Abstract
Pregnancy is associated with profound maternal metabolic changes, necessary for the growth and development of the fetus, mediated by reproductive signals acting on metabolic organs. However, the role of brown adipose tissue (BAT) in regulating gestational metabolism is unknown. We show that BAT phenotype is lost in murine pregnancy, while there is a gain of white adipose tissue (WAT)-like features. This is characterised by reduced thermogenic capacity and mitochondrial content, accompanied by increased levels of markers of WAT and lipid accumulation. Surgical ablation of BAT prior to conception caused maternal and fetal hyperlipidemia, and consequently larger fetuses. We show that BAT phenotype is altered from day 5 of gestation, implicating early pregnancy factors, which was confirmed by reduced expression of BAT markers in progesterone challenged oophorectomised mice. Moreover, in vitro data using primary BAT cultures show a direct impact of progesterone on expression of Ucp1. These data demonstrate that progesterone mediates a phenotypic change in BAT, which contributes to the gestational metabolic environment, and thus overall fetal size.
Introduction
Pregnancy is associated with a gradual metabolic shift from normal maternal serum concentrations of triglycerides, free fatty acids, and glucose at the time of conception to hyperlipidemia and insulin resistance in the third trimester. These changes support the growth and development of the fetus. The importance of maternal-fetal energy transfer is exemplified by several pathophysiological conditions. Under-nutrition or obesity during pregnancy can increase the risk of babies being born with intrauterine growth restriction or large for gestational age1–4. Furthermore, altered metabolism as a result of maternal obesity is associated with an increased risk of gestational diabetes and pre-eclampsia5–7, and predisposes the infant to metabolic disease in later life8–10.
Maternal lipid homeostasis is dependent upon the coordination of metabolically active organs. For example, during early pregnancy, increased insulin sensitivity in white adipose tissue (WAT) results in lipid accumulation, whereas towards term, in conjunction with placental hormones, there is insulin resistance and lipid catabolism11. However, little is known about the prevalence and function of brown adipose tissue (BAT) during pregnancy. Adult humans possess classical brown adipocytes and beige cells of white adipocyte origin, both of which are thermogenic and can contribute to whole body energy metabolism (reviewed in ref. 12). Interestingly, women have been found to possess larger volumes of BAT than men13,14. It is not known whether BAT is involved in regulating the gestational metabolic profile. Nonetheless it has been hypothesised that there is a decrease in BAT-mediated energy expenditure during pregnancy to divert energy to the developing fetus and/or lactation (reviewed in refs 15 and 16), although the data to support this are inconsistent. Research into the effects of reproductive hormones on BAT in the context of pregnancy has largely focused on estrogen17, 18 with limited studies of progesterone and prolactin19, 20.
In this report, we examine the impact of pregnancy on BAT, the role of BAT in regulating the maternal and fetal metabolic environment, as well as alterations in BAT phenotype mediated by early pregnancy signals.
Results
BAT undergoes morphometric and functional changes during pregnancy
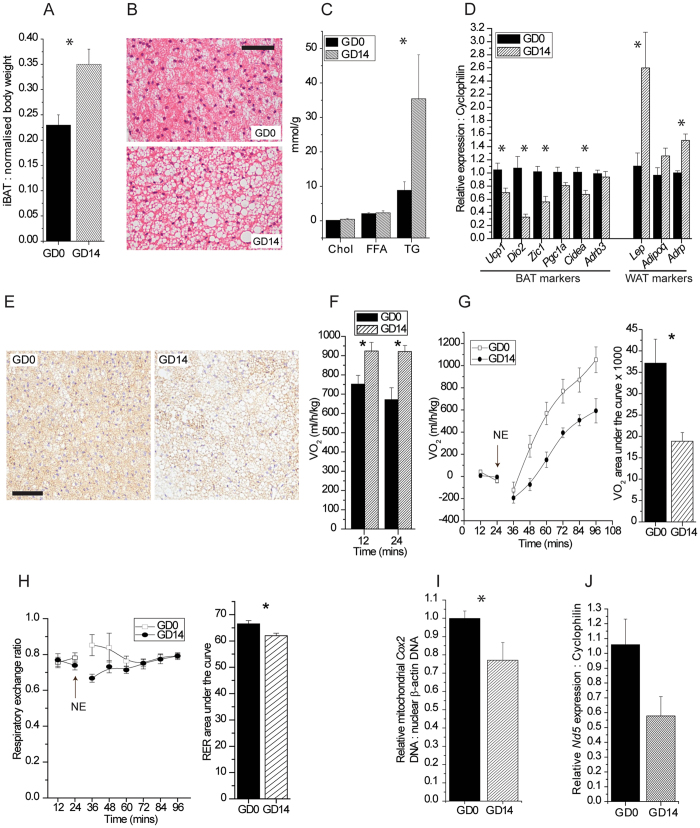
To understand the impact of brown adipose tissue (BAT) on pregnancy, a period associated with profound metabolic change, we first identified a point during gestation to study BAT biology that follows significant alterations in serum lipid concentrations. Longitudinal sampling of sera over the course of pregnancy identified gestational day (GD) 14 as a point that follows significant increases in serum cholesterol, HDL, and triglyceride concentrations (see Supplementary Fig. S1); fasting serum glucose concentrations were not different at this time point (data not shown). Interscapular BAT (iBAT) normalised against total body weight without the uterine horn was found to be significantly heavier by 52% at GD14 relative to non-pregnant controls (GD0) (Fig. 1A, and Supplementary Figure S2). H&E staining and lipid biochemical analyses revealed that the iBAT at GD14 contained enlarged lipid droplets (Fig. 1B), and that there was a 4-fold increase in triglyceride (TG) concentrations, respectively (Fig. 1C). iBAT cholesterol and free fatty acids (FFA) concentrations were unchanged. Profiling of classical phenotypic markers of brown and white adipose tissue revealed significantly decreased relative gene expression of BAT markers Ucp1, Dio2, Zic1, and Cidea, accompanied by significant increases in white adipose tissue (WAT) markers Lep and Adrp. Gene expression of the β3-adrenergic receptor was unchanged (Fig. 1D). Immunohistochemical analysis of GD14 BAT revealed decreased levels of UCP1 (Fig. 1E). Cumulatively, these data point to a decrease in overall BAT phenotype accompanied by ‘whitening’ of the tissue at GD14.
Figure 1.
Pregnancy mediates changes in the morphometry and function of BAT. (A) Interscapular BAT (iBAT) weight expressed as a percent of total body weight without uterine horn at gestational days 0 (GD0) and 14 (GD14). (B) H&E staining of iBAT. Scale bar, 100 µm. (C) Concentrations of total cholesterol (Chol), free fatty acids (FFA) and triglycerides (TG), normalised to protein, in iBAT. (D) Expression of genes related to BAT and WAT phenotype. (E) Immunohistochemical staining for UCP1 in iBAT. Scale bar, 100 µm. (F) Basal measurement of O2 consumption in terminally anaesthetised mice. (G) Measurement of norepinephrine (NE)-mediated O2 consumption in terminally anaesthetised mice over time with O2 consumption total area under the curve. To facilitate comparisons, responses are expressed as increases over pre-NE values. (H) NE-mediated changes in respiratory exchange ratio (RER) over time with RER total area under the curve. (I) qPCR analysis of DNA levels of mitochondrial Cox2 normalised to nuclear β-actin in iBAT. (J) Expression of the mitochondrial-encoded gene Nd5 in iBAT.
To test whether the reduction of BAT phenotype translated to a decrease in basal and inducible thermogenic BAT function, indirect calorimetry was used to measure O2 consumption in terminally anaesthetised mice at GD14. Basal O2 consumption was found to be significantly higher than at GD0 (Fig. 1F). However, pregnancy at GD14 significantly blunted the acute response to the β3-adrenergic receptor agonist norepinephrine (NE), as inducible O2 consumption was reduced by 49% (Fig. 1G) and the respiratory exchange ratio by 7.3% (Fig. 1H). Relative iBAT mitochondrial Cox2 DNA levels were significantly decreased at GD14, and mRNA expression of the mitochondrial gene Nd5 was also markedly reduced, albeit not significantly (Fig. 1I,J). Taken together with the UCP1 expression result, these data indicate reduced BAT thermogenic capacity.
Ablation of maternal BAT perturbs gestational metabolism and fetal growth
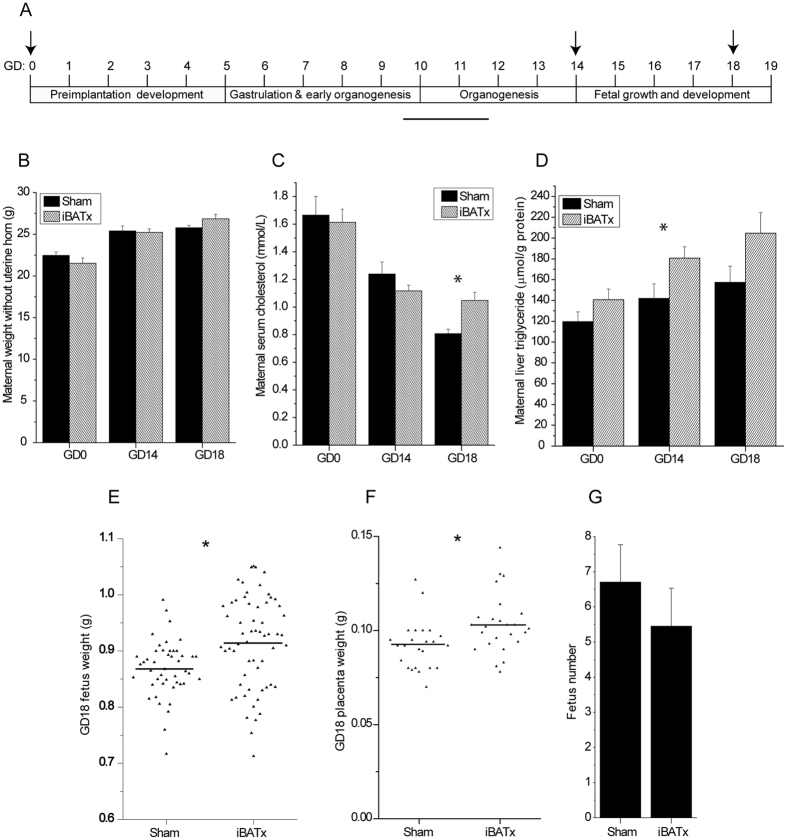
We hypothesised that the gestational loss of classical BAT phenotype and function with accompanied whitening is involved in supporting the maternal and fetal metabolic environment. A surgical approach was employed to ablate the interscapular BAT depot (iBATx) of female mice prior to conception, to enable assessment of the relative contribution of BAT to gestational metabolism. Gestational days 14 and 18 were chosen as time points of interest to capture the impact of BAT ablation prior to, and at the end of, the period of rapid fetal growth and development (Fig. 2A). Ablation of BAT had no effect on maternal weight; however, serum cholesterol levels were significantly increased by 29.7% at GD18 (Fig. 2B,C). Serum triglycerides, FFA and fasted glucose levels remained unchanged (see Supplementary Fig. S3). In the BAT-ablated group, maternal hepatic triglycerides were increased by 17.6%, 27.2% and 30.0% at GD0, GD14 and GD18, respectively, although the change was only significant for GD14 (Fig. 2D). No differences were observed in liver cholesterol and FFA levels (see Supplementary Fig. S3). Importantly, there was no evidence of compensatory ‘browning’ of WAT in the iBATx mice (see Supplementary Fig. S5).
Figure 2.
Surgical ablation of interscapular BAT alters maternal and fetal metabolic profiles. (A) Schema summarising the key stages of mouse pregnancy over time and the time-points selected for the study of pregnancy and metabolism in iBAT ablated (iBATx) or sham-operated mice, as indicated by the arrows. (B) Maternal weight without uterine horn (Sham, n = 5–10; iBATx, n = 6–9). (C) Maternal serum cholesterol levels (Sham, n = 6–9; iBATx, n = 7–10). (D) Maternal liver triglyceride levels (Sham, n = 7–10; iBATx, n = 9–12). Data are represented as mean ± SEM, *p<0.05 for Sham versus iBATx comparison at a single pregnancy time-point as determined by unpaired Student’s t-test. (E) Weight of fetuses at GD18 (Sham, n = 46 fetuses from 7 mothers; iBATx, n = 51 fetuses from 9 mothers). (F) Weight of placentas at GD18 (Sham, n = 24 placentas from 4 mothers; iBATx, n = 25 placentas from 5 mothers). (G) Average number of fetuses per pregnancy at GD18 (Sham n = 7; iBATx n = 9). *p<0.05 as determined by unpaired Student’s t-test.
The impact of BAT ablation on fetal parameters was evident at GD18, as the average fetal and placental weight increased by 7.8% and 12.3% respectively (Fig. 2E,F), whereas at GD14 no differences were observed (see Supplementary Fig. S4). Therefore, at a macroscopic level, fetal growth is influenced by maternal BAT. There was no significant difference in the average number of fetuses per pregnancy between the two groups at GD18 indicating that the increase in fetal and placental weight in the iBATx group is due to the decrease of a factor or function associated with brown fat during pregnancy (Fig. 2G).
Ablation of maternal BAT perturbs maternal and fetal lipid homeostasis
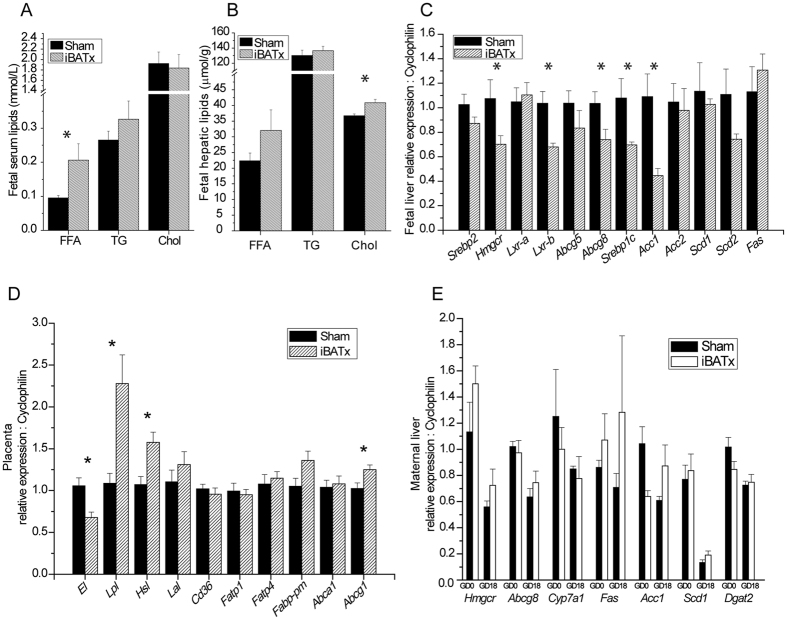
The mouse fetus undergoes a rapid growth phase between GD14 and parturition, which coincides with our observation that at GD18, the fetuses are heavier from iBATx mothers. This may be as a result of altered fetal or maternal lipid homeostasis and fuel availability. Biochemical analysis of FFA concentrations in the serum of fetuses at GD18 from iBATx mothers revealed a significant increase of 117% (Fig. 3A), whereas triglyceride and total cholesterol levels remained unchanged. GD18 fetal hepatic cholesterol concentrations were significantly increased by 11.4% and there was a trend for increased FFA in the iBATx group (Fig. 3B).
Figure 3.
Surgical ablation of maternal interscapular BAT results in fetal hyperlipidemia. Serum concentrations (A) and normalised hepatic levels (B) of total free fatty acids (FFA), triglycerides (TG) and total cholesterol of GD18 fetuses from sham-operated (n = 4) or iBATx (n = 5–6) mothers. Expression of genes related to lipid homeostasis in (C) GD18 fetal liver (n = 4 per group), (D) GD18 placenta (Sham, n = 7; iBATx, n = 10)) and (E) GD0 and 18 maternal liver (Sham, n = 6–7; iBATx, n = 7–8). Liver and serum were pooled from fetuses from the same mother. Data are represented as mean ± SEM, *p<0.05 as determined by unpaired Student’s t-test.
To determine the source of the increased FFA levels, the expression of genes involved in lipid metabolism was studied in GD18 fetal and maternal liver as well as placenta. Fatty acid gene expression targets were either unchanged or significantly decreased, e.g. Srebp1c and Acc1 in the fetal liver (Fig. 3C). The expression of placental lipases was studied, as they may be involved in the hydrolysis of serum triglycerides to fatty acids, which are transported across into the fetoplacental unit. Indeed, the expression of Lpl and Hsl were significantly increased by 2.3- and 1.6-fold respectively (Fig. 3D). There was a trend for increased gene expression of maternal hepatic enzymes Acc1 and Scd1 involved in generating FA in iBATx mothers (Fig. 3E). These data suggest that the increased FFA in the fetuses from the iBATx mice is not due to de novo lipogenesis, but rather increased transport across the placenta as indicated by the upregulation of placental lipolysis.
Early gestational alterations in BAT phenotype are mediated by a progesterone signal
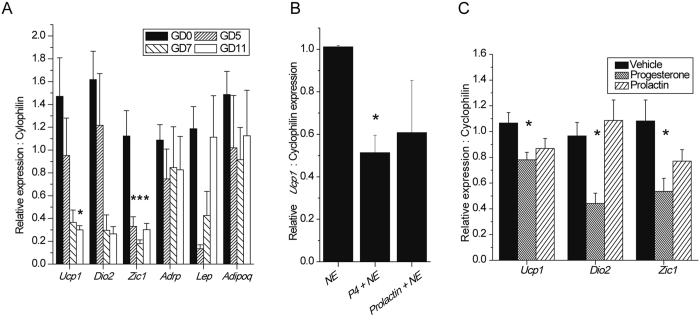
To delineate the gestational signal that alters the BAT phenotype, the expression profiles of BAT and WAT markers were studied in the iBAT from pregnant mice at GD5 and GD7, pre-placentation time points, and GD11, post-placentation. Ucp1 and Dio2 gene expression was reduced considerably from as early as GD7, and Zic1 was significantly reduced from as early as GD5, indicating that pre-placentation factors are involved in mediating gestational gene expression changes in BAT. The gene expression of the WAT marker leptin was markedly decreased at GD5 and GD7, returning to control levels by GD11. The gene expression of the WAT marker adiponectin did not change at the time-points studied (Fig. 4A). These data are supported by the fact that BAT weight is unchanged at GD5 (see Supplementary Fig. S6), therefore lipid accumulation does not occur until the later stages of pregnancy. To identify the pre-placentation hormone that is able to mediate the early gestational changes in BAT phenotype, an in vitro approach was taken. Differentiated primary brown adipocytes were treated with 500 nM progesterone or 550 ng/ml prolactin overnight, which are physiological concentrations10, 21, ± a 2 hour treatment with 1 μM of the β3-adrenergic agonist NE. Ucp1 gene expression was used as a quantifiable reporter due to it being a functional marker of brown adipose tissue and because its induction is measurable following a 2-hour acute challenge. Progesterone significantly inhibited NE-mediated induction of Ucp1 by 49%, whereas prolactin did not exert any significant effects (Fig. 4B). This result demonstrated that the pre-placentation hormone progesterone can blunt the induction of the BAT marker Ucp1. Finally, we sought to recapitulate the in vitro data in an in vivo model. Exogenous progesterone was administered chronically over two weeks by an osmotic pump to oophorectomised mice, which resulted in a significant relative reduction in the expression of Ucp1, Dio2 and Zic1. There was a trend for prolactin to inhibit Ucp1 and Zic1 expression, but not to the extent of progesterone (Fig. 4C), the concentration of which rises early in pregnancy (see Supplementary Fig. S7). Concentrations of cholesterol, triglycerides, FFA and glucose, as well as mouse weight were unchanged (data not shown).
Figure 4.
Progesterone mediates a reduction in the expression of thermogenic genes in brown adipose tissue. (A) Expression of genes related to BAT and WAT in iBAT at GD0 (n = 4), GD5 (n = 5), GD7 (n = 5) and GD11 (n = 5). (B) Relative Ucp1 expression of cultured BAT following treatment with 1 μM norepinephrine (NE) ± 500 nM progesterone (P4) or 550 ng/ml prolactin normalised to the 1 μM norepinephrine treated group (n = 4). (C) Normalised relative gene expression of BAT targets in oophorectomised mice challenged with vehicle (n = 7), progesterone (n = 6) or prolactin (n = 6) for 14 days via osmotic pumps. All data are represented as mean ± SEM. *p<0.05 as determined by one-way ANOVA.
These data are consistent with progesterone being the endocrine signal that mediates the gestational changes in BAT phenotype.
Discussion
Fluxes in energy metabolism during pregnancy are vital for the controlled growth of the developing fetus. We have demonstrated throughout this study the importance of pregnancy-mediated adaptations to BAT, which contribute to the regulation of energy metabolism in both the mother and the fetus. We conclusively show at a macroscopic level that the iBAT was hypertrophied at GD14 of pregnancy due to an increase in triglyceride concentrations and lipid droplet size. This was accompanied by a decrease in the levels of markers of classical BAT phenotype and a parallel increase in WAT markers, giving the tissue a whitened phenotype.
To ascertain if the diminished BAT phenotype resulted in decreased BAT function, we assessed the capacity of β3-adrenergic agonist-stimulated thermogenesis at GD14 using indirect calorimetry, avoiding the muscular contribution to O2 consumption by terminally anaesthetising the mice. As previously reported22, we observed that basal O2 consumption was greater during pregnancy, which may be a consequence of increased metabolic activity associated with maintaining pregnancy. However, we demonstrated that norepinephrine-mediated O2 consumption was blunted at GD14, despite the higher basal O2 consumption. This was paralleled by a reduction in the norepinephrine (NE)-mediated RER value. The levels of mitochondrial markers were also reduced at GD14 and, when combined with reduced UCP1 expression and increased lipid concentrations, could be responsible for the abrogation of BAT function during pregnancy. These results demonstrate that BAT undergoes adaptive changes characterised by a loss of classical thermogenic phenotype and function accompanied by whitening during pregnancy. Similarly, whitening of BAT with a loss of BAT phenotype and function has been observed in diet-induced obese mice23.
Evidence for a functional role of gestationally modified BAT was provided by experiments where iBAT, which represents approximately 60% of total BAT24, was surgically ablated prior to conception. Days 14 and 18 of gestation were chosen to study the impact of BAT ablation, as they lie before and towards the end of the period of rapid fetal growth25, 26. Removal of BAT had no effect on maternal body weight. This is not surprising, as mice lacking UCP1 housed below thermoneutrality do not become obese and O2 consumption remains unchanged27–29. However, the iBATx group had significantly higher levels of serum cholesterol and hepatic triglyceride concentrations. This is consistent with the view that gestationally adapted BAT contributes to whole body lipid metabolism during pregnancy.
It is well established that the metabolic status of the mother can affect the developing fetus30–33. We therefore studied the consequences of iBAT ablation on the fetal compartment. Fetal and placental weight were increased at GD18 in the iBATx group as a result of a decrease of a factor or function associated with brown fat during pregnancy that contributes to the regulation of fetal growth. There are striking similarities between maternal obesity, which is associated with hypertriglyceridemia and an increased risk for babies to be born large-for-gestational age4, 7, 31, 34, 35, and our findings in iBAT ablated mice that have raised maternal hepatic triglyceride levels and heavier GD18 fetuses. This emphasises the importance of BAT in regulating systemic maternal lipid levels during a critical period of fetal development.
Biochemical analyses of the serum from the iBATx GD18 fetuses revealed elevated FFA which is likely responsible for the increased placental and fetal weight. Indeed, FFA are readily converted to precursors of other molecules that modulate cell growth and metabolism, and incorporated into cellular structures36. We hypothesised that the increased FFA levels originated from the maternal serum, as gene expression analysis of lipid homeostasis targets in the fetal liver were not increased and, in some cases, were reduced, possibly as a measure to rebalance the elevated fetal serum FFA levels. The probable source of the increased fetal serum FFA is hydrolysed triglycerides from the placenta, as the expression of placental lipases was significantly increased in GD18 iBATx mice. This may be a homeostatic response to reduce the elevated levels of GD14 maternal triglycerides (Fig. 2D), emphasising the sensitive nature of the maternal-fetal lipid metabolic axis and the important role BAT plays in this. iBATx fetal liver cholesterol concentrations were also significantly increased at GD18, reflecting the GD18 iBATx maternal hypercholesterolemic environment and increased levels of the placental cholesterol transporter Abcg1. However, GD18 iBATx fetal liver gene expression targets involved in cholesterol synthesis were decreased, indicating a homeostatic response to the aberrant hepatic cholesterol concentrations. The data presented here show a direct link between maternal BAT activity and fetal energy metabolism and growth. A recent study in humans has proposed that there is a crosstalk between maternal BAT and fetal adiposity37. In light of our study, the impact of maternal BAT ablation upon offspring metabolism in the long term warrants further investigation, particularly since adipogenesis is a potential target for fetal programming38.
Pregnancy factors, including reproductive hormones, alter a range of physiological systems to adapt them to the gestational needs of the mother and fetus. The impact of pregnancy hormones on BAT has been studied extensively, but the data are conflicting. Estrogen has been shown to both increase and decrease BAT activity17, 18, 39–41. This may be a reflection of species and strain specific differences as well estrogen delivery strategies used. In addition, previous studies of progesterone and prolactin effects indicate that both hormones can influence BAT phenotype and activity19, 42–47. In this study, we investigated three different model systems to identify an early gestational signal capable of eliciting phenotypic changes in the BAT seen during pregnancy. Firstly, by studying the temporal expression of BAT markers at different stages of pregnancy, we were able to define GD5 and GD7 as time-points where the expression of classical BAT markers Ucp1, Dio2 and Zic1 were reduced. At GD5 and GD7, the gestational factors are likely to be pre-placentation hormones secreted by the ovary and pituitary gland such as progesterone and prolactin respectively, whereas post-placentation, GD10 onwards, would include hormones secreted from the placenta, such as estrogen. The expression of the WAT markers leptin and adiponectin were not increased, revealing that gestational whitening of BAT, as observed at GD14, follows the reduction in the expression of classical BAT markers. Differentiated primary BAT cultures were treated with physiological concentrations of progesterone or prolactin in combination with the β3-adrenergic agonist NE. NE-mediated Ucp1 induction was consistently blunted by the progesterone treatment, demonstrating that progesterone is a pregnancy signaling molecule that can act on brown adipocytes to modulate the expression of thermogenic targets. This result is also strongly indicative of a direct effect of progesterone on the brown adipocyte. Finally, the expression of Ucp1, Dio2 and Zic1 were all reduced by exogenous progesterone delivered by osmotic pumps in oophorectomised mice reconfirming that progesterone is a key gestational signal involved in altering BAT phenotype. This is the first study to show that the reproductive hormone progesterone consistently decreases BAT phenotype and is therefore a key mediator of the gestational metabolic switch. To corroborate our findings, experiments would be required to show that maternal BAT does not change in the absence of progesterone. However, due to the physiological importance of progesterone in maintaining pregnancy, these studies are not feasible. Further work is needed to investigate whether our findings have implications for use of progesterone in in vitro fertilisation techniques, or for women taking progesterone supplements as hormonal contraception or for the prevention for pre-term birth.
Human studies have shown that women possess greater volumes of BAT relative to men, which decrease with age14, yet few studies have sought to understand why this may be. The data presented here demonstrate that the early gestational factor progesterone switches the phenotype of brown adipose tissue, which is necessary to support the maternal and fetal metabolic milieu. Moreover, this is the first study to our knowledge that demonstrates a direct relationship between maternal BAT activity and fetal growth. These data support the notion that BAT has an underappreciated gender-specific role in women that has evolved to modify lipid metabolism during pregnancy, and underscores BAT as a potential therapeutic target of maternal obesity and as a consequence, fetal macrosomia.
Methods
Animal studies
Animal studies were performed using C57BL/6 mice from Charles River UK Ltd., maintained on a standard breeding diet (RM3) and allowed free access to food and water. They were housed in stable conditions (12 hour light/dark cycle; 22–24 °C). Mice were acclimatised to the animal house for 1 week prior to experiments.
For pregnancy experiments, 7–8 week old female mice were mated with males. Virgin mice were used as controls (gestational day (GD) 0). GD1 was considered to be the day the copulatory plug was identified. For all animal studies, mice were sacrificed after a 4 hour fast, and maternal and fetal serum and tissues were collected and snap-frozen immediately or fixed in 10% neutral buffered formalin for 24 hours.
Metabolic studies
Indirect calorimetry was carried out using the LabMaster cage system (TSE). GD0 and GD14 mice were terminally anaesthetised by intraperitoneal injection of 90 mg/kg pentobarbital (Merial Animal Health Limited). Once fully sedated, they were placed in the metabolic cages and allowed to acclimatise for 20 minutes, to establish a constant metabolic baseline. Norepinephrine (NE; 1 mg/kg; Sigma-Aldrich) was then administered by subcutaneous injection at the nape. The O2 consumption and CO2 production was measured in each cage sequentially every 12 minutes, and retrospectively corrected for body weight without pups. Respiratory exchange ratio was calculated according to manufacturer’s software.
BAT ablation surgery
7–8 week old female mice were anaesthetised via isoflurane vapour (Abbott Animal Health) and the interscapular BAT (iBAT) was surgically removed. Sham-operated mice underwent the same procedure without the removal of iBAT. Mice were single-housed for 2 weeks during the recovery period, and then paired with males until visual confirmation of a plug, following which they were monitored until sacrifice at GD14 or GD18 of pregnancy, along with GD0 controls.
Osmotic pump implant surgery
7–8 week old female mice were anaesthetised via isoflurane vapour and underwent bilateral oophorectomy. After a two-week recovery period, the mice were anaesthetised and 14-day release osmotic minipumps (Alzet model 2002; Charles River) were implanted subcutaneously in the dorsal region. Osmotic pumps contained progesterone (250 µg/day; Sigma-Aldrich), prolactin (7 µg/day; PeproTech) or vehicle only (20% (w/v) 2-hydroxypropyl-beta-cyclodextrin, Sigma Aldrich). After 2 weeks, animals were sacrificed as described above.
Histology and immunohistochemistry
Fixed iBAT sections were embedded in paraffin and sectioned at 5 µm onto poly-L-lysine coated slides. Sections were stained with haematoxylin and eosin for histological analysis. Immunohistochemical analysis was performed as previously described48. Sections were incubated overnight at 4 °C with rabbit anti-mouse primary antibody to UCP1 diluted 1:10,000 in phosphate buffered saline. The UCP1 antibody was a kind gift from Barbara Cannon.
Lipid measurements
Serum and tissue cholesterol, LDL-cholesterol, HDL-cholesterol, TG, FFA and total proteins were measured using an Unicel DxC 800 autoanalyzer (Beckman-Coulter) using dedicated kits from Beckman-Coulter, excluding FFA which were measured using a kit from Wako Diagnostics (Neuss). Liver and brown adipose tissue were extracted in a 0.125 M potassium phosphate buffer and normalised to the total protein content.
Primary adipocyte culture and differentiation
iBAT was removed from 10 day old C57BL/6 pups and pooled. Primary adipocytes were isolated and differentiated as previously described49. Cells were seeded and grown in growth media consisting of DMEM/10% NCS with (final concentrations) 20 nM insulin (Actrapid, Novonordisk) and 1nM triiodothyronine (Sigma-Aldrich), until 70% confluent. The adipocytes were differentiated for 48 hours in the above media supplemented with 500 µM isobutylmethylxanthine, 2 µM dexamethasone, 50 µM indomethacin and 1 µM rosiglitazone (Sigma-Aldrich). On the morning of an experiment the media was replaced with growth media.
mRNA analysis
Total RNA was extracted from frozen tissue using the RNeasy Mini Kit (Qiagen), and cDNA synthesised using the Superscript II kit (Thermo Fisher Scientific), according to manufacturer’s instructions. Semi-quantitative real-time PCR with SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich) was used to detect and amplify target cDNA. Relative gene expression was calculated using the ∆∆ Ct method. Genes of interest were normalised to the housekeeping gene Cyclophilin B. See Supplementary Experimental Procedures online for a table of primers and sequences. Basal expression of Ucp1 was undetectable in several of the cultures, and thus the results of the hormonal treatments were calculated as relative to the NE treated group, as previously described43 (see Supplementary Fig. S8 for example).
Mitochondrial DNA analysis
Frozen iBAT samples were homogenised in Qiazol (Qiagen) and incubated for 5 minutes at room temperature, after which chloroform (Sigma-Aldrich) was added. Following centrifugation, total DNA was precipitated from the interphase and the phenol phase with 100% ethanol. After further centrifugation, the resulting DNA pellet was washed in 0.1M sodium citrate, followed by 75% (v/v) ethanol. The DNA pellet was air-dried, resuspended in 8mM sodium hydroxide, and neutralised by the addition of HEPES buffer and 100 mM EDTA, and stored at −20 °C until use. Relative mitochondrial content was assessed by comparing the Ct of mitochondrial Cox2 relative to nuclear β-actin, as described above.
Statistics
Data are presented as mean ± standard error of the mean (SEM). Statistical analysis was performed using Origin Pro software (OriginLab). An unpaired Student’s t-test was used for comparison between two groups. One-way ANOVA was used when comparing more than two groups, followed by Tukey’s post-hoc analysis. A p-value of <0.05 was considered significant. Data was assessed for normality by carrying out standard distribution plots. Where there were outliers, sensitivity analysis was performed and analysis repeated with the outlier(s) removed.
Study Approval
All experiments were performed in accordance with the Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012 and approved by King’s College London’s Animal Welfare and Ethical Review Body and the Home Office.
Electronic supplementary material
Acknowledgements
This study was funded by the Wellcome Trust and the Guy’s and St Thomas’ Charity. C.W. is also funded by Tommy’s Charity and the National Institute of Health Research (NIHR) Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.. We thank Dr. Sam Virtue for his expert assistance in indirect calorimetric studies, Dr. Paul Seed for guidance on statistical analysis and Dr. Bryn Owen and members of the Maternal and Fetal Disease Group, KCL, for helpful discussion.
Author Contributions
Conceptualisation, S.A.-H. and C.W.; Methodology, S.M. and S.A.-H.; Investigation, S.M., A.M., G.P., C.H., J.S., E.J. and S.A.-H.; Writing- original draft, S.M. and S.A.-H.; Writing- reviewing and editing, S.M., S.A.-H. and C.W.; Funding acquisition, C.W.; Supervision, S.A.-H. and C.W.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Shadi Abu-Hayyeh and Catherine Williamson contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10979-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shadi Abu-Hayyeh, Email: shadi.abu-hayyeh@kcl.ac.uk.
Catherine Williamson, Email: catherine.williamson@kcl.ac.uk.
References
- 1.Yu Z, et al. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell EA, et al. Maternal nutritional risk factors for small for gestational age babies in a developed country: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2004;89:F431–435. doi: 10.1136/adc.2003.036970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godfrey K, Robinson S, Barker DJ, Osmond C, Cox V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ. 1996;312:410–414. doi: 10.1136/bmj.312.7028.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191:964–968. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Chu SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 7.Gaillard R, et al. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 2013;21:1046–1055. doi: 10.1002/oby.20088. [DOI] [PubMed] [Google Scholar]
- 8.O'Reilly JR, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinol (Oxf) 2013;78:9–16. doi: 10.1111/cen.12055. [DOI] [PubMed] [Google Scholar]
- 9.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008;112:999–1006. doi: 10.1097/AOG.0b013e31818a5d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mingrone G, et al. Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care. 2008;31:1872–1876. doi: 10.2337/dc08-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71:1256S–1261S. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- 12.Kajimura S, Spiegelman BM, Seale P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfannenberg C, et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–1793. doi: 10.2337/db10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 16.Trayhurn P. Thermogenesis and the energetics of pregnancy and lactation. Can J Physiol Pharmacol. 1989;67:370–375. doi: 10.1139/y89-060. [DOI] [PubMed] [Google Scholar]
- 17.Puerta ML, Nava MP, Abelenda M, Fernandez A. Inactivation of brown adipose tissue thermogenesis by oestradiol treatment in cold-acclimated rats. Pflugers Arch. 1990;416:659–662. doi: 10.1007/BF00370611. [DOI] [PubMed] [Google Scholar]
- 18.Martinez de Morentin PB, et al. Pregnancy induces resistance to the anorectic effect of hypothalamic malonyl-CoA and the thermogenic effect of hypothalamic AMPK inhibition in female rats. Endocrinology. 2015;156:947–960. doi: 10.1210/en.2014-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Cuenca S, et al. Sex steroid receptor expression profile in brown adipose tissue. Effects of hormonal status. Cell Physiol Biochem. 2007;20:877–886. doi: 10.1159/000110448. [DOI] [PubMed] [Google Scholar]
- 20.Chan E, Swaminathan R. Role of prolactin in lactation-induced changes in brown adipose tissue. Am J Physiol. 1990;258:R51–56. doi: 10.1152/ajpregu.1990.258.1.R51. [DOI] [PubMed] [Google Scholar]
- 21.Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114:1326–1331. doi: 10.1097/AOG.0b013e3181c2bde8. [DOI] [PubMed] [Google Scholar]
- 22.McQueenie JRSaJ. Limits to Sustained Metabolic Rate: The Link between Food Intake, Basal Metabolic Rate, and Morphology in Reproducing Mice, Mus musculus. Physiol Zool69, 746–769 (1996).
- 23.Shimizu I, et al. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest. 2014;124:2099–2112. doi: 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bal NC, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–1579. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulandavelu S, et al. Embryonic and neonatal phenotyping of genetically engineered mice. ILAR J. 2006;47:103–117. doi: 10.1093/ilar.47.2.103. [DOI] [PubMed] [Google Scholar]
- 26.Ko MS. Embryogenomics: developmental biology meets genomics. Trends Biotechnol. 2001;19:511–518. doi: 10.1016/S0167-7799(01)01806-6. [DOI] [PubMed] [Google Scholar]
- 27.Enerback S, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, et al. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111:399–407. doi: 10.1172/JCI200315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kontani Y, et al. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005;4:147–155. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 30.Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol. 2008;198(416):e411–416. doi: 10.1016/j.ajog.2007.10.796. [DOI] [PubMed] [Google Scholar]
- 31.Olmos PR, et al. Maternal hypertriglyceridemia: A link between maternal overweight-obesity and macrosomia in gestational diabetes. Obesity (Silver Spring) 2014;22:2156–2163. doi: 10.1002/oby.20816. [DOI] [PubMed] [Google Scholar]
- 32.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189:1698–1704. doi: 10.1016/S0002-9378(03)00828-7. [DOI] [PubMed] [Google Scholar]
- 33.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merzouk H, Meghelli-Bouchenak M, Loukidi B, Prost J, Belleville J. Impaired serum lipids and lipoproteins in fetal macrosomia related to maternal obesity. Biol Neonate. 2000;77:17–24. doi: 10.1159/000014190. [DOI] [PubMed] [Google Scholar]
- 35.Kim SY, Sharma AJ, Sappenfield W, Wilson HG, Salihu HM. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obstet Gynecol. 2014;123:737–744. doi: 10.1097/AOG.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Innis SM. Fatty acids and early human development. Early Hum Dev. 2007;83:761–766. doi: 10.1016/j.earlhumdev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Perez, B. et al. Angiopoietin-like protein 8 (ANGPTL8) in pregnancy: a brown adipose tissue-derived endocrine factor with a potential role in fetal growth. Transl Res (2016). [DOI] [PubMed]
- 38.Desai M, Beall M, Ross MG. Developmental origins of obesity: programmed adipogenesis. Curr Diab Rep. 2013;13:27–33. doi: 10.1007/s11892-012-0344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richard D. Effects of ovarian hormones on energy balance and brown adipose tissue thermogenesis. Am J Physiol. 1986;250:R245–249. doi: 10.1152/ajpregu.1986.250.2.R245. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen SB, Bruun JM, Kristensen K, Richelsen B. Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen. Biochem Biophys Res Commun. 2001;288:191–197. doi: 10.1006/bbrc.2001.5763. [DOI] [PubMed] [Google Scholar]
- 41.Puerta M, Abelenda M, Nava MP, Fernandez A. Reduced noradrenaline responsiveness of brown adipocytes isolated from estradiol-treated rats. Can J Physiol Pharmacol. 1993;71:858–861. doi: 10.1139/y93-129. [DOI] [PubMed] [Google Scholar]
- 42.Monjo M, Rodriguez AM, Palou A, Roca P. Direct effects of testosterone, 17 beta-estradiol, and progesterone on adrenergic regulation in cultured brown adipocytes: potential mechanism for gender-dependent thermogenesis. Endocrinology. 2003;144:4923–4930. doi: 10.1210/en.2003-0537. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez AM, Monjo M, Roca P, Palou A. Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes. Cell Mol Life Sci. 2002;59:1714–1723. doi: 10.1007/PL00012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viengchareun S, et al. Prolactin receptor signaling is essential for perinatal brown adipocyte function: a role for insulin-like growth factor-2. PLoS One. 2008;3 doi: 10.1371/journal.pone.0001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearce S, et al. Prolactin, the prolactin receptor and uncoupling protein abundance and function in adipose tissue during development in young sheep. J Endocrinol. 2005;184:351–359. doi: 10.1677/joe.1.05732. [DOI] [PubMed] [Google Scholar]
- 46.Viengchareun S, et al. Prolactin potentiates insulin-stimulated leptin expression and release from differentiated brown adipocytes. J Mol Endocrinol. 2004;33:679–691. doi: 10.1677/jme.1.01563. [DOI] [PubMed] [Google Scholar]
- 47.Pearce S, et al. Prolactin, prolactin receptor and uncoupling proteins during fetal and neonatal development. Proc Nutr Soc. 2003;62:421–427. doi: 10.1079/PNS2003246. [DOI] [PubMed] [Google Scholar]
- 48.Leonardsson G, et al. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci USA. 2004;101:8437–8442. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cannon B, Nedergaard J. Cultures of adipose precursor cells from brown adipose tissue and of clonal brown-adipocyte-like cell lines. Methods Mol Biol. 2001;155:213–224. doi: 10.1385/1-59259-231-7:213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.