotic components (%) | 0.123 | 0.431 | −0.002 | 0.990 | −0.384 | 0.011 |
| Lipidic components (%) | −0.114 | 0.467 | 0.140 | 0.370 | 0.171 | 0.273 |
| Necrotic components (%) | −0.171 | 0.273 | −0.064 | 0.682 | 0.406 | 0.007 |
| Calcified components (%) | 0.107 | 0.497 | 0.058 | 0.712 | 0.034 | 0.830 |
| Lipidic plus Necrotic components (%) | −0.179 | 0.251 | −0.002 | 0.990 | 0.417 | 0.005 |
The relationships of the parameters were investigated by Spearman's correlation coefficient.
Fig. 2.
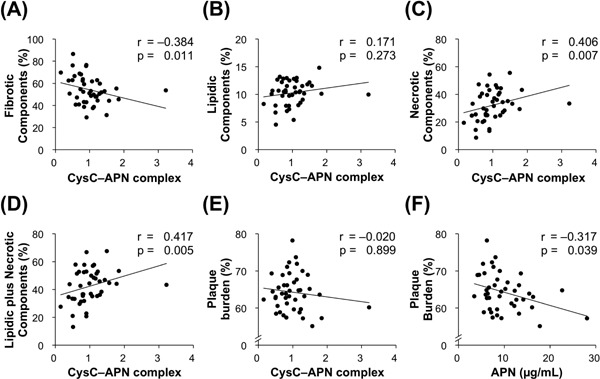
CysC–APN complex levels correlate with coronary plaque instability.
(A–D) Serum CysC–APN complex levels negatively correlated with fibrotic components and positively correlated with both necrotic and lipidic plus necrotic components (n = 43).
(E and F) Plaque burden was negatively correlated with serum APN levels, but not with serum CysC–APN complex levels (n = 43).
Factors Affecting Plaque Burden and Components
We next performed univariate and multivariate analyses to determine the factors affecting the plaque burden and components (Table 5). Univariate analysis showed that the plaque burden was positively correlated with BMI (r = 0.474, p = 0.002) and negatively correlated with systolic blood pressure (r = −0.313, p = 0.041) and APN. Fibrotic components were negatively correlated with CysC–APN complex levels and FBS (r = −0.301, p = 0.050). Necrotic components and lipidic plus necrotic components positively correlated with FBS (necrotic: r = 0.352, p = 0.021, lipidic plus necrotic: r = 0.313, p = 0.041) and CysC–APN complex levels. Lipidic components were positively correlated with immunoreactive insulin (r = 0.442, p = 0.004) (data not shown). However, none of the lipid parameters were significantly correlated with either plaque burden or any plaque component. In the multivariate analysis, BMI was identified as the most important positive factor for plaque burden (β= 0.369, p = 0.018). CysC–APN complex levels were identified as the strongest negative factor for fibrotic components (β = −0.285, p = 0.069) and the strongest positive factor for necrotic (β = 0.276, p = 0.076) and lipidic plus necrotic (β = 0.287, p = 0.067) components.
Table 5. Correlation between plaque characteristics and clinical parameters.
| Plaque burden |
Fibrotic components |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||
| r | p | β | p | r | p | β | p | |
| Age | −0.050 | 0.751 | −0.081 | 0.606 | ||||
| BMI | 0.474 | 0.002 | 0.369 | 0.018 | −0.115 | 0.468 | ||
| Systolic blood pressure | −0.313 | 0.041 | −0.194 | 0.168 | −0.056 | 0.720 | ||
| Diastolic blood pressure | −0.072 | 0.645 | −0.055 | 0.728 | ||||
| Fasting blood sugar | 0.029 | 0.854 | −0.301 | 0.050 | −0.085 | 0.581 | ||
| IRI | 0.111 | 0.491 | −0.248 | 0.117 | ||||
| HOMA-R | 0.110 | 0.495 | −0.261 | 0.100 | ||||
| HbA1c (NGSP) | −0.073 | 0.643 | −0.069 | 0.663 | ||||
| Total cholesterol | 0.005 | 0.976 | −0.117 | 0.462 | ||||
| Triglyceride | 0.164 | 0.293 | −0.249 | 0.107 | ||||
| HDL-C | −0.290 | 0.060 | 0.012 | 0.940 | ||||
| LDL-C | 0.158 | 0.317 | −0.030 | 0.851 | ||||
| hsCRP | 0.138 | 0.398 | 0.154 | 0.343 | ||||
| serum creatinine | −0.075 | 0.633 | 0.073 | 0.641 | ||||
| eGFR | 0.071 | 0.652 | −0.069 | 0.661 | ||||
| CysC | −0.064 | 0.682 | −0.002 | 0.990 | ||||
| APN | −0.317 | 0.039 | −0.201 | 0.190 | 0.123 | 0.431 | ||
| CysC–APN complex | −0.020 | 0.899 | −0.384 | 0.011 | −0.285 | 0.069 | ||
| Necrotic components |
Lipidic plus Necrotic components |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||
| r | p | β | p | r | p | β | p | |
| Age | 0.027 | 0.865 | 0.040 | 0.797 | ||||
| BMI | 0.152 | 0.337 | 0.169 | 0.286 | ||||
| Systolic blood pressure | 0.053 | 0.737 | 0.032 | 0.838 | ||||
| Diastolic blood pressure | 0.051 | 0.746 | 0.031 | 0.842 | ||||
| Fasting blood sugar | 0.352 | 0.021 | 0.120 | 0.433 | 0.313 | 0.041 | 0.009 | 0.572 |
| IRI | 0.230 | 0.149 | 0.292 | 0.064 | ||||
| HOMA-R | 0.262 | 0.098 | 0.305 | 0.053 | ||||
| HbA1c (NGSP) | 0.099 | 0.529 | 0.054 | 0.732 | ||||
| Total cholesterol | 0.147 | 0.355 | 0.136 | 0.389 | ||||
| Triglyceride | 0.287 | 0.062 | 0.277 | 0.072 | ||||
| HDL-C | 0.028 | 0.860 | −0.011 | 0.942 | ||||
| LDL-C | 0.039 | 0.809 | 0.045 | 0.779 | ||||
| hsCRP | −0.196 | 0.226 | −0.145 | 0.373 | ||||
| serum creatinine | −0.150 | 0.339 | −0.121 | 0.438 | ||||
| eGFR | 0.148 | 0.343 | 0.122 | 0.436 | ||||
| CysC | −0.064 | 0.682 | −0.002 | 0.990 | ||||
| APN | −0.171 | 0.273 | −0.179 | 0.251 | ||||
| CysC–APN complex | 0.406 | 0.007 | 0.276 | 0.076 | 0.417 | 0.005 | 0.287 | 0.067 |
The relationships among parameters were investigated by Spearman's correlation coefficient (univariate) and multiple regression analysis (multivariate).
Discussion
In this study, we investigated CysC–APN binding both in vivo and in vitro and found that serum CysC–APN complex levels negatively correlated with stable fibrotic coronary plaque components and positively correlated with unstable necrotic or lipidic plus necrotic coronary plaque components in CAD patients.
We have demonstrated the CysC–APN interaction by ELISA using recombinant APN and human immunoglobulin-conjugated CysC33). We also investigated the effects of CysC on the functions of APN using mice and cultured cells19). In mice, CysC injections reduced the clearance rate of plasma APN, leading to elevated plasma APN levels. In cultured human umbilical vein endothelial cells, CysC eliminated the suppressive effect of APN on the adhesion molecules mRNA expression induced by tumor necrosis factor-α. In the present study, we demonstrated that the CysC–APN complex is present in human serum. We further confirmed this interaction in vitro by immunoprecipitation and immunoblot analysis using epitope-tagged recombinant proteins, which is more convincing than ELISA.
CysC belongs to the endogenous inhibitors of the cathepsin family proteins23), which degrade elastin and collagen, the major matrix components of the vascular wall. Previous reports showing reduced CysC levels in human atherosclerotic lesions34) and accelerated atherosclerosis in CysC-deficient mice35–37) have suggested the anti-atherogenic roles of intracellular CysC. Plasma CysC levels are strongly associated with an increased risk of CAD, particularly for secondary CAD events28, 29, 38). Wen et al. have reported the relationship between serum CysC levels and the presence of a carotid plaque30). Therefore, serum CysC and intracellular CysC should have different functions in atherosclerotic mechanisms, i.e., serum CysC may cause a reduction in serum APN clearance and suppress the vasculoprotective effects of APN through the formation of the CysC–APN complex, which can explain the paradox that hyperadiponectinemia is associated with increased CAD risk in patients with renal impairment39).
In this study, we evaluated coronary plaque components using iMAP®-IVUS in male CAD patients with a relatively normal renal function. Currently, three radiofrequency signal-based IVUS (RF-IVUS) systems are available, allowing us to obtain more detailed information on plaque components. Virtual histology IVUS (VH-IVUS) was the first released RF-IVUS system, and then, integrated backscatter IVUS (IB-IVUS) and iMap®-IVUS were launched. Although these three modalities provide similar results on plaque phenotypes, there are some differences in their classifications. Yamada et al. reported that the “lipid pool” assessed by IB-IVUS was recognized as a “necrotic” component by iMap®-IVUS and that “fibrosis” and “calcification” evaluated by IB-IVUS correlated well with “fibrotic” and “calcified” by iMap®-IVUS, respectively40). It is advantageous to use iMap®-IVUS not only for identifying vulnerable plaques41) but also for distinguishing between ACS and non-ACS patients because more lipidic and necrotic components and less fibrotic components are found in ACS42).
Serum CysC–APN complex levels, but not serum APN or CysC levels, were significantly negatively correlated with fibrotic components and positively correlated with necrotic or lipidic plus necrotic components in this study. On the other hand, serum APN levels negatively correlated with only plaque burden, which is in agreement with our previous observation32). We have shown that serum APN levels are reduced in obese patients and that hypoadiponectinemia might be an independent risk factor for CAD15). Because BMI was the strongest factor affecting plaque burden in the multiple regression analysis (Table 5), hypoadiponectinemia due to obesity might accelerate the progression of a coronary plaque. Sawada et al. have reported that low plasma APN levels are associated with the presence of a vulnerable plaque in stable CAD male patients17); Otake et al. have demonstrated that low serum APN levels are linked to an increased necrotic core in ACS patients but not in stable angina patients43). Because CysC-bound APN is supposed to be a biologically inactive APN, serum CysC–APN complex levels might correlate with plaque vulnerability more sensitively than serum APN levels in stable CAD male patients. Hence, we propose that serum CysC–APN complex levels are a novel biomarker of coronary plaque instability.
Dyslipidemia is a well-established risk factor for ACS. Nasu et al. have reported a positive relationship between serum LDL-C levels and plaque vulnerability in stable angina patients without any lipid-lowering treatment44). In the present study, however, there was no obvious correlation between any lipid parameter and plaque burden or plaque components. Systolic blood pressure, also known as a positive risk factor for atherosclerosis, is negatively correlated with plaque burden. These unexpected results might be due to the medical treatments the enrolled patients were receiving. As shown in Table 1, 56% and 67% of the patients had already been treated with statins and anti-hypertensive agents, respectively. However, serum CysC–APN complex levels were still predictors for coronary plaque instability in these patients. We found it useful to measure serum CysC–APN complex levels in fully medicated stable CAD patients. A simple and reliable system for measuring serum CysC–APN complex levels would be a non-invasive method to predict plaque vulnerability and would be useful for the treatment and prevention of ACS.
This study has some limitations. We conducted a cross-sectional, single-center study, with a relatively small number of patients. The patients had already been treated with medication at enrollment. Further larger case-control or prospective studies including female patients are needed to obtain additional information on the clinical significance of serum CysC–APN complex levels.
Conclusion
Serum CysC–APN levels can be a useful biomarker for predicting coronary plaque instability in stable CAD male patients.
Acknowledgements
We thank Dr. John Hulleman (UT Southwestern Medical Center) and Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan) for generously providing a FLAG-CysC expression vector and a mouse anti-human adiponectin monoclonal antibody, respectively.
Funding
This study was supported in part by a “Grantin-Aid for Scientific Research (B)” (JSPS/KAKENHI #JP15H04762) to S.K. and “Grant-in-Aid for Research Activity Start-Up” (JSPS/KAKENHI #JP24890111) and the Mochida Memorial Foundation for Medical and Pharmaceutical Research to H.Y.
Conflict of Interest
None declared.
Author Contributions
A.M., H.Y., K.K., S.O., and N.M. performed research; T.M. performed clinical work; H.Y. and S.K. designed research; A.M., H.Y., and S.K. wrote the manuscript.
Abbreviations
- APN
adiponectin
- CAD
coronary artery disease
- CysC
cystatin C
- IVUS
intravascular ultrasound
References
- 1). WHO: Global status report on noncommunicable diseases 2014 [Google Scholar]
- 2). Group UPDSU: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet, 1998; 352: 837-853 [PubMed] [Google Scholar]
- 3). He J, Whelton PK: Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J, 1999; 138: 211-219 [DOI] [PubMed] [Google Scholar]
- 4). Saito Y, Shirai K, Sasaki N, Shinomiya M, Yoshida S: Prognosis of hypercholesterolemic patients taking pravastatin for five years: the Chiba Lipid Intervention Program (CLIP) Study. J Atheroscler Thromb, 2002; 9: 99-108 [DOI] [PubMed] [Google Scholar]
- 5). Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM: Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol, 2000; 20: 1262-1275 [DOI] [PubMed] [Google Scholar]
- 6). Stary HC: Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol, 2000; 20: 1177-1178 [DOI] [PubMed] [Google Scholar]
- 7). Virmani R, Burke AP, Farb A, Kolodgie FD: Pathology of the vulnerable plaque. J Am Coll Cardiol, 2006; 47: C13-18 [DOI] [PubMed] [Google Scholar]
- 8). Nissen SE, Yock P: Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation, 2001; 103: 604-616 [DOI] [PubMed] [Google Scholar]
- 9). Sathyanarayana S, Carlier S, Li W, Thomas L: Characterisation of atherosclerotic plaque by spectral similarity of radiofrequency intravascular ultrasound signals. Euro-Intervention, 2009; 5: 133-139 [DOI] [PubMed] [Google Scholar]
- 10). Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, Nagai M, Matsuzawa Y, Funahashi T: Adiponectin as a biomarker of the metabolic syndrome. Circ J, 2004; 68: 975-981 [DOI] [PubMed] [Google Scholar]
- 11). Hirose H, Takayama M, Iwao Y, Kawabe H: Effects of Aging on Visceral and Subcutaneous Fat Areas and on Homeostasis Model Assessment of Insulin Resistance and Insulin Secretion Capacity in a Comprehensive Health Checkup. J Atheroscler Thromb, 2016; 23: 207-215 [DOI] [PubMed] [Google Scholar]
- 12). Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y: Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol, 2000; 20: 1595-1599 [DOI] [PubMed] [Google Scholar]
- 13). Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T: Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension, 2004; 43: 1318-1323 [DOI] [PubMed] [Google Scholar]
- 14). Kumada M: Association of Hypoadiponectinemia With Coronary Artery Disease in Men. Arterioscler Thromb Vasc Biol, 2002; 23: 85-89 [DOI] [PubMed] [Google Scholar]
- 15). Kihara S, Matsuzawa Y: Fat Distribution and Cardiovascular Disease Risk. Curr Cardiovasc Risk Rep, 2015; 9: 8 [Google Scholar]
- 16). Shibata R, Ouchi N, Murohara T: Adiponectin and cardiovascular disease. Circ J, 2009; 73: 608-614 [DOI] [PubMed] [Google Scholar]
- 17). Sawada T, Shite J, Shinke T, Otake H, Tanino Y, Ogasawara D, Kawamori H, Kato H, Miyoshi N, Yoshino N, Kozuki A, Hirata K: Low plasma adiponectin levels are associated with presence of thin-cap fibroatheroma in men with stable coronary artery disease. Int J Cardiol, 2010; 142: 250-256 [DOI] [PubMed] [Google Scholar]
- 18). Arita Y: Adipocyte-Derived Plasma Protein Adiponectin Acts as a Platelet-Derived Growth Factor-BB-Binding Protein and Regulates Growth Factor-Induced Common Postreceptor Signal in Vascular Smooth Muscle Cell. Circulation, 2002; 105: 2893-2898 [DOI] [PubMed] [Google Scholar]
- 19). Komura N, Kihara S, Sonoda M, Maeda N, Tochino Y, Funahashi T, Shimomura I: Increment and impairment of adiponectin in renal failure. Cardiovasc Res, 2010; 86: 471-477 [DOI] [PubMed] [Google Scholar]
- 20). Niinaga R, Yamamoto H, Yoshii M, Uekita H, Yamane N, Kochi I, Matsumoto A, Matsuoka T, Kihara S: Marked elevation of serum M2BP-adiponectin complex in men with coronary artery disease. Atherosclerosis, 2016; 253: 70-74 [DOI] [PubMed] [Google Scholar]
- 21). Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K: Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest, 2007; 117: 375-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Yamamoto H, Kuroda N, Uekita H, Kochi I, Matsumoto A, Niinaga R, Funahashi T, Shimomura I, Kihara S: E-selectin ligand-1 (ESL-1) is a novel adiponectin binding protein on cell adhesion. Biochem Biophys Res Commun, 2016; 470: 425-430 [DOI] [PubMed] [Google Scholar]
- 23). Smith ER: Cystatin C - More than a filtration marker? Atherosclerosis, 2013; 230: 73-75 [DOI] [PubMed] [Google Scholar]
- 24). Dharnidharka VR, Kwon C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis, 2002; 40: 221-226 [DOI] [PubMed] [Google Scholar]
- 25). Marwyne MN, Loo CY, Halim AG, Norella K, Sulaiman T, Zaleha MI: Estimation of glomerular filtration rate using serum cystatin C in overweight and obese subjects. Med J Malaysia, 2011; 66: 313-317 [PubMed] [Google Scholar]
- 26). Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, Investigators C-E : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med, 2012; 367: 20-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis, 2008; 51: 395-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Ix JH, Shlipak MG, Chertow GM, Whooley MA: Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation, 2007; 115: 173-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med, 2005; 352: 2049-2060 [DOI] [PubMed] [Google Scholar]
- 30). Wen Y, Xia D, Wang Y, Zhang H, Li H, Ali G, Gao Y, Li J, Sun W, Li L: Cystatin C is Associated With Plaque Phenotype and Plaque Burden. Kidney Blood Press Res, 2016; 41: 197-207 [DOI] [PubMed] [Google Scholar]
- 31). Nguyen A, Hulleman JD: Evidence of Alternative Cystatin C Signal Sequence Cleavage Which Is Influenced by the A25T Polymorphism. PloS One, 2016; 11: e0147684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Matsuo N, Matsuoka T, Onishi S, Yamamoto H, Kato A, Makino Y, Kihara S: Impact of Remnant Lipoprotein on Coronary Plaque Components. J Atheroscler Thromb, 2015; 22: 783-795 [DOI] [PubMed] [Google Scholar]
- 33). Masaie H, Oritani K, Yokota T, Takahashi I, Shirogane T, Ujiie H, Ichii M, Saitoh N, Maeda T, Tanigawa R, Oka K, Hoshida Y, Tomiyama Y, Kanakura Y: Adiponectin binds to chemokines via the globular head and modulates interactions between chemokines and heparan sulfates. Exp Hematol, 2007; 35: 947-956 [DOI] [PubMed] [Google Scholar]
- 34). Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP: Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol, 2004; 24: 1359-1366 [DOI] [PubMed] [Google Scholar]
- 35). Bengtsson E, To F, Grubb A, Hakansson K, Wittgren L, Nilsson J, Jovinge S: Absence of the protease inhibitor cystatin C in inflammatory cells results in larger plaque area in plaque regression of apoE-deficient mice. Atherosclerosis, 2005; 180: 45-53 [DOI] [PubMed] [Google Scholar]
- 36). Bengtsson E, To F, Hakansson K, Grubb A, Branen L, Nilsson J, Jovinge S: Lack of the cysteine protease inhibitor cystatin C promotes atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol, 2005; 25: 2151-2156 [DOI] [PubMed] [Google Scholar]
- 37). Sukhova GK, Wang B, Libby P, Pan JH, Zhang Y, Grubb A, Fang K, Chapman HA, Shi GP: Cystatin C deficiency increases elastic lamina degradation and aortic dilatation in apolipoprotein E-null mice. Circ Res, 2005; 96: 368-375 [DOI] [PubMed] [Google Scholar]
- 38). Koenig W, Twardella D, Brenner H, Rothenbacher D: Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem, 2005; 51: 321-327 [DOI] [PubMed] [Google Scholar]
- 39). Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, Pereira AA, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ: Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol, 2006; 17: 2599-2606 [DOI] [PubMed] [Google Scholar]
- 40). Yamada R, Okura H, Kume T, Neishi Y, Kawamoto T, Miyamoto Y, Imai K, Saito K, Hayashida A, Yoshida K: A comparison between 40 MHz intravascular ultrasound iMap imaging system and integrated backscatter intravascular ultrasound. J Cardiol, 2013; 61: 149-154 [DOI] [PubMed] [Google Scholar]
- 41). Koga S, Ikeda S, Miura M, Yoshida T, Nakata T, Koide Y, Kawano H, Maemura K: iMap-Intravascular Ultrasound Radiofrequency Signal Analysis Reflects Plaque Components of Optical Coherence Tomography-Derived Thin-Cap Fibroatheroma. Circ J, 2015; 79: 2231-2237 [DOI] [PubMed] [Google Scholar]
- 42). Kozuki A, Shinke T, Otake H, Shite J, Matsumoto D, Kawamori H, Nakagawa M, Nagoshi R, Hariki H, Inoue T, Nishio R, Hirata K: Feasibility of a novel radiofrequency signal analysis for in-vivo plaque characterization in humans: comparison of plaque components between patients with and without acute coronary syndrome. Int J Cardiol, 2013; 167: 1591-1596 [DOI] [PubMed] [Google Scholar]
- 43). Otake H, Shite J, Shinke T, Watanabe S, Tanino Y, Ogasawara D, Sawada T, Hirata K, Yokoyama M: Relation between plasma adiponectin, high-sensitivity C-reactive protein, and coronary plaque components in patients with acute coronary syndrome. Am J Cardiol, 2008; 101: 1-7 [DOI] [PubMed] [Google Scholar]
- 44). Nasu K, Terashima M, Habara M, Ko E, Ito T, Yokota D, Ishizuka S, Kurita T, Kimura M, Kinoshita Y, Asakura Y, Tsuchikane E, Katoh O, Suzuki T: Impact of cholesterol metabolism on coronary plaque vulnerability of target vessels: a combined analysis of virtual histology intravascular ultrasound and optical coherence tomography. JACC Cardiovasc Interv, 2013; 6: 746-755 [DOI] [PubMed] [Google Scholar]


