Abstract
Previous studies have demonstrated the role of hydroquinone (HQ), a hydroxylated benzene metabolite, in modulating various immune responses; however, its role in macrophage-mediated inflammatory responses is not fully understood. In this study, the role of HQ in inflammatory responses and the underlying molecular mechanism were explored in macrophages. HQ down-regulated the expression of interferon (IFN)-β mRNA in LPS-stimulated RAW264.7 cells without any cytotoxicity and suppressed interferon regulatory factor (IRF)-3-mediated luciferase activity induced by TIR-domain-containing adapter-inducing interferon-β (TRIF) and TANK-binding kinase 1 (TBK1). A mechanism study revealed that HQ inhibited IRF-3 phosphorylation induced by lipopolysaccharide (LPS), TRIF, and AKT by suppressing phosphorylation of AKT, an upstream kinase of the IRF-3 signaling pathway. IRF-3 phosphorylation is highly induced by wild-type AKT and poorly induced by an AKT mutant, AKT C310A, which is mutated at an inhibitory target site of HQ. We also showed that HQ inhibited IRF-3 phosphorylation by targeting all three AKT isoforms (AKT1, AKT2, and AKT3) in RAW264.7 cells and suppressed IRF-3-mediated luciferase activities induced by AKT in HEK293 cells. Taken together, these results strongly suggest that HQ inhibits the production of a type I IFN, IFN-β, by targeting AKTs in the IRF-3 signaling pathway during macrophage-mediated inflammation.
Keywords: AKT, Hydroquinone, IFN-β, Inflammation, IRF-3, Macrophage
INTRODUCTION
Inflammation is a complex biological process that protects our body from invasion by various pathogens, such as bacteria, viruses, fungi, and other microorganisms. Inflammation is characterized by several hallmarks, including pain, redness, swelling, heat, and loss of function [1]. While inflammation is an innate immune response and a defense mechanism to protect our bodies against various dangers, chronic inflammation or repeated and prolonged inflammation has been regarded as a leading cause of various human diseases, including inflammatory/autoimmune diseases, such as rheumatoid arthritis, atherosclerosis, lupus erythematosus, and sepsis; metabolic diseases; and even cancers [2,3]. Several types of innate immune cells are actively involved in the inflammatory process; among these, macrophages are some of the most critical factors in inducing an inflammatory response.
Macrophages are the first defense cells to initiate an inflammatory response by detecting pathogens. Macrophages recognize conserved pathogen structures and molecules, known as pathogen-associated molecular patterns (PAMPs), such as cell wall components and nucleic acids, which are essential for pathogen survival and virulence [4,5]. This recognition is accomplished by molecular interaction between PAMPs and conserved pattern recognition receptors (PRRs) in macrophages, such as toll-like receptors (TLRs), RIG-I-like receptors (RLRs), and sensor proteins [6,7,8]. Through PAMP recognition by PRRs, especially TLRs, macrophages immediately induce inflammatory responses by releasing type I interferons (IFNs), such as IFN-α and -β, and proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1b, and IL-6 [9,10,11,12]. Pro-inflammatory cytokines are thought to remove pathogens in an indirect manner, e.g., by recruiting more immune cells to inflamed lesions and killing pathogen-infected cells. On the other hand, type I IFNs remove pathogens in a more direct manner than do pro-inflammatory cytokines, e.g., by preventing pathogen replication and proliferation to reduce pathogen numbers. The molecular mechanism of the macrophage-mediated inflammatory response has been extensively studied. PAMPs binding to macrophage PRRs initiate rapid signal transduction cascades activating a variety of intracellular signaling molecules, such as Src; spleen tyrosine kinase (Syk); and mitogen-activated protein kinases (MAPKs), such as extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinase (JNK), and p38. These signaling molecules activate inflammatory transcription factors, such as nuclear factor-kappa-B (NF-κB) and activator protein (AP)-1 [13,14,15,16,17]. Therefore, the intracellular signaling molecules in macrophages that are activated during inflammatory responses could be pharmaceutical targets to prevent and treat inflammatory and infectious diseases. Many studies have concentrated on identifying and testing novel anti-inflammatory and anti-infectious agents targeting signaling molecules in macrophages.
Hydroquinone (benzene-1,4-diol; HQ; Fig. 1) is a benzenederived metabolite that is a major component of industrial chemicals; cigarette smoke; and some foods, such as coffee, tea, and beer [18,19]. Because cigarette smoking induces allergenic and inflammatory reactions, possibly due to the presence of benzene metabolites, which are strong haptens [20], previous studies have demonstrated HQ to be an immunotoxicological substance in allergenic and inflammatory diseases [21]. A number of recent studies have reported anti-inflammatory activities of HQ, including suppression of the expression of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, by targeting AKT, which is a critical signaling molecule in the NF-kB pathway in lipopolysaccharide (LPS)-activated macrophages [22,23,24,25,26]. In addition, HQ suppressed the production and release of inflammatory mediators, such as nitric oxide (NO) and prostaglandin E2 (PGE2), and ameliorated hepatitis symptoms in an LPS/D-galactosamine-induced hepatitis mouse model [27]. These results strongly suggest that HQ and possibly benzene-derived metabolites are not simple toxic agents, but potential immunosuppressants with anti-inflammatory activities in macrophage-mediated inflammation. Despite these previous studies, new anti-inflammatory roles of HQ and the molecular mechanisms underlying macrophage-mediated inflammatory responses are still poorly understood.
Fig. 1. Chemical structure of hydroquinone (HQ).
In this study, we investigated a new role of HQ during inflammatory responses and the molecular mechanisms by which HQ modulates inflammatory responses in macrophages using a well-established, in vitro-activated macrophage platform, LPS-stimulated RAW264.7 cells. The results of this study provide strong evidence of a previously unknown role of HQ in suppressing the expression of a type I IFN, IFN-β, a critical cytokine in preventing pathogen proliferation, in macrophages by targeting AKT kinases in the interferon regulatory factor (IRF)-3 signaling pathway.
METHODS
Materials
HQ (Fig. 1), lipopolysaccharide (LPS, E. coli 0111:B4), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and polyethylenimine (PEI) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). RAW264.7 and HEK293 cells were purchased from ATCC (Rockville, MD, USA). Roswell Park Memorial Institute (RPMI) 1640, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), streptomycin, penicillin, and L-glutamine were purchased from Gibco (Grand Island, NY, USA). TRI reagent® was purchased from Molecular Research Center Inc. (Cincinnati, OH, USA). MuLV reverse transcriptase was purchased from Thermo-Fisher Scientific (Waltham, MA, USA). Primers used for semiquantitative polymerase chain reaction (PCR) were synthesized, and PCR premix was purchased from Bioneer Inc. (Daejeon, Korea). Antibodies specific for phosphorylated and total forms of IRF-3, TBK-1, AKT, hemagglutinin (HA) tag, and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). Enhanced chemiluminescence system was purchased from AbFrontier (Seoul, Korea). AKT gene constructs (AKT1, AKT2, AKT3, and AKT KD) used in this study were purchased from Addgene (Cambridge, MA, USA). TRIF and TKB1 constructs were used as previously reported [28].
Cell culture
RAW264.7 and HEK293 cells were maintained in RPMI 1640 medium and DMEM, respectively, containing 10% heat-inactivated FBS, 100 mg/ml streptomycin, 100 U/ml penicillin, and 2 mM L-glutamine at 37℃ in a 5% CO2 humidified incubator. The cells were subcultured with new cell culture media three times a week to maintain the cells.
Semi-quantitative PCR
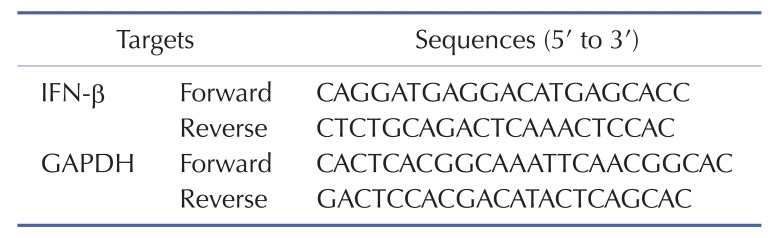
To determine the level of IFN-β mRNA expression, RAW264.7 (1×106 cells/ml) cells were pretreated with escalating doses of HQ (0, 25, 50, and 75 µM) for 30 min and then incubated with LPS (1 µg/ml) for 6 h. Total RNA was extracted with TRI reagent® according to the manufacturer's instructions. cDNA was immediately synthesized from 1 mg of total RNA by MuLV reverse transcriptase according to the manufacturer's instructions and used for semi-quantitative PCR as previously described [3,29]. The primer sequences for the semi-quantitative PCR are listed in Table 1.
Table 1. Primer sequences used for semi-quantitative PCR in this study.
IRF-3-luciferase reporter gene assay
HEK293 cells (1×106 cells/ml) were co-transfected with IFN-β-Luc and β-gal along with TIR-domain-containing adapter-inducing interferon-β (TRIF), TANK-binding kinase 1 (TBK1), or an AKT construct for 24 h using PEI in 24 well plates and treated with escalating doses of HQ (0, 25, 50, and 75 µM) for another 24 h. The cells were next lysed by freezing and thawing, and the luciferase activity in the lysate was measured with a luciferase assay as reported previously [3,30,31]. All luciferase activities were normalized to b-galactosidase activities.
Cell viability assay
The cytotoxic effect of HQ on RAW264.7 cells (1×106 cells/ml) was determined by treating cells with escalating doses of HQ (0, 5, 10, 25, 50, and 75 µM) for 24 h and performing an MTT assay as previously reported [32,33]. Briefly, cell culture medium (100 ml) was mixed with MTT solution (10 µl; 10 mg/ml in PBS, pH 7.4) and incubated for 4 h at 37℃. A 15% sodium dodecyl sulfate solution was directly added to the mixture to stop the reaction, and the mixture was further incubated at 37℃ for 24 h. The OD at 570 nm was determined using a Spectramax 250 microplate reader.
Preparation of total cell lysates and Western blot analysis
Total lysates of RAW264.7 cells were prepared as previously described [31,34]. Briefly, RAW264.7 cells were pretreated with the indicated doses of HQ, incubated with LPS (1 µg/ml) for the indicated time, and washed three times with PBS. The cells were lysed by a sonication on ice for 1 min in cold cell lysis buffer (20 mM Tris–HCl, pH 7.4, 2 mM EDTA, 2 mM ethyleneglycotetraacetic acid, 50 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1% Triton X-100, 10% glycerol, 10 µg/ml aprotinin, 10 µg/ml pepstatin, 1 mM benzimide, and 2 mM PMSF). Clear total lysates were collected by centrifugation at 16,000 × g for 10 min at 4℃, discarding the pellet, and storing immediately at −80℃ until use. For Western blot analysis, total cell lysates were subjected to SDS-polyacrylamide electrophoresis and transferred to polyvinylidene fluoride membrane at 4℃ and 250 mA for 1 h. Total and phosphorylated forms of target proteins IRF-3, TBK1, AKTs, and β-actin were detected by antibodies specific for each target and visualized with an enhanced chemiluminescence system according to the manufacturer's instructions as reported previously [35].
Statistical analysis
All data in this study are presented as the mean and standard deviation of at least three independent experiments. To determine statistical significance among experimental groups, all results were analyzed by ANOVA/Scheffe's post hoc test and Kruskal-Wallis/Mann-Whitney test. p<0.05 was considered statistically significant, and all statistical analyses were performed with SPSS.
RESULTS AND DISCUSSION
Previous studies have reported that HQ has various immunomodulatory activities. HQ has been shown to ameliorate the symptoms of allergic diseases by regulating immune responses. HQ significantly reduced IL-12 production in LPS-stimulated macrophages, which suppress immune responses mediated by type-2 helper T-cells associated with the development of allergic disease by suppressing NF-κB activity [21]. In addition, HQ reduced the production of some cytokines and cytotoxic molecules, such as IL-2, IL-1β, and NO, in macrophages [22,26]. HQ was also reported to induce IL-4 production from CD4+ T-cells and increase serum immunoglobulin E level in antigen-primed mice [36]. Recent studies have demonstrated that HQ is also actively involved in modulating inflammatory responses in macrophages. HQ significantly decreased the production of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, as well as inflammatory molecules, such as NO, in LPS-activated macrophages by suppressing the NF-kB signaling pathway [22,24,25,37]. Although these studies suggest that HQ modulates a wide range of immune responses in immune cells, the role of HQ in immune responses, especially macrophage-mediated inflammatory responses, is poorly understood. Therefore, we investigated the role of HQ in macrophage-induced inflammatory responses and the underlying molecular mechanism using LPS-stimulated RAW264.7 cells.
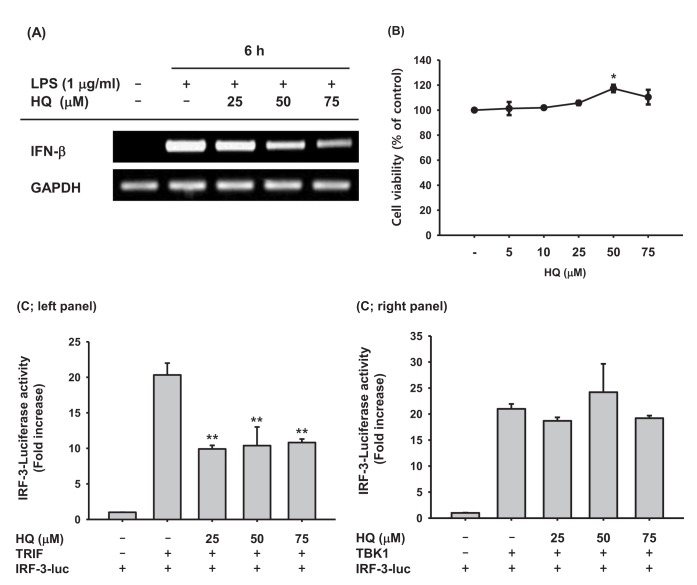
Upon recognizing invading pathogens, macrophages immediately initiate inflammatory responses in a cooperative manner by producing pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, and anti-pathogen cytokines, such as type I IFNs. Pro-inflammatory cytokines help remove invading pathogens indirectly by recruiting circulating immune cells to the inflamed lesions through vasodilation and killing pathogen-infected cells. Type I IFNs directly remove invading pathogens by suppressing transcription and translation of pathogen components to inhibit pathogen replication and proliferation, resulting in pathogen removal. Because previous studies have successfully demonstrated that HQ significantly reduced the production of pro-inflammatory cytokines and molecules [22,25], the effect of HQ on IFN-β production was examined in the present study. HQ suppressed IFN-β mRNA expression in a dose-dependent manner in LPS-stimulated RAW264.7 cells (Fig. 2A) but did not have any cytotoxic effects at low (5 µM) to high (75 µM) doses (Fig. 2B). These results indicate that decreased IFN-b production due to HQ is not a result of cytotoxicity, but is due to HQ-specific inhibition of IFN-β transcription. Since HQ decreased IFN-β transcription in LPS-stimulated RAW264.7 cells, we examined the effects of HQ on the transcriptional activity of IRF-3, the key transcription factor inducing IFN-β expression, using a luciferase reporter gene. TRIF and TBK1 have been reported to induce IFN-β mRNA expression by activating IRF-3 [15,38], and TRIF and TBK1 induced IRF-3-mediated luciferase activity in HEK293 cells. HQ at all doses significantly suppressed TRIF-induced, IRF-3-mediated luciferase activity (Fig. 2C; left panel), but it did not suppress TBK1-induced, IRF-3-mediated luciferase activity (Fig. 2C; right panel). Although HQ down-regulated IFN-β mRNA expression in a dose-dependent manner (Fig. 2A), suppression of IRF-3-mediated luciferase activity by HQ was not dose dependent; all HQ doses statistically significantly suppressed IRF-3-mediated luciferase activity. Why the effects of HQ on IFN-β mRNA expression and IRF-3-mediated luciferase activity are not consistent remains unclear, so further study is required.
Fig. 2. Effect of HQ on the mRNA expression of IFN-β.
(A) RAW264.7 cells (1×106 cells/ml) pretreated with the indicated doses of HQ for 30 min were incubated with LPS (1 µg/ml) for 6 h. Expression levels of IFN-β and GAPDH mRNA were determined by semi-quantitative PCR. (B) RAW264.7 cells (1×106 cells/ml) were treated with the indicated doses of HQ for 24 h, and cell viability was determined by MTT assay. (C) HEK293 cells (1×106 cells/ml) cotransfected with IRF-3-Luc (1.0 µg/ml), β-gal (0.1 µg/ml), and either (Left panel) TRIF (1.0 µg/ml) or TBK1 (1.0 µg/ml) for 24 h were treated with the indicated doses of HQ for another 24 h. Luciferase activity was measured with a luminometer. All luciferase activities were normalized to β-galactosidase activity. *p<0.05, **p<0.01 compared to the control.
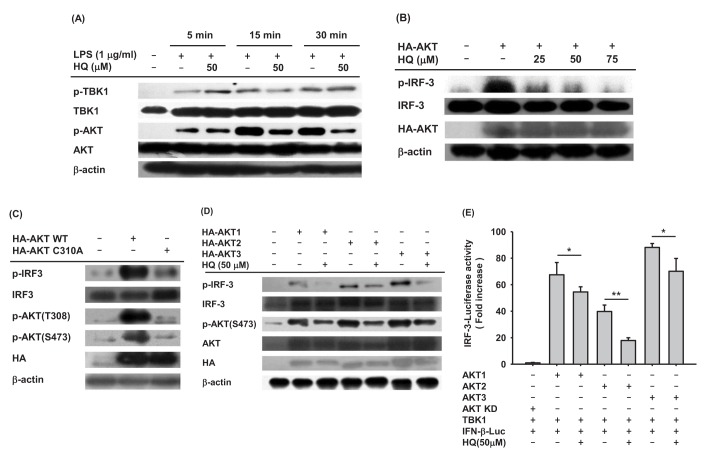
Since HQ effectively suppressed IRF-3 activity induced by TRIF and TBK1 (Fig. 2C), we next investigated the effect of HQ on IRF-3 activity in macrophages by Western blot analysis. The effect of HQ on IRF-3 activity was examined in LPS-stimulated RAW264.7 cells, and HQ dramatically reduced IRF-3 phosphorylation at 15 and 30 min after LPS treatment in RAW264.7 cells (Fig. 3A). To confirm this result, the effect of HQ on IRF-3 activity was examined in TRIF-transfected RAW264.7 cells since HQ significantly suppressed IRF-3-mediated luciferase activity induced by TRIF more than that induced by TBK1 (Fig. 2C). As expected, HQ at all doses effectively reduced IRF-3 phosphorylation in TRIF-transfected RAW264.7 cells (Fig. 3B). These results strongly suggest that HQ suppresses IRF-3 activation by inhibiting its phosphorylation and consequently decreases IRF-3 activity, resulting in down-regulated IFN-β mRNA expression in LPS-stimulated RAW264.7 cells.
Fig. 3. Effect of HQ on the activation of IRF-3.
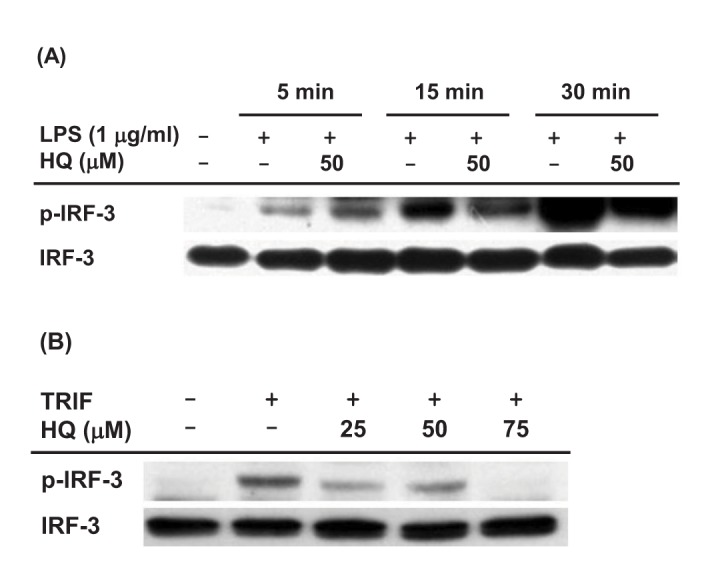
(A) RAW264.7 cells (1×106 cells/ml) pretreated with either HQ (50 µM) or vehicle for 30 min were incubated with LPS (1.0 µg/ml) for the indicated time. Phosphorylated and total levels of IRF-3 in total cell lysates were determined by Western blot analysis. (B) RAW264.7 cells (1×106 cells/ml) transfected with TRIF (1.0 µg/ml) for 24 h were treated with the indicated doses of HQ for 24 h, and phosphorylated and total levels of IRF-3 in total cell lysates were determined by Western blot analysis.
A number of previous studies have established that many intracellular molecules in the IRF-3 signaling pathway, such as AKT, TRIF, TNF receptor associated factor 3 (TRAF3), TNAK, TBK1, and inhibitor of nuclear factor kappa-B kinase ε (IKKε), are activated in IRF-3 signaling [14,15]. Since HQ suppressed IRF-3 activation in LPS-stimulated RAW264.7 cells, we next analyzed the upstream signaling molecules targeted by HQ. To examine which intracellular molecules in the IRF-3 signaling pathway are targeted by HQ, the phosphorylation of critical upstream molecules in the IRF-3 signaling pathway, such as TBK1 and AKTs, were determined in the LPS-stimulated RAW264.7 cells treated with HQ. HQ dramatically reduced AKT phosphorylation from 15 to 30 min. On the other hand, HQ did not suppress TBK1 phosphorylation in LPS-stimulated RAW264.7 cells (Fig. 4A). This result is consistent with previous findings that HQ did not suppress the IRF-3-mediated luciferase activity in TBK1-transfected cells (Fig. 2C right panel) and suggests that HQ suppresses IRF-3 activity by targeting AKT rather than TBK1. To confirm that HQ suppressed AKT phosphorylation (Fig. 4A), RAW264.7 cells transfected with AKT were treated with HQ, and IRF-3 phosphorylated was examined by Western blot. As expected, IRF-3 phosphorylation induced by AKT transfection was dramatically decreased by HQ (Fig. 4B). To further confirm that AKT is a target for HQ-mediated suppression of IRF-3 activation in LPS-stimulated RAW264.7 cells, RAW264.7 cells were transfected with either wild-type AKT or mutant AKT with cysteine 310 substituted for alanine (AKT C310A). HQ directly targets and suppresses AKT phosphorylation at both threonine 308 and serine 473 [24]. IRF-3 phosphorylation in these cells was examined by Western blot analysis. Wild-type AKT dramatically induced IRF-3 phosphorylation in RAW264.7 cells, whereas AKT C310A poorly increased IRF-3 phosphorylation (Fig. 4C). These results indicate that HQ suppresses IRF-3 activation by targeting AKT at cysteine 310 in LPS-stimulated RAW264.7 cells. To date, three different AKT isoforms (AKT1, AKT2, and AKT3) have been identified in mice and humans, but their roles in modulating immune responses have not yet been elucidates. To determine which AKT is the target for HQ-mediated inhibition of IRF-3 activation in LPS-stimulated macrophages, RAW264.7 cells were transfected with each AKT isoform in the absence or presence of HQ, and IRF-3 phosphorylation was examined by Western blot. HQ inhibited phosphorylation of all three AKTs, resulting in suppressed IRF-3 phosphorylation in all RAW264.7 cells transfected with AKT isoforms (Fig. 4D). As previously reported, HQ suppresses AKT1 activation by targeting cysteine 310, abolishing phosphorylation at threonine 308 and serine 473 [24]. This mechanism was confirmed for HQ-mediated suppression of IRF-3 activation in LPS-stimulated macrophages (Fig. 4C). Cysteine 310 is evolutionarily well conserved among AKT isoforms. AKT2 and AKT3 have cysteine residues at 311 and 307, respectively, suggesting that HQ targets cysteine 311 of AKT2 and cysteine 307 of AKT3 to suppress AKT phosphorylation and IRF-3 activity in LPS-stimulated macrophages. Although this mechanism is reasonable, it needs to be confirmed in further investigation. Since HQ suppressed phosphorylation of all three AKT isoforms and reduced IRF-3 phosphorylation in LPS-stimulated RAW264.7 cells, we further investigated the effect of HQ on the suppression of IRF-3 activation with different AKT isoforms using a luciferase reporter gene. IRF-3-mediated luciferase activities were determined in HEK293 cells transfected with each AKT isoform in the absence or presence of HQ, and HQ significantly suppressed the IRF-3-mediated luciferase activities induced by each AKT (Fig. 4E). These results strongly indicate that HQ suppresses IRF-3 activation, which is critical for expression of IFN-b mRNA, by targeting all three AKT isoforms in LPS-stimulated macrophages.
Fig. 4. Effect of HQ on AKT activation in IRF3 pathway.
(A) RAW264.7 cells (1×106 cells/ml) pretreated with either HQ (50 µM) or vehicle for 30 min were incubated with LPS (1.0 µg/ml) for the indicated time. Phosphorylated and total levels of TBK1 and AKT in total cell lysates were determined by Western blot analysis. (B) RAW264.7 cells (1×106 cells/ml) transfected with HA-AKT (1.0 µg/ml) for 24 h were treated with the indicated doses of HQ for another 24 h, and phosphorylated and total levels of IRF-3 and HA in total cell lysates were determined by Western blot analysis. (C) RAW264.7 cells (1×106 cells/ml) were transfected with either HA-AKT (1.0 µg/ml) or HA-AKT C310A (1.0 µg/ml) for 48 h. Phosphorylated and total levels of IRF-3, AKT, and HA in total cell lysates were determined by Western blot analysis. (D) RAW264.7 cells (1×106 cells/ml) transfected with either HA-AKT1 (1.0 µg/ml), HA-AKT2 (1.0 µg/ml), or HA-AKT3 (1.0 µg/ml) for 24 h were treated with either HQ (50 µM) or vehicle for another 24 h. Phosphorylated and total levels of IRF-3, AKT, and HA in the total cell lysates were determined by Western blot analysis. (E) HEK293 cells (1×106 cells/ml) cotransfected with TBK1 (1.0 µg/ml), IRF-3-Luc (1.0 µg/ml), β-gal (0.1 µg/ml), and HA-AKT1 (1.0 µg/ml), HA-AKT2 (1.0 µg/ml), HA-AKT3 (1.0 µg/ml), or HA-AKT KD (kinase domain deletion mutant) for 24 h were treated with either HQ (50 µM) or vehicle for another 24 h. Luciferase activity was measured with a luminometer. All luciferase reporter gene activities were normalized to β-galactosidase activity. *p<0.05, **p<0.01 compared to the control.
CONCLUSION
The present study aimed to explore the anti-inflammatory activity of HQ in activated macrophages and provided new evidence that HQ effectively suppresses expression of IFN-β mRNA, which plays a crucial role in inhibiting pathogens from replicating and proliferating in host cells. This inhibitory activity was accomplished by targeting AKTs, which are critical upstream kinases in the IRF-3 signaling pathway, suppressing IRF-3 activity, which is required for macrophages to produce type I IFNs, as described in Fig. 5. These results suggest that HQ could be a novel agent to prevent and treat inflammatory and infectious diseases by preventing the production of type I IFNs during macrophage-mediated inflammatory responses.
Fig. 5. Proposed model for HQ-mediated suppression of IFN-β production by targeting AKT kinases in the IRF-3 signaling pathway during macrophage-mediated inflammatory responses.
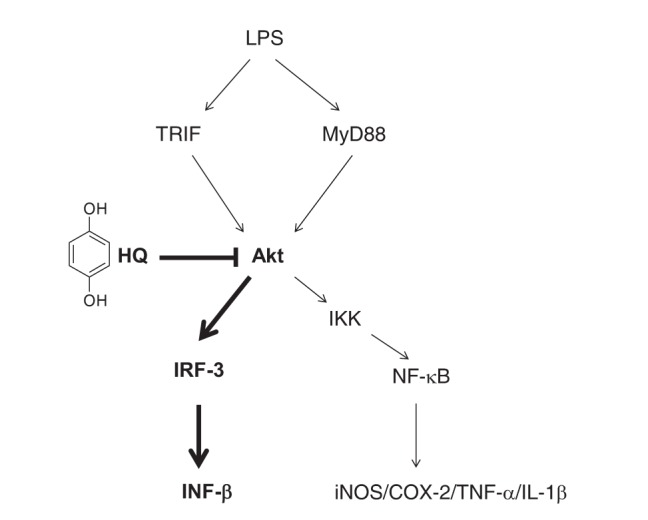
Bold denotes a signaling molecule targeted by HQ in macrophages during inflammatory responses.
ACKNOWLEDGEMENTS
This work was supported by the Postdoctoral Research Program of Sungkyunkwan University (2016).
Footnotes
Author contributions: Y.K., H.G.K., S.Y.H., D.J., and W.S.Y. performed experiments. J.I.K. performed statistical analysis. J.H.K., Y.S.Y., and J.Y.C. supervised and coordinated the study. H.G.K., J.H.K., Y.S.Y., and C.K.S. wrote the manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur M, Singh M, Silakari O. Inhibitors of switch kinase ‘spleen tyrosine kinase’ in inflammation and immune-mediated disorders: a review. Eur J Med Chem. 2013;67:434–446. doi: 10.1016/j.ejmech.2013.04.070. [DOI] [PubMed] [Google Scholar]
- 3.Baek KS, Yi YS, Son YJ, Yoo S, Sung NY, Kim Y, Hong S, Aravinthan A, Kim JH, Cho JY. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res. 2016;40:437–444. doi: 10.1016/j.jgr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 6.Pandey S, Kawai T, Akira S. Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors. Cold Spring Harb Perspect Biol. 2014;7:a016246. doi: 10.1101/cshperspect.a016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao H, Gonzalezgugel E, Cheng L, Richbourgh B, Nie L, Liu C. The roles of interferon-inducible p200 family members IFI16 and p204 in innate immune responses, cell differentiation and proliferation. Genes Dis. 2015;2:46–56. doi: 10.1016/j.gendis.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 9.Massarotti EM. Clinical and patient-reported outcomes in clinical trials of abatacept in the treatment of rheumatoid arthritis. Clin Ther. 2008;30:429–442. doi: 10.1016/j.clinthera.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Vo VA, Lee JW, Chang JE, Kim JY, Kim NH, Lee HJ, Kim SS, Chun W, Kwon YS. Avicularin inhibits lipopolysaccharide-induced inflammatory response by suppressing ERK phosphorylation in RAW 264.7 macrophages. Biomol Ther (Seoul) 2012;20:532–537. doi: 10.4062/biomolther.2012.20.6.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 12.Kwon IS, Yim JH, Lee HK, Pyo S. Lobaric acid inhibits VCAM-1 expression in TNF-α-stimulated vascular smooth muscle cells via modulation of NF-κB and MAPK signaling pathways. Biomol Ther (Seoul) 2016;24:25–32. doi: 10.4062/biomolther.2015.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byeon SE, Yi YS, Oh J, Yoo BC, Hong S, Cho JY. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012;2012:512926. doi: 10.1155/2012/512926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi YS, Son YJ, Ryou C, Sung GH, Kim JH, Cho JY. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014;2014:270302. doi: 10.1155/2014/270302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu T, Yi YS, Yang Y, Oh J, Jeong D, Cho JY. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediators Inflamm. 2012;2012:979105. doi: 10.1155/2012/979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung GH, Yoo BC, Cho JY. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014;2014:352371. doi: 10.1155/2014/352371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S, Kim Y, Jeong D, Kim JH, Kim S, Son YJ, Yoo BC, Jeong EJ, Kim TW, Lee IH, Cho JY. Pyrrole-derivative of chalcone, (E)-3-phenyl-1-(2-pyrrolyl)-2-propenone, inhibits inflammatory responses via inhibition of Src, Syk, and TAK1 kinase activities. Biomol Ther (Seoul) 2016;24:595–603. doi: 10.4062/biomolther.2016.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeCaprio AP. The toxicology of hydroquinone−relevance to occupational and environmental exposure. Crit Rev Toxicol. 1999;29:283–330. doi: 10.1080/10408449991349221. [DOI] [PubMed] [Google Scholar]
- 19.McDonald TA, Holland NT, Skibola C, Duramad P, Smith MT. Hypothesis: phenol and hydroquinone derived mainly from diet and gastrointestinal flora activity are causal factors in leukemia. Leukemia. 2001;15:10–20. doi: 10.1038/sj.leu.2401981. [DOI] [PubMed] [Google Scholar]
- 20.Moerloose KB, Pauwels RA, Joos GF. Short-term cigarette smoke exposure enhances allergic airway inflammation in mice. Am J Respir Crit Care Med. 2005;172:168–172. doi: 10.1164/rccm.200409-1174OC. [DOI] [PubMed] [Google Scholar]
- 21.Kim E, Kang BY, Kim TS. Inhibition of interleukin-12 production in mouse macrophages by hydroquinone, a reactive metabolite of benzene, via suppression of nuclear factor-kappaB binding activity. Immunol Lett. 2005;99:24–29. doi: 10.1016/j.imlet.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Kim AR, Cho JY, Lee JY, Choi JS, Chung HY. Hydroquinone modulates reactivity of peroxynitrite and nitric oxide production. J Pharm Pharmacol. 2005;57:475–481. doi: 10.1211/0022357055731. [DOI] [PubMed] [Google Scholar]
- 23.Pyatt DW, Yang Y, Stillman WS, Cano LL, Irons RD. Hydroquinone inhibits PMA-induced activation of NFkappaB in primary human CD19+ B lymphocytes. Cell Biol Toxicol. 2000;16:41–51. doi: 10.1023/a:1007644620655. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Lee YG, Lee J, Yang KJ, Kim AR, Kim JY, Won MH, Park J, Yoo BC, Kim S, Cho WJ, Cho JY. Akt Cys-310-targeted inhibition by hydroxylated benzene derivatives is tightly linked to their immunosuppressive effects. J Biol Chem. 2010;285:9932–9948. doi: 10.1074/jbc.M109.074872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Q, Kinneer K, Ye J, Chen BJ. Inhibition of nuclear factor kappaB by phenolic antioxidants: interplay between antioxidant signaling and inflammatory cytokine expression. Mol Pharmacol. 2003;64:211–219. doi: 10.1124/mol.64.2.211. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Kim JY, Lee YG, Shin WC, Chun T, Rhee MH, Cho JY. Hydroquinone, a reactive metabolite of benzene, reduces macrophage-mediated immune responses. Mol Cells. 2007;23:198–206. [PubMed] [Google Scholar]
- 27.Cho JY. Suppressive effect of hydroquinone, a benzene metabolite, on in vitro inflammatory responses mediated by macrophages, monocytes, and lymphocytes. Mediators Inflamm. 2008;2008:298010. doi: 10.1155/2008/298010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Yang WS, Yu T, Yi YS, Park JG, Jeong D, Kim JH, Oh JS, Yoon K, Kim JH, Cho JY. Novel anti-inflammatory function of NSC95397 by the suppression of multiple kinases. Biochem Pharmacol. 2014;88:201–215. doi: 10.1016/j.bcp.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Keung MH, Chan LS, Kwok HH, Wong RN, Yue PY. Role of microRNA-520h in 20(R)-ginsenoside-Rg3-mediated angiosuppression. J Ginseng Res. 2016;40:151–159. doi: 10.1016/j.jgr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi YS, Cho JY, Kim D. Cerbera manghas methanol extract exerts anti-inflammatory activity by targeting c-Jun N-terminal kinase in the AP-1 pathway. J Ethnopharmacol. 2016;193:387–396. doi: 10.1016/j.jep.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Baek KS, Hong YD, Kim Y, Sung NY, Yang S, Lee KM, Park JY, Park JS, Rho HS, Shin SS, Cho JY. Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J Ginseng Res. 2015;39:155–161. doi: 10.1016/j.jgr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerlier D, Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 33.Park JG, Son YJ, Aravinthan A, Kim JH, Cho JY. Korean Red Ginseng water extract arrests growth of xenografted lymphoma cells. J Ginseng Res. 2016;40:431–436. doi: 10.1016/j.jgr.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong D, Yi YS, Sung GH, Yang WS, Park JG, Yoon K, Yoon DH, Song C, Lee Y, Rhee MH, Kim TW, Kim JH, Cho JY. Anti-inflammatory activities and mechanisms of Artemisia asiatica ethanol extract. J Ethnopharmacol. 2014;152:487–496. doi: 10.1016/j.jep.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Lee J, Rhee MH, Yu T, Baek KS, Sung NY, Kim Y, Yoon K, Kim JH, Kwak YS, Hong S, Kim JH, Cho JY. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J Ginseng Res. 2015;39:61–68. doi: 10.1016/j.jgr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee MH, Chung SW, Kang BY, Kim KM, Kim TS. Hydroquinone, a reactive metabolite of benzene, enhances interleukin-4 production in CD4+ T cells and increases immunoglobulin E levels in antigen-primed mice. Immunology. 2002;106:496–502. doi: 10.1046/j.1365-2567.2002.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S, Oh MH, Kim BS, Kim WI, Cho HS, Park BY, Park C, Shin GW, Kwon J. Upregulation of heme oxygenase-1 by ginsenoside Ro attenuates lipopolysaccharide-induced inflammation in macrophage cells. J Ginseng Res. 2015;39:365–370. doi: 10.1016/j.jgr.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen T, Yang WS, Yi YS, Sung GH, Rhee MH, Poo H, Kim MY, Kim KW, Kim JH, Cho JY. AP-1/IRF-3 targeted anti-inflammatory activity of andrographolide isolated from andrographis paniculata. Evid Based Complement Alternat Med. 2013;2013:210736. doi: 10.1155/2013/210736. [DOI] [PMC free article] [PubMed] [Google Scholar]