Abstract
Centella asiatica (L.) Urb. has been used as an herbal brain tonic for mental disorders and enhancing memory, but no review of the overall evidence of C. asiatica and cognitive function has been conducted. This study aims to determine the effects of C. asiatica on cognitive function and its related properties. The current systematic review includes five randomized controlled trials (RCTs) conducted to determine the effect of C. asiatica alone and six RCTs conducted to determine the effect of C. asiatica-containing products. Meta-analysis indicated that there are no significant differences in all cognitive function domains of C. asiatica when compared to placebo. However, it could improve mood by increasing alert scores [SMD: 0.71 (95% CI; 0.01 to 1.41); I2 = 30.5%] and decreasing anger scores at 1 hour after treatment [SMD: −0.81 (95%CI; −1.51 to −0.09); I2 = 36.6%]. None of the studies reported adverse effects of C. asiatica. In conclusion, there is not strong evidence to support the use of C. asiatica for cognitive function improvement in each cognitive domain. C. asiatica could improve alertness and relieve anger. However, some limitations should be aware including dose regimen, plant preparation, standardization, and product variation. Future well-designed clinical trials using suitable doses of standardized C. asiatica are still needed.
Introduction
Cognition can be defined as the group of mental processes that lead to knowledge through thought, experience, and the senses. Cognitive function consists of various domains including attention and concentration, executive function, information processing speed, language, visuospatial skill, working memory, verbal memory, and visual memory1. Diseases, drugs, chemicals, genetics, and aging can all cause declines in cognitive ability leading to cognitive impairment. Cognitive impairment may result in dementia or Alzheimer’s disease.
Acetylcholinesterase inhibitors (AChEIs) have been recommended as a first-line treatment for Alzheimer’s disease. However, AChEIs are also associated with various adverse events. To avoid these, herbal medicines such as Ginkgo (Gingko biloba L.), Curcuma longa L., Melissa officinalis L. and Bacopa monnieri L. Wettst have been increasingly used as alternatives to prevent or treat cognitive impairment2–5.
Centella asiatica (L.) Urban., (family Apiaceae), commonly known as asiatic pennywort or gotu kola, is a plant that has been used as an AChEI alternative. It is a perennial, herbaceous creeper with kidney shaped leaves commonly found and cultivated in Asian countries6, 7. It has been used since ancient times in Ayurvedic traditions under the name of mandukaparni6–8. This plant functions as an herb, spice, vegetable, and juice as well as in nutraceutical and cosmetic products. C. asiatica has been added to the Thailand National List of Essential Medicines for its antipyretic and wound healing properties9. It has also been selected as one of the five medicinal plants to be developed as a “champion herbal product” to generate income for the country10.
C. asiatica contains several active ingredients with the most important group being pentacyclic triterpenes, which includes asiaticoside, madecassoside, asiatic acid, and madecassic acid8. C. asiatica and its pentacyclic triterpenes are commonly used for their antipyretic, wound healing, anti-wrinkle, and anti-inflammation effects11. Important indications for C. asiatica in Ayurveda include its use for cognitive properties as a brain tonic, in the treatment of mental disorders, and as a memory-enhancing agent6, 7, 12. C. asiatica was shown to improve neuronal morphology and learning performance and enhance memory retention in animal models13, 14. Several mechanisms of action of C. asiatica were demonstrated for enhancing cognitive function, such as the inhibition of acetylcholinesterase activity, reduction of phospholipase A2 (PLA2) activity, protection against ß-amyloid formation, and protection from brain damage15–17. Furthermore, C. asiatica has also shown anti-stress, antidepressant, anxiolytic and anti-seizure properties in pre-clinical studies18–20. In animal models, asiaticoside and asiatic acid showed neuroprotective, antidepressive, and anxiolytic effects20–23. Learning and memory improvements facilitated by asiatic acid have been observed in passive and active avoidance tests24. From these data and its use in traditional medicine, C. asiatica is selected as one of the active ingredients in nutraceutical products for improving brain function.
A number of randomized controlled studies have investigated the clinical effect of C. asiatica on cognitive function25, 26. However, no study has summarized the overall evidence of C. asiatica on cognitive function and its related properties. Therefore, this study aims to systematically review all available evidence to determine the efficacy and safety of C. asiatica on cognitive function and its related properties including effects on mood and quality of life (QoL).
Results
Study selection
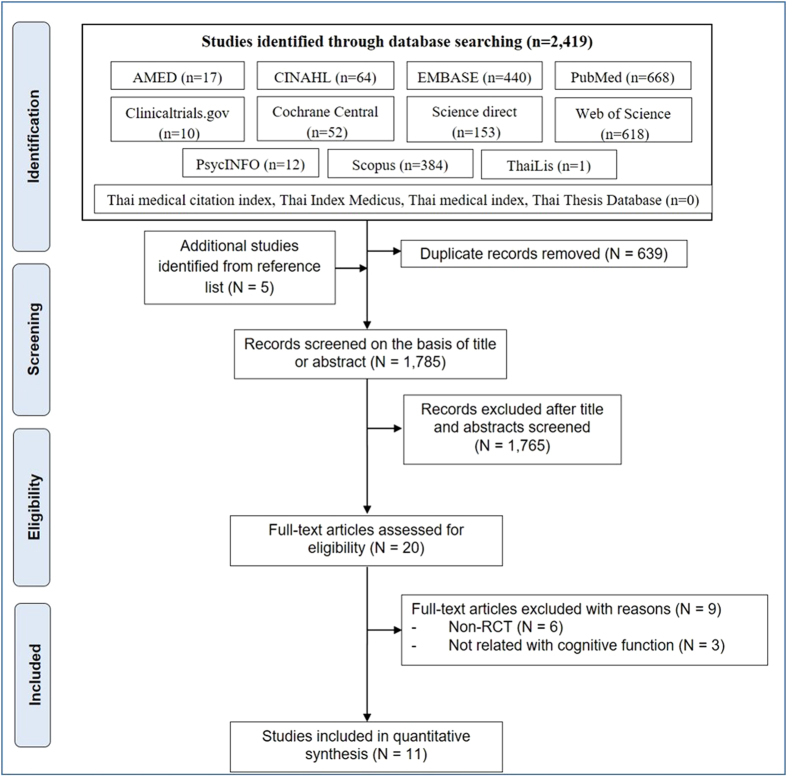
A total of 2,419 articles were identified from the database searches, and five articles were added based on our review of the reference lists. Of the articles, 693 were excluded because of duplication. A total of 1,785 titles and abstracts were screened. Of the screened titles and abstracts, 20 full-text articles were reviewed, of which only 11 articles were included in the systematic review. The flow of included studies is depicted in Fig. 1.
Figure 1.
Flow of included studies.
Study characteristics
Of the 11 included studies, five studies (45%) compared C. asiatica alone to placebo, and six studies (54%) compared a combination of C. asiatica versus other herbs. For combination products, three of the six studies (50%) used mix herbs as the active ingredients, two of the six studies (33%) used Gingko biloba as the major compound, and one study used a combination of vitamins and herbs (Table 1). Only one study (9%) did not report the Latin binomial nomenclature of the herbal ingredients27. Standardization methods were reported in three studies (27%)26, 28, 29 but only two studies quantitatively described the standardization26, 29. Nine studies (81%) were conducted using double-blind parallel designs, one used an open-labeled parallel design, and one used a cross-over design. Most studies (91%) were conducted in healthy volunteers, while one study was conducted in children with attention deficit hyperactive disorder. Other information (herbal supplement type, dosage form, plant preparation, dose of C. asiatica, standardization method, study characteristics, intervention and patient characteristics) is summarized in Table 1.
Table 1.
Characteristics of Centella asiatica (L.) Urb. supplements and included studies.
| Author | Herbal supplement type | Dosage form | Report Latin name (Raw material authentication) | Plant part | C. asiatica Preparation (solvent for extraction) | Standardization | Dose of C. asiatica per day (mg) | Standard compound content per day (mg) | Standard PT# content per day (mg) | Pharmacy (P)/Manufacturer (M) production |
|---|---|---|---|---|---|---|---|---|---|---|
| Bradwejn, 200032 | Single | Mixture | Yes (No) | NR | Powder | NR | 12,000 | N/A | N/A | NR |
| Dev, 200925 | Single | Capsule | Yes (No) | NR | Powder | NR | 5,000–8,000 | N/A | N/A | Yes (M) |
| Mato, 201128 | Single | Capsule | Yes (Yes) | Aerial | Extract (water) | Standardized using TPC, AS, AA | 250 500 750 | TPC = 7.48, AS = 0.27, AA = 12.22 TPC = 14.19, AS = 0.55, AA = 24.45 TPC = 22.43, AS = 0.82, AA = 36.67 | 22.49 25.00 37.49 | Yes (P) |
| Rao, 197735 | Single | Tablet | Yes (No) | NR | Powder | NR | 500 | N/A | N/A | NR |
| Wattanathorn, 200826 | Single | Capsule | Yes (Yes) | Aerial | Extract (water) | Standardized using TPC, AS, AA | 250 500 750 | TPC = 7.48, AS = 0.27, AA = 12.22 TPC = 14.19, AS = 0.55, AA = 24.45 TPC = 22.43, AS = 0.82, AA = 36.67 | 22.49 25.00 37.49 | Yes (P) |
| Carlson, 200733 | Combination (G. biloba a) | Softgel capsule | Yes (No) | NR | NR | NR | 204 | N/A | N/A | Yes (M) |
| Harris, 201127 | Combination (Vitaminsa) | Tablet | No (No) | NR | Extract (NR) | NR | NR | N/A | N/A | Yes (M) |
| Katz, 201130 | Combination (Mix herba) | Mixture | Yes (Yes) | NR | Extract (NR) | Standardized By Thin layer chromatography | NR | N/A | N/A | Yes (P) |
| Lewis, 201434 | Combination (G. biloba a) | Capsule plus tablet | Yes (No) | Leaf | NR | NR | 40 | N/A | N/A | Yes (M) |
| Sarokte, 201329 | Combination (Mix herba) | Powder | Yes (Yes) | NR | Powder | NR | 1,000 | N/A | N/A | NR |
| Udani, 201331 | Combination (Mix herba) | Capsule | Yes (No) | NR | Extract (NR) | NR | 100 | N/A | N/A | Yes (M) |
| Author | RCTs design | Participants | Inclusion age | Group | No. Participant | M:F | Mean age | Intake Duration | Interval Assessed | |
| Bradwejn, 200032 | DB, parallel | Healthy | 18–45 | C. asiatica 12 g single oral Placebo | 20 20 | 21:19 | NR | single oral | 0, 30, 60, 90 min | |
| Dev, 200925 | DB, parallel | Healthy | 35–50 | C. asiatica 3–4 g OD (50 mg/Kg) male C. asiatica 3–4 g OD (50 mg/Kg) female Placebo male Placebo female | 10 11 9 10 | 10:0 0:11 9:0 0:10 | 43.3 ± 3.6 44.2 ± 5.9 40.1 ± 4.6 44.2 ± 4.8 | 60 days | 0, 40, 60, 90 days | |
| Mato, 201128 | DB, parallel | Healthy | 55–80 | C. asiatica extract 250 mg OD C. asiatica extract 500 mg OD C. asiatica extract 750 mg OD Placebo | 20 20 20 20 | 1:19 1:19 1:19 1:19 | 64.6 ± 4.5 64.2 ± 5.1 66.8 ± 4.7 65.7 ± 4.8 | 90 days | 0, 30, 60, 90, 120 days | |
| Rao, 197735 | DB, parallel | Mentally retarded children | 7–18 | C. asiatica 500 mg OD Placebo | 15 12 | 23:7 | 13.3 | 180 days | 0, 90, 180 days | |
| Wattanathorn, 200826 | DB, parallel | Healthy | Elderly | C. asiatica extract 250 mg OD C. asiatica extract 500 mg OD C. asiatica extract 750 mg OD Placebo | 7 7 7 7 | 1:6 1:6 1:6 1:6 | 67.3 ± 1.4 62.0 ± 4.3 64.8 ± 2.7 65.9 ± 5.1 | 60 days | 0, 60 min, 30, 60 days | |
| Carlson, 200733 | DB, parallel | Healthy | 65–85 | Ginkgo biloba containing supplement (C. asiatica 68 mg/day)* Placebo | 42 36 | 21:21 21:15 | 73.1 ± 4.8 72.1 ± 6.0 | 120 days | 0, 120 days | |
| Harris, 201127 | DB, parallel | Healthy man | 50–69 | Multivitamin + mineral + herb (C. asiatica 10–200 mg/day)* Placebo | 25 25 | 25:0 25:0 | 62.1 ± 3.8 62.9 ± 7.0 | 56 day | 0, 56 days | |
| Katz, 201130 | DB, parallel | ADHD children | 6–12 | Compound herbal preparation (C. asiatica extract included)* Placebo | 73 19 | 55:18 15:4 | 9.8 ± 1.6 9.4 ± 2.0 | 120 days | 0, 120 days | |
| Lewis, 201434 | DB, parallel | Healthy | 60+ | Ginkgo Synergy® 2 cap* + Choline 4 tab (C. asiatica included) OPC Synergy®2 cap* + Catalyn® 4 tab* Placebo | 33 31 33 | 8:24 7:24 12:21 | 67.6 ± 6.3 68.5 ± 6.7 70.3 ± 8.3 | 90 day | 0, 90, 180 days | |
| Sarokte, 201329 | Open label, parallel | Healthy | 10–16 | MedhyaRasaya 4 g/day (C. asiatica 1 g/day) with milk Yogic practices Control (no intervention) | 30 30 30 | 13:17 18:12 15:15 | NR NR NR | 90 day | 0, 90 day | |
| Udani, 201331 | DB, crossover | Healthy | 35–65 | SuperUlam* (C. asiatica extract 100 mg) single oral Placebo | 20 | 10:10 | 47.7 | single oral | 0, 1, 2, 3, 4, 5 hours | |
NR = Not report, N/A = Not applicable, a = major component (as mentioned in article). UA = Ursolic acid, AS = Asiaticoside, AA = Asiatic acid, TPC = Total phenolic content. #PT = Pentacyclic triterpenes are consist of asiaticoside, asiatic acid and ursolic acid. DB = double blind, OD = once daily, ADHD = Attention deficit hyperactive disorder, *Commercial product, RCTs = Randomized controlled trials. NR = not report, M:F = Male:Female.
Quality of included studies
Three of the studies (27%)29–31 had a high risk of bias, seven studies (64%) were unclear25, 27, 28, 32–35, and one study (9%) had a low risk of bias26. Although, all studies stated that they were randomized controlled trials, four of the trials (36%) were found to have unclear risk of bias for “sequence generation” because there was no description of the sequence generation methods. Most studies (72.7%) did not describe the “allocation concealment” method. In the bias domain of “blinding”, one study was an open-label study which was categorized as having a high risk of bias. All double-blind studies included had low risk. Furthermore, incomplete outcome data, selective outcome reporting, other sources of bias risk, and JADAD scores for each study are presented in Table 2.
Table 2.
Methodological quality assessment of the included studies.
| Author | Risk of bias domain | JADAD Score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective outcome reporting | Other sources of bias | Overall risk of bias | |||
| Investigator | Participants | ||||||||
| C. asiatica alone | |||||||||
| Bradwejn, 200032 | U | U | L | L | L | L | L | U | 3 |
| Dev, 200925 | L | U | L | L | L | L | L | U | 5 |
| Mato, 201128 | L | U | L | L | L | L | L | U | 5 |
| Rao, 197735 | U | U | L | L | L | L | L | U | 4 |
| Wattanathorn, 200826 | L | L | L | L | L | L | L | L | 5 |
| Combination product contained with C. asiatica | |||||||||
| Carlson, 200733 | U | U | L | L | L | L | U | U | 3 |
| Harris, 201127 | U | U | L | L | L | L | L | U | 4 |
| Katz, 201030 | L | L | L | L | U | L | H | H | 4 |
| Lewis, 201434 | L | U | L | L | L | L | U | U | 5 |
| Sarokte, 201329 | L | U | H | H | L | L | L | H | 1 |
| Udani, 201331 | L | L | L | L | L | L | H | H | 5 |
L = Low risk, U = Unclear, H = High risk.
Effects of C. asiatica in cognitive function
Of the included studies, 60 cognitive function tests were described, but only 27 of the tests had sufficient data for a meta-analysis. The 27 cognitive function tests were each categorized into specific cognitive domains for the purpose of evaluating the cognitive improvement effect of C. asiatica 1. The domains included 1) overall cognitive status, 2) attention and concentration, 3) executive function, 4) working memory, 5) information processing speed, 6) language, 7) verbal memory, 8) visuospatial skill, and 9) visual memory (Table 3). The meta-analysis indicated no significant difference between C. asiatica and comparators (placebo) on any cognitive function domain [Overall cognitive status SMD: 0.49 (95%CI; −0.49 to 1.48), I 2 = 87.9%: Attention and concentration (score) SMD: 0.37 (95%CI; −0.48 to 1.22), I 2 = 77.0%: Attention and concentration (time) SMD: 0.01 (95%CI; −0.66 to 0.68), I 2 = 0.0%: Exclusive function (score) SMD: 0.17 (95%CI; −0.19 to 0.53), I 2 = 0.0%: Information processing (score) SMD: 0.51 (95%CI; −0.41 to 1.44), I 2 = 77.7%: Information processing (time) SMD: −0.23 (95%CI; −1.02 to 0.56), I 2 = 24.2%: Language SMD: 0.28 (95%CI; −0.62 to 1.17), I 2 = 83.0%: Visuospatial skill SMD: 0.61 (95%CI; −0.18 to 0.61), I 2 = 0.0%: Working memory (score) SMD: 0.61 (95%CI; −0.25 to 1.48), I 2 = 76.9%: Working memory (time) SMD: 0.61 (95%CI; −0.59 to 1.80), I 2 = 69.0%: Verbal memory SMD: 0.14 (95%CI; −0.43 to 0.71), I 2 = 61.6% and Visual memory SMD: 0.15 (95%CI; −0.28 to 0.58), I 2 = 22.1%]. All results are presented in Table 4. However, the findings in some trials indicated that C. asiatica alone may improve working memory. Significant positive effects were found on numeric working memory tests (Appendix D) (i.e., decreased working time) after patients received 750 mg (37.49 mg of pentacyclic triterpenes) of C. asiatica water extract for 1 hour [MD: 218.36 (95%CI; 39.73 to 397.0)]26. Moreover, the combination products also revealed possible effects on some cognitive function tests (Appendix D) associated with attention and concentration (overall attention test in attention deficit hyperactive disorder children) [MD: 16.8 (95%CI; 9.82 to 23.78)]30, executive function (trail making test B in healthy elderly participant) [MD: −16.92 (95%CI; −27.14 to −6.70)]33 and information processing speed (variability test in attention deficit hyperactive disorder children [MD: 23.90 (95%CI; 12.80 to 35.00)])30.
Table 3.
Cognitive, mood, and quality of life tests included in the meta-analysis.
| Function | Domain | Domain type | Test | Included studies |
|---|---|---|---|---|
| Cognitive | Overall cognitive status | Over all | Mini mental status examination (MMSE) Mini mental status examination (MMSE) | Carlson et al., 2007; Sarokte et al., 2013 |
| Intelligence quotient (IQ) | Rao et al., 1977 | |||
| Over all cognitive function | Udani, 2013 | |||
| Attention and concentration | Accuracy/Score | Digit vigilant test (accuracy) | Wattanathorn et al., 2008 | |
| Sustained attention | Udani, 2013 | |||
| Broad attention | Dev et al., 2009 | |||
| Over all attention | Katz et al., 2010 | |||
| Time | Digit vigilant test (time) | Wattanathorn et al., 2008 | ||
| React time | Udani, 2013 | |||
| Executive function | Accuracy/Score | Symbol digit modalities | Carlson et al., 2007 | |
| Executive process | Dev et al., 2009 | |||
| Time | Trail Making Test B (TMT-B) | Lewis et al., 2014 | ||
| Cognitive flexibility | Udani, 2013 | |||
| Information processing speed | Accuracy/Score | Processing speed | Dev et al., 2009 | |
| Variability | Katz et al., 2010 | |||
| Time | Choice reaction time | Wattanathorn et al., 2008 | ||
| Processing speed | Udani, 2013 | |||
| Language | Over all | Controlled Oral Word Association test | Carlson et al., 2007; Lewis et al., 2014 | |
| Visuospatial skill | Over all | Spatial memory (accuracy) | Wattanathorn et al., 2008 | |
| Judgment of line orientation | Carlson et al., 2007 | |||
| Visual spatial thinking | Dev et al., 2009 | |||
| Working memory | Accuracy/Score | Numeric working memory (accuracy) | Wattanathorn et al., 2008 | |
| Working memory | Dev et al., 2009 | |||
| Short term memory picture | Sarokte et al., 2013 | |||
| Time | Working memory | Udani, 2013 | ||
| Numeric working memory (time) | Wattanathorn et al., 2008 | |||
| Verbal memory | Over all | Word recognition (accuracy) | Wattanathorn et al., 2008 | |
| Serial recall effect test - words | Sarokte et al., 2013 | |||
| List Learning | Carlson et al., 2007 | |||
| Visual memory | Over all | Picture recognition (accuracy) | Wattanathorn et al., 2008 | |
| Benton Visual retention | Carlson et al., 2007 | |||
| Delayed recall | Dev, 2009 | |||
| Mood | Mood | Over all | Profile of mood status (POMS) Profile of mood status (POMS) Mood rating | Udani, 2013; Harris et al., 2011; Bradwejn et al., 2000 |
| Mood scale | Over all | Bond-Lader mood scale Visual analog mood scale (VAMS) | Wattanathorn et al., 2008; Harris et al., 2011 | |
| Quality of life (QoL) | Total QoL | Over all | SF-36 | Carlson et al., 2007 |
| General health questionnair | Harris et al., 2011 | |||
| Physical | Over all | SF-36 (physical function) | Mato et al., 2011 | |
| Total physical | Udani, 2013 |
The same domain was pooled together for meta-analysis.
Table 4.
Result of primary and secondary outcomes.
| Domain | Inc. trial | N | Standardized mean difference [95% CI] | p-value | Heterogeneity (%I 2) | Pooled studies |
|---|---|---|---|---|---|---|
| Primary outcomes | ||||||
| Over all cognitive status | ||||||
| Outcomes at the end of study (All) | 3 | 153 | 0.49 [−0.49, 1.48] | 0.327 | 87.9 | Rao et al., 1977; Carlson et al., 2007; Sarokte et al., 2013 |
| Outcomes at the end of study (DB only) | 2 | 93 | −0.01 [−0.52, 0.51] | 0.976 | 29.0 | Rao et al., 1977; Carlson et al., 2007 |
| Outcomes at the end of study (Combination only) | 2 | 126 | 0.56 [−0.95, 2.08] | 0.465 | 93.9 | Carlson et al., 2007; Sarokte et al., 2013 |
| Outcomes at the end of study (C. asiatica only)# | 1 | 27 | 4.30 [−5.42, 14.02] | 0.386 | — | Rao et al., 1977 |
| 5 hr after ingestion (Combination only)# | 1 | 20 | −0.11 [−6.61, 4.51] | 0.711 | — | Udani, 2013 |
| Attention and concentration | ||||||
| Attention (Score) | ||||||
| Outcomes at the end of study | 3 | 146 | 0.37 [−0.48, 1.22] | 0.395 | 77.0 | Wattanathorn et al., 2008; Dev et al., 2009; Katz et al., 2010 |
| 1 month ingestion (C. asiatica only) | 2 | 54 | 0.05 [−0.49, 0.58] | 0.862 | 0.00 | Wattanathorn et al., 2008; Dev et al., 2009 |
| 2 month ingestion (C. asiatica only) | 2 | 54 | −0.01 [−0.55, 0.52] | 0.962 | 0.00 | Wattanathorn et al., 2008; Dev et al., 2009 |
| Outcomes at the end of study (Combinantion only)*,# | 1 | 92 | 16.8 [9.82, 23.78] | 0.000 | — | Katz et al., 2010 |
| 1 hr after ingestion | 2 | 34 | −0.13 [−0.81, 0.54] | 0.698 | 0.00 | Wattanathorn et al., 2008; Udani, 2013 |
| 1 hr after ingestion (C. asiatica only)# | 1 | 14 | −4.76 [−34.90, 25.40] | 0.757 | — | Wattanathorn et al., 2008 |
| 1 hr after ingestion (Combination only)# | 1 | 20 | −1.25 [−11.12, 8.62] | 0.804 | — | Udani, 2013 |
| Attention (time) | ||||||
| 1 hr after ingestion | 2 | 34 | 0.01 [−0.66, 0.68] | 0.977 | 0.00 | Wattanathorn et al., 2008; Udani, 2013 |
| 1 hr after ingestion (C. asiatica only)# | 1 | 14 | 6.88 [−38.74, 52.50] | 0.758 | — | Wattanathorn et al., 2008 |
| 1 hr after ingestion (Combination only)# | 1 | 20 | −0.90 [−9.34, 7.54] | 0.834 | — | Udani, 2013 |
| Executive function | ||||||
| Executive function (Score) | ||||||
| Outcomes at the end of study | 2 | 118 | 0.17 [−0.19, 0.53] | 0.357 | 0.00 | Carlson et al., 2007; Dev et al., 2009 |
| Outcomes at the end of study (C. asiatica only)# | 1 | 40 | 14.43 [−8.63, 37.49] | 0.220 | — | Dev et al., 2009 |
| Outcomes at the end of study (Combination only)# | 1 | 78 | 0.70 [−3.03, 4.43] | 0.713 | — | Carlson et al., 2007 |
| Executive function (Time) | ||||||
| 5 hr after ingestion (Combination only)# | 1 | 20 | −3.25 [−10.53, 4.03] | 0.381 | — | Udani, 2013 |
| Outcomes at the end of study (Combination only)*,# | 1 | 48 | −16.92 [−27.14, −6.70] | 0.001 | — | Lewis et al., 2014 |
| Information processing speed | ||||||
| Information processing (Score) | ||||||
| Outcomes at the end of study | 2 | 132 | 0.51 [−0.41, 1.44] | 0.277 | 77.7 | Dev et al., 2009; Katz et al., 2010 |
| Outcomes at the end of study (C. asiatica only)# | 1 | 40 | 0.49 [−7.63, 8.61] | 0.906 | — | Dev et al., 2009 |
| Outcomes at the end of study (Combination only)*,# | 1 | 92 | 23.90 [12.80, 35.00] | 0.000 | — | Katz et al., 2010 |
| Information processing (Time) | ||||||
| 1 hr after ingestion | 2 | 34 | −0.23 [−1.02, 0.56] | 0.572 | 24.2 | Wattanathorn et al., 2008; Udani, 2013 |
| 1 hr after ingestion (C. asiatica only)# | 1 | 14 | 36.97 [−134.2, 208.1] | 0.672 | — | Wattanathorn et al., 2008 |
| 1 hr after ingestion (Combination only)# | 1 | 20 | −6.25 [−15.63, 3.13] | 0.192 | — | Udani, 2013 |
| Language | ||||||
| Outcomes at the end of study (Combination only)# | 2 | 126 | 0.28 [−0.62, 1.17] | 0.545 | 83.0 | Carlson et al., 2007; Lewis et al., 2014 |
| Visuospatial skill | ||||||
| Outcomes at the end of study | 3 | 132 | 0.61 [−0.18, 0.61] | 0.347 | 0.00 | Carlson et al., 2007; Wattanathorn et al., 2008; Dev et al., 2009 |
| Outcomes at the end of study (Healthy, elderly) | 2 | 92 | 0.14 [−0.27, 0.55] | 0.514 | 0.00 | Carlson et al., 2007; Wattanathorn et al., 2008 |
| Outcomes at the end of study (C. asiatica only) | 2 | 54 | 0.30 [−0.24, 0.84] | 0.279 | 0.00 | Wattanathorn et al., 2008; Dev et al., 2009 |
| Working memory | ||||||
| Working memory (Score) | ||||||
| Outcomes at the end of study | 3 | 114 | 0.61 [−0.25, 1.48] | 0.167 | 76.9 | Wattanathorn et al., 2008; Dev et al., 2009; Sarokte et al., 2013 |
| Outcomes at the end of study (C. asiatica only) | 2 | 54 | 0.19 [−0.35, 0.72] | 0.488 | 0.0 | Wattanathorn et al., 2008; Dev et al., 2009 |
| Working memory (time) | ||||||
| 1 hr after ingestion | 2 | 34 | 0.61 [−0.59, 1.80] | 0.319 | 69.0 | Wattanathorn et al., 2008; Udani, 2013 |
| 1 hr after ingestion (C. asiatica only)* | 1 | 14 | 218.36 [39.73, 397.0] | 0.017 | — | Wattanathorn et al., 2008 |
| 1 hr after ingestion (Combination only) | 1 | 20 | 0.60 [−8.51, 9.71] | 0.897 | — | Udani, 2013 |
| Verbal memory | ||||||
| Outcomes at the end of study | 3 | 152 | 0.14 [−0.43, 0.71] | 0.635 | 61.6 | Carlson et al., 2007; Wattanathorn et al., 2008; Sarokte et al., 2013 |
| Outcomes at the end of study (Healthy, eldery) | 2 | 92 | −0.15 [−0.56, 0.26] | 0.473 | 0.00 | Carlson et al., 2007; Wattanathorn 2008 |
| Outcomes at the end of study (Combination only) | 2 | 138 | 0.23 [−0.51, 0.97] | 0.543 | 78.8 | Carlson et al., 2007; Sarokte et al., 2013 |
| Outcomes at the end of study (C. asiatica only) | 1 | 14 | −2.07 [12.26, 8.12] | 0.691 | — | Wattanathorn et al., 2008 |
| Visual memory | ||||||
| Outcomes at the end of study | 3 | 132 | 0.15 [−0.28, 0.58] | 0.487 | 22.1 | Carlson et al., 2007; Dev et al., 2009; Wattanathorn et al., 2008 |
| Outcomes at the end of study (C. asiatica only) | 2 | 54 | 0.37 [−0.24, 0.98] | 0.235 | 18.8 | Wattanathorn et al., 2008; Dev et al., 2009 |
| Secondary outcomes | ||||||
| Mood (self-report from participants) | ||||||
| Bond-Lader mood scale/VAMS | ||||||
| Outcomes at the end of study (Alert)* | 2 | 64 | 0.71 [0.01, 1.41] | 0.046 | 30.5 | Wattanathorn et al., 2008; Harris et al., 2011 |
| Outcomes at the end of study (Alert) (C. asiatica only)*,# | 1 | 14 | 9.38 [1.71, 17.05] | 0.017 | — | Wattanathorn et al., 2008 |
| Outcomes at the end of study (Alert) (Combination only)# | 1 | 50 | 7.20 [−0.98, 15.38] | 0.085 | — | Harris et al., 2011 |
| Outcomes at the end of study (Content) | 2 | 64 | 0.30 [−0.19, 0.80] | 0.227 | 0.00 | Wattanathorn et al. 2008; Harris et al., 2011 |
| Outcomes at the end of study (Content) (C. asiatica only)# | 1 | 14 | 2.38 [−2.77, 7.53] | 0.365 | — | Wattanathorn et al., 2008 |
| Outcomes at the end of study (Content) (Combination only)# | 1 | 50 | 3.90 [−4.57, 12.37] | 0.367 | — | Harris et al., 2011 |
| Outcomes at the end of study (Clam) | 2 | 64 | 0.60 [−0.30, 1.50] | 0.194 | 53.5 | Wattanathorn et al., 2008; Harris et al., 2011 |
| Outcomes at the end of study (Clam)* (C. asiatica only)# | 1 | 14 | 2.37 [0.33, 4.41] | 0.023 | — | Wattanathorn 2008 |
| Outcomes at the end of study (Clam) (Combination only)# | 1 | 50 | 3.60 [−4.19, 11.39] | 0.365 | — | Harris et al., 2011 |
| POMS and mood rating (self-report from participants) | ||||||
| Tension | ||||||
| 1 hr after ingestion | 2 | 59 | −0.05 [−0.56, 0.46] | 0.846 | 0.00 | Bradwejn et al., 2000; Udani, 2013 |
| 2 hr after ingestion | 2 | 59 | 0.30 [−0.99, 1.58] | 0.651 | 80.8 | Bradwejn et al., 2000; Udani, 2013 |
| Outcomes at the end of study (Combination only)# | 1 | 50 | −1.70 [−4.62, 1.22] | 0.253 | — | Harris et al., 2011 |
| Depression | ||||||
| 1 hr after ingestion | 2 | 59 | 0.09 [−1.53, 1.71] | 0.916 | 87.8 | Bradwejn et al., 2000; Udani, 2013 |
| 2 hr after ingestion | 2 | 59 | 0.33 [−1.42, 2.08] | 0.710 | 89.0 | Bradwejn et al., 2000; Udani, 2013 |
| Outcomes at the end of study (Combination only)# | 1 | 50 | −1.00 [−5.05, 3.05] | 0.628 | — | Harris et al., 2011 |
| Angor | ||||||
| 1 hr after ingestion* | 2 | 59 | −0.81 [−1.51, −0.09] | 0.026 | 36.6 | Bradwejn et al., 2000; Udani, 2013 |
| 2 hr after ingestion | 2 | 59 | 0.27 [−0.35, 0.89] | 0.386 | 26.4 | Bradwejn et al., 2000; Udani, 2013 |
| Outcomes at the end of study (Combination only)# | 1 | 50 | −2.90 [−7.29, 1.49] | 0.196 | — | Harris et al., 2011 |
| Vigor | ||||||
| 1 hr after ingestion | 2 | 59 | −0.25 [−1.68, 1.19] | 0.737 | 85.0 | Bradwejn et al., 2000; Udani, 2013 |
| 2 hr after ingestion | 2 | 59 | −0.16 [−1.10, 0.78] | 0.735 | 66.5 | Bradwejn et al., 2000; Udani, 2013 |
| Outcomes at the end of study (Combination only)# | 1 | 50 | 0.70 [−2.88, 4.28] | 0.701 | — | Harris et al., 2011 |
| Fatigue | ||||||
| 1 hr after ingestion | 2 | 59 | 0.39 [−0.42, 1.20] | 0.345 | 54.1 | Bradwejn et al., 2000; Udani, 2013 |
| 2 hr after ingestion | 2 | 59 | 0.26 [−0.53, 1.05] | 0.640 | 52.6 | Bradwejn et al., 2000; Udani, 2013 |
| Outcomes at the end of study (Combination only)# | 1 | 50 | −1.30 [−4.06, 1.46] | 0.355 | — | Harris et al., 2011 |
| Confusion | ||||||
| 1 hr after ingestion | 2 | 59 | −0.48 [−1.65, 0.70] | 0.427 | 76.6 | Bradwejn et al., 2000; Udani, 2013 |
| 2 hr after ingestion | 2 | 59 | 0.11 [−0.40, 0.62] | 0.675 | 0.00 | Bradwejn et al., 2000; Udani, 2013 |
| Outcomes at the end of study (Combination only)# | 1 | 50 | −0.90 [−3.26, 1.46] | 0.454 | — | Harris et al., 2011 |
| Quality of life | ||||||
| Outcomes at the end of study (Physical) | 2 | 60 | 0.21 [−0.30, 0.72] | 0.417 | 0.00 | Mato et al., 2011; Harris et al., 2011 |
| Outcomes at the end of study (Total) | 2 | 128 | 0.04 [−0.87, 0.95] | 0.931 | 84.4 | Carlson et al., 2007; Harris et al., 2011 |
*Significant (p < 0.05).
#Presented as mean difference (not standardized mean difference).
Combination only = Only combination product, C. asiatica only = C. asiatica alone product.
Outcomes at the end of study = Outcomes measured at the longest following up.
*Significant (p < 0.05), CI = confident interval.
#Presented as mean difference (not standardized mean difference).
All = pooled all data, Combination only = Only combination product, C. asiatica only = C. asiatica alone product, DB = Double blind, Score = Score unit, Time = Time unit, Healthy = Healthy volunteer, Elderly = elderly volunteer.
Outcomes at the end of study = Outcomes measured at the longest following up time.
For secondary outcomes, C. asiatica could increase self-reported alert scores [SMD: 0.71 (95%CI; 0.01 to 1.41), I 2 = 30.5%]. Furthermore, ingestion of C. asiatica water extract (750 mg/day) for 2 months showed an increase in self-reported alertness [MD: 9.38 (95%CI; 1.71 to 17.05)] and self-reported calmness [MD: 2.37 (95%CI; 0.33 to 4.41)]. C. asiatica also decreased self-reported anger scores at 1 hour after treatment [SMD: −0.81 (95%CI; −1.51 to −0.09), I 2 = 36.6%]. However, no other significant differences for mood or quality of life could be identified. Other findings of all outcomes are presented in Table 4.
Adverse effects
Adverse effects or toxicity associated with C. asiatica were also evaluated based on the included articles. No adverse effects were reported in any studies looking at C. asiatica alone. However, for studies of combination products, four studies reported mild adverse events of C. asiatica-containing products. Two studies reported adverse event rates comparable to the placebo rate31, 34, while another two studies reported lower rates of adverse event for C. asiatica-containing products30, 33. Common adverse events were gastrointestinal discomfort, flatulence, nausea, headache, decreased appetite, sedation, and rash. Hepatotoxicity, which has been reported in one previous case report36, was not observed in any of the included RCTs.
Discussion
This systematic review and meta-analysis provides a comprehensive summary of the effects of C. asiatica on cognitive function.
Current evidence does not support the effects of C. asiatica alone on overall cognitive function. However, ingestion of C. asiatica water extract (750 mg/day) for 1 hour may improve working memory, as shown in the positive effect on the numeric working memory test26 by a decrease in working time. This finding does not agree with a recent quasi-experimental study which found a statistically significant improvement in the memory domain of patients who had vascular cognitive impairment treated with C. asiatica extract (1,000 mg/day) when compared to patients treated with 3 mg/day of folic acid37. In that study, however, the dose of C. asiatica was higher than nine of the eleven trials included in this meta-analysis. Thus, the non-significant differences in overall cognitive function between C. asiatica and its comparators observed in this review might be due to the dosages used in the included studies. In traditional use and experimental evidence38, at least 3 grams of C. asiatica needs to be used to improve cognitive function. However, only two included studies29, 32 used doses greater than 3 g of C. asiatica per day, while the rest used lower doses.
The combination of C. asiatica with other herbs also showed non-significant improvements in overall cognitive function. However, the combination products in other studies have revealed that there arepossible effects on attention and concentration30, executive function34 and information processing speed30. The improvement in cognitive function from the combination products might be due to the synergistic effects of C. asiatica with other herbs or the effects of other herbs in C. asiatica-containing products such as G. biloba 31, 34. G. biloba is a well-known herbal medicine used for cognitive impairment. From previous systematic reviews and meta-analyses, G. biloba exhibited potential benefits for cognitive improvement in mild cognitive impairment or Alzheimer’s patients2, 5. Moreover, beneficial effects on cognitive function of Withania somnifera (L.) Dunal, Spirulina (Arthrospira platensis) and paeoniflorin (monoterpene glucoside) have been exhibited in different pre-clinical models30. None of the studies reported details on which parts of C. asiatica were used in the combination or how the combinations were prepared. Thus, thefindings could not show the direct effect of C. asiatica on cognitive function, and there is currently a lack of persuasive evidence to confirm a cognitive enhancing effect of C. asiatica.
For secondary outcomes, C. asiatica consumption was associated with improvements in self-reported alertness (after 2 months of ingestion) and with reductions in self-reported anger (after 1 hour of ingestion). Moreover, C. asiatica alone (750 mg/day for 2 months) induced alertness and calmness. These improvements in alertness and calmness may facilitate cognitive function by improving working memory, attention and concentration, executive function and information processing speed, and memory capacity and by reducing the time to solve problems. These results also support the traditional use of C. asiatica as a brain tonic. However, the positive effects may be caused by the other herbs in the combination products, so firm conclusions on the efficacy of C. asiatica cannot be drawn. There were also no significant differences between C. asiatica and placebo for physical or total QoL scores. From the safety data, C. asiatica seems to be safe since there were no serious adverse events reported in any of the included articles.
This meta-analysis included both C. asiatica alone and C. asiatica combined with other herbs. There were differences among the included studies such as differences in the part of C. asiatica used, dosage forms, extraction procedures, preparation, and outcome measurements. However, based on the objectives, all cognitive function data were collected from the RCTs that used any type of C. asiatica. The authors believe that the analysis is valid to address the objectives. Using the standardized mean difference (SMD), allowed the effect of C. asiatica on cognitive function to be assessed across the various types of cognitive function measurements used in the included studies. SMD converts data from different scales to a common scale. However, the standardization causes the original information for each measurement to be lost, so the findings cannot be interpreted in common units. They can only provide the level of significance of the effect of C. asiatica compared to the comparators39.
A classification defined by previous studies was used to determine the domains of cognitive function and pool the findings together1, 37. This classification has been used in several studies37, 40–42 to classify the domains of cognitive function and pool their findings. Thus, it is believed that the approach is appropriate for this meta-analysis. As no validation study of the classification was conducted, future studies may look into this issue.
This review identified limitations in the reporting of clinical studies of C. asiatica. Most of the included studies did not report details on the parts of the C. asiatica plant used in the products, the standardization methods, the active marker contents, or the methods for preparing the products. Only three of eleven (27%) trials26, 28, 30 reported standardization methods of the plant extract, and only two trials (18%) reported the amount of the active compounds (asiaticoside and asiatic acid) contained in the C. asiatica extract26, 28. Moreover, none of the studies reported qualitative analyses (such as HPLC fingerprints) of the C. asiatica in their studies. Clinical trials of herbal medicine should use standardized products as interventions and should report the detail of each intervention according to the CONSORT statement for reporting herbal medicinal interventions43. Furthermore, the place, conditions, and season of cultivation as well as the parts of the plant used can affect the pentacyclic triterpene (asiaticoside, asiatic acid, madecassoside, madecassic acid) contents of the C. asiatica raw material44. Lack of herbal standardization in clinical trials may affect the quality of studies and explain the variations in the clinical effects across studies. Interpretation of the findings of this systematic review should be done with cautions due to the lack of information about standardization.
Another consideration is that the doses of C. asiatica in each study were different, ranging from 40–12,000 mg/day. Variations in C. asiatica preparation were also observed. For C. asiatica alone, three of five trials used dry C. asiatica powder ranging from 500–12,000 mg/day while two trials used C. asiatica water extract ranging from 250–750 mg/day. Furthermore, the doses used in most of the included studies were lower than the traditional dose recommendation for cognitive improvement (3 g/day of C. asiatica powder)38. In the combination products, the dose of C. asiatica was very low (40–204 mg/day) compared with the main active component except in one study that used C. asiatica 1,000 mg/day29. Additionally, the dose and preparation of C. asiatica in some combination products was not clear. These limitations may affect the pooled data of C. asiatica in each cognitive domain. Moreover, the observed findings did not support a direct effect of C. asiatica containing products on cognitive function. There is currently a lack of persuasive evidence to confirm a cognitive enhancing effect of C. asiatica.
Based on this review, future well-designed clinical trials are warranted to evaluate the effects of C. asiatica products on cognitive function and mood as well as its safety. Standardized doses of C. asiatica products should be investigated over short-term and long-term periods of ingestion for effects in each specific cognitive domain, especially working memory, attention and concentration, executive function, and information processing speed.
In conclusion, the findings revealed that there is no strong evidence to support the effect of C. asiatica on overall cognitive function improvement. However, C. asiatica may improve working memory. A combination of C. asiatica with other herbs may improve attention and concentration, executive function, and information processing speed. C. asiatica may also improve mood disorders in terms of self-reported alertness and reductions in self-reported anger. Issues with dosage and preparation standardization need to be considered when these findings are applied. Future well-designed clinical trials are needed to assess the effects of standardized C. asiatica on cognitive function and mood as well as safety.
Methods
This systematic review was conducted according to the Cochrane Collaboration framework guidelines39 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement45. The review protocol was registered with PROSPERO (registration number: CRD42015023595).
Search strategies and study selection
An electronic search was conducted for original articles from inception to September 2016 using a number of electronic databases including AMED, CINAHL, Cochrane Central Register of clinical trial, EMBASE, PubMed, Psycinfo, Science direct, Scopus, www.clinicaltrials.gov, ThaiLis, Thai Index Medicus, Thai Medical Index, and Thai Thesis Database. Strategic search terms were C. asiatica name, OR active compound from C. asiatica (such as asiaticoside, madecassoside, asiatic acid, madecassic acid), OR C. asiatica containing products combined with cognitive function or memory and its related properties including mood and quality of life. Details of the search strategies are described in appendix A. Eligibility criteria were 1) published and unpublished randomized controlled trials in patients or healthy volunteers and 2) reported effects of C. asiatica or a combination of C. asiatica with other herbs in humans. No language restriction was applied. To ensure that the search would be thorough, reference lists were reviewed to identify potential studies not indexed in above mentioned databases. Furthermore, corresponding authors of identified studies were consulted for additional studies as sources. Titles and abstracts were screened according to the eligibility criteria. Full-text articles of the potential studies were retrieved from database or corresponding authors and were subsequently assessed independently by two researchers (PP, PD) for inclusion in the meta-analysis. Disagreements between the independent researchers were settled by discussion and consensus with a third independent researcher (NC).
Data extraction and quality assessment
Data extraction was undertaken using a standard data extraction form. Extracted data included study design, characteristics of participants, characteristics of intervention and comparator, duration of herbal use, follow-up time, cognitive function tests, and cognitive function outcomes. Data for cognitive function tests included the name of the cognitive function test, the cognitive function domain, the outcome measures, and the outcome scale. For this meta-analysis, each cognitive test was categorized into one specific domain of cognitive function following a previous study1. This approach avoids over-weighting effects and provides consistency for the evaluation of the effect of C. asiatica on cognition across studies (Appendix B, C). A primary outcome of interest was the clinical effect of C. asiatica on cognitive function in each domain (Appendix B) including attention and concentration, executive function, information processing speed, language, visuospatial skill, working memory, verbal memory, and visual memory as well as overall cognitive status. In addition, secondary outcomes were mood, quality of life, and adverse events reported across each intervention. Where relevant data were unavailable, it was sought directly from the corresponding authors.
The quality of included studies was assessed using the Cochrane risk of bias tool39 and JADAD score46. Sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias were evaluated. Data search, data extraction, and quality assessment were performed by PP and PD. Disagreements between the reviewers were settled through discussion and consensus.
Statistical analysis
To determine the cognitive effect of C. asiatica, data for individual cognitive function tests were compared between C. asiatica and its comparator using standardized mean difference (SMD) or mean difference (MD) with a 95% confidence interval (CI). Heterogeneity was assessed by the I 2-statistic47. Thresholds of I 2 were interpreted in accordance with the magnitude and direction of effects and strength of evidence of heterogeneity. I 2 values of more than 50% indicated substantial heterogeneity. Data from included studies were pooled using the Der Simonian and Laird random-effects model48. The software used for data analysis was STATA version 12 (STATA Corp, College Station, TX, USA).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
The authors thank Miss Nutchakarn Vorachai and Mr Siriwat Na Ranong for providing support in the systematic review process. We also would like to thank Professor Dr. Daniel D Reidpath from Monash University Malaysia and Alicia Ritscher from University of Wisconsin-Madison, the United States of America for English editing. This systematic review received financial support from the Thai Traditional Medical Knowledge Fund.
Author Contributions
P.P. participated in the study concept and design, data acquisition, data analysis, data interpretation, manuscript drafting, critical revision of the manuscript, and the final review of the manuscript. P.D. participated in the study concept and design, data acquisition, data analysis, data interpretation, manuscript drafting, critical revision of the manuscript, and the final review of the manuscript. S.S. participated in data analysis and the final review of the manuscript. T.D. participated in the study concept and design critical revision of the manuscript, and the final review of the manuscript. C.K. participated in the study concept and design critical revision of the manuscript, and the final review of the manuscript. R.S. participated in the study concept and design critical revision of the manuscript, and the final review of the manuscript. A.C. participated in the study concept and design critical revision of the manuscript, and the final review of the manuscript. N.C. participated in the study concept and design, data acquisition, data analysis, data interpretation, manuscript drafting, critical revision of the manuscript, and the final review of the manuscript.
Competing Interests
AC is currently a government official under the department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, Nonthaburi, Thailand. Other authors declare no financial relationships with any organizations in the previous three years that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the submitted work exist.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Piyameth Dilokthornsakul, Email: piyamethd@nu.ac.th.
Nathorn Chaiyakunapruk, Email: nathorn.chaiyakunapruk@monash.edu.
References
- 1.Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104:2222–2233. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- 2.Brondino N, et al. Curcumin as a therapeutic agent in dementia: a mini systematic review of human studies. Scientific World Journal. 2014;2014 doi: 10.1155/2014/174282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kongkeaw C, Dilokthornsakul P, Thanarangsarit P, Limpeanchob N, Norman Scholfield C. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. J Ethnopharmacol. 2014;151:528–535. doi: 10.1016/j.jep.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 4.May BH, et al. Herbal medicine for dementia: a systematic review. Phytother Res. 2009;23:447–459. doi: 10.1002/ptr.2656. [DOI] [PubMed] [Google Scholar]
- 5.Yang G, Wang Y, Sun J, Zhang K, Liu J. Ginkgo Biloba for Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr Top Med Chem. 2016;16:520–528. doi: 10.2174/1568026615666150813143520. [DOI] [PubMed] [Google Scholar]
- 6.Brinkhaus B, Lindner M, Schuppan D, Hahn EG. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine. 2000;7:427–448. doi: 10.1016/S0944-7113(00)80065-3. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. (World Health Organization, Geneva, Switzerland, 1999).
- 8.Inamdar EK, Yeole RD, Ghogare AB, Souza NJ. Determination of biologically active constituents in Centella asiatica. J Chromatogr A. 1996;742:124–130. doi: 10.1016/0021-9673(96)00237-3. [DOI] [Google Scholar]
- 9.National Drug Committee. The national list of essential drug, A.D. 2015 [in Thai], http://www.nlem.in.th (2015).
- 10.Department for Development of Thai Traditional and Alternative Medicine. The 10th national herb exhibition, http://www.dtam.moph.go.th/index.php?option=com_content&view=article&id=344:pr0175&catid=8&Itemid=114 (2013).
- 11.Coldren CD, et al. Gene expression changes in the human fibroblast induced by Centella asiatica triterpenoids. Planta Med. 2003;69:725–732. doi: 10.1055/s-2003-42791. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya AD, Devasagayam TP. Current status of herbal drugs in India: an overview. J Clin Biochem Nutr. 2007;41:1–11. doi: 10.3164/jcbn.2007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao SB, Chetana M, Uma Devi P. Centella asiatica treatment during postnatal period enhances learning and memory in mice. Physiol Behav. 2005;86:449–457. doi: 10.1016/j.physbeh.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Veerendra Kumar MH, Gupta YK. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J Ethnopharmacol. 2002;79:253–260. doi: 10.1016/S0378-8741(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 15.Barbosa NR, Pittella F, Gattaz WF. Centella asiatica water extract inhibits iPLA2 and cPLA2 activities in rat cerebellum. Phytomedicine. 2008;15:896–900. doi: 10.1016/j.phymed.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee PK, Kumar V, Houghton PJ. Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytother Res. 2007;21:1142–1145. doi: 10.1002/ptr.2224. [DOI] [PubMed] [Google Scholar]
- 17.Soumyanath A, et al. Centella asiatica Extract Improves Behavioral Deficits in a Mouse Model of Alzheimer’s Disease: Investigation of a Possible Mechanism of Action. Int J Alzheimers Dis. 2012;2012 doi: 10.1155/2012/381974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta YK, Veerendra Kumar MH, Srivastava AK. Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol Biochem Behav. 2003;74:579–585. doi: 10.1016/S0091-3057(02)01044-4. [DOI] [PubMed] [Google Scholar]
- 19.Visweswari G, Prasad KS, Chetan PS, Lokanatha V, Rajendra W. Evaluation of the anticonvulsant effect of Centella asiatica (gotu kola) in pentylenetetrazol-induced seizures with respect to cholinergic neurotransmission. Epilepsy Behav. 2010;17:332–335. doi: 10.1016/j.yebeh.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Wijeweera P, Arnason JT, Koszycki D, Merali Z. Evaluation of anxiolytic properties of Gotukola–(Centella asiatica) extracts and asiaticoside in rat behavioral models. Phytomedicine. 2006;13:668–676. doi: 10.1016/j.phymed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Chen SW, et al. Anxiolytic-like effect of asiaticoside in mice. Pharmacol Biochem Behav. 2006;85:339–344. doi: 10.1016/j.pbb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Liang X, et al. Antidepressant-like effect of asiaticoside in mice. Pharmacol Biochem Behav. 2008;89:444–449. doi: 10.1016/j.pbb.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Xu CL, et al. Asiaticoside: attenuation of neurotoxicity induced by MPTP in a rat model of Parkinsonism via maintaining redox balance and up-regulating the ratio of Bcl-2/Bax. Pharmacol Biochem Behav. 2012;100:413–418. doi: 10.1016/j.pbb.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Nasir MN, et al. Effects of asiatic acid on passive and active avoidance task in male Spraque-Dawley rats. J Ethnopharmacol. 2011;134:203–209. doi: 10.1016/j.jep.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Dev RDO, Mohamed S, Hambali Z, Samah BA. Comparison on cognitive effects of Centella asiatica in healthy middle age female and male volunteers. Eur J Sci Res. 2009;31:553–565. [Google Scholar]
- 26.Wattanathorn J, et al. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J Ethnopharmacol. 2008;116:325–332. doi: 10.1016/j.jep.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 27.Harris E, et al. The effect of multivitamin supplementation on mood and stress in healthy older men. Hum Psychopharmacol. 2011;26:560–567. doi: 10.1002/hup.1245. [DOI] [PubMed] [Google Scholar]
- 28.Mato L, et al. Centella asiatica Improves Physical Performance and Health-Related Quality of Life in Healthy Elderly Volunteer. Evid Based Complement Alternat Med. 2011;2011 doi: 10.1093/ecam/nep177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarokte AS, Rao MV. Effects of Medhya Rasayana and Yogic practices in improvement of short-term memory among school-going children. Ayu. 2013;34:383–389. doi: 10.4103/0974-8520.127720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz M, Levine AA, Kol-Degani H, Kav-Venaki L. A compound herbal preparation (CHP) in the treatment of children with ADHD: a randomized controlled trial. J Atten Disord. 2010;14:281–291. doi: 10.1177/1087054709356388. [DOI] [PubMed] [Google Scholar]
- 31.Udani JK. Effects of SuperUlam on Supporting Concentration and Mood: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/238454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradwejn J, Zhou Y, Koszycki D, Shlik J. A double-blind, placebo-controlled study on the effects of Gotu Kola (Centella asiatica) on acoustic startle response in healthy subjects. J Clin Psychopharmacol. 2000;20:680–684. doi: 10.1097/00004714-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Carlson JJ, et al. Safety and efficacy of a ginkgo biloba-containing dietary supplement on cognitive function, quality of life, and platelet function in healthy, cognitively intact older adults. J Am Diet Assoc. 2007;107:422–432. doi: 10.1016/j.jada.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Lewis JE, et al. A double-blind, randomized clinical trial of dietary supplementation on cognitive and immune functioning in healthy older adults. BMC Complement Altern Med. 2014;14 doi: 10.1186/1472-6882-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao MVRA, Srinivasan K, Rao TK. The effect of Centella asiatica on the general mental ability of mentally retarded children. Indian J Psychiatry. 1977;77:54–59. [Google Scholar]
- 36.Jorge OA, Jorge AD. Hepatotoxicity associated with the ingestion of Centella asiatica. Rev Esp Enferm Dig. 2005;97:115–124. doi: 10.4321/S1130-01082005000200006. [DOI] [PubMed] [Google Scholar]
- 37.Farhana KM, Malueka RG, Wibowo S, Gofir A. Effectiveness of Gotu Kola Extract 750 mg and 1000 mg Compared with Folic Acid 3 mg in Improving Vascular Cognitive Impairment after Stroke. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/2795915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bone, K. & Mills, S. Principles and Practice of Phytotherapy. 657–670 (Churchill Livingstone Elsevier, 2013).
- 39.Higgins, J. P. & Green, S. Cochrane handbook for systematic reviews of interventions version 5.1.0 (The Cochrane Collaboration, 2011).
- 40.Cullen B, O’Neill B, Evans JJ, Coen RF, Lawlor BA. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78:790–799. doi: 10.1136/jnnp.2006.095414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ott BR, et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2015;30:348–358. doi: 10.1007/s11606-014-3115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teoh, S. L. et al. Chicken Essence for Cognitive Function Improvement: A Systematic Review and Meta-Analysis. Nutrients8, doi:10.3390/nu8010057 (2016). [DOI] [PMC free article] [PubMed]
- 43.Gagnier JJ, et al. Recommendations for reporting randomized controlled trials of herbal interventions: Explanation and elaboration. J Clin Epidemiol. 2006;59:1134–1149. doi: 10.1016/j.jclinepi.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 44.Puttarak P, Panichayupakaranant P. Factors affecting the content of pentacyclic triterpenes in Centella asiatica raw materials. Pharm Biol. 2012;50:1508–1512. doi: 10.3109/13880209.2012.685946. [DOI] [PubMed] [Google Scholar]
- 45.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(264–269) doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 46.Jadad AR, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 47.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.