Abstract
Mucor irregularis is an emerging fungal pathogen that cause cutaneous infection and could cause death. However, little is known about its mechanism of pathogenesis. There is evidence suggesting virulence vary with mating types in fungi, including the Mucorales. Here, we characterized the mating type locus of M. irregularis and the mating type ratio of 17 clinical isolates in China. Genomic data indicated M. irregularis is heterothallic having two mating types – bearing either SexP or SexM allele. Also, we employed a mice model to study the inflammation and pathological effects of different mating types. The comparison of the inflammatory response, cytokine profiles and Th-1, Th-2 and Th-17 cells numbers in each mating type treated mice showed that the severity and disease progress were enhanced in (+) mating type treated mice. One (+/0) mutant strain, with multiple mutations at the mating locus, had defects in sexual mating ability but appeared to be more virulent than the (−) mating type. Although (+) mating type appeared to be more virulent, most of our clinical isolates presented belonged to (−) mating type. Our findings support the involvement of MAT genes in sexual fertility, and the influence of mating type on the severity of cutaneous infection.
Introduction
Mucormycosis is an invasive fungal infection that almost invariably occurs in immunocompromised patients and it is the third most common life-threatening fungal infection following aspergillosis and candidiasis1. Mucormycosis is usually caused by a group of angioinvasive fungi in the order of Mucorales. Mucor irregularis Stchigel, Cano, Guarro & E. Álvarez (synonym: Rhizomucor variabilis R.Y. Zheng & G.Q. Chen), a causative agent of mucormycosis, was firstly reported in 1991, on the hand of a woman in China with a primary cutaneous infection2. Since then, dozens of M. irregularis infection have been reported in China3–6, India7, 8, Japan9, 10, USA11, 12, Australia13 and France14. Mucormycosis caused by M. irregularis are characterized by progressive central facial swelling, ulceration and midline facial destruction with pathological features of inflammation, necrosis, ultimately leading to severe disfigurement and even death if left untreated4, 5, 15 (see Supplementary Fig. S1). However, little is known about the pathogenic mechanism of M. irregularis that is critically important for the prevention and treatment of mucormycosis.
Sexual reproduction is ubiquitous across fungi and important to the fitness of species. It has been noted that sexual reproduction coupled with meiosis and recombination plays an important role in the evolution of pathogenic fungi and could have significance influence on virulence16. For any fungus that can reproduce through a sexual cycle, different mating types are required to form sexual/gametes fusion17. In fungi, the mating process is controlled by a sex-specific region of the genome known as the mating type locus (MAT), and the MAT loci determination in fungi has predominantly been studied in ascomycetes and basidiomycetes. However, sexual reproduction has not been demonstrated in the Mucorales until recently, for example, Rhizopus miehei, Mucor circinelloides, Phycomyces blakesleeanus, Rhizopus oryzae and other human pathogenic members of the Mucorales18–20. These studies revealed that the mating system in Mucorales were regulated by divergent alleles of a single gene - SexM/SexP, which consist of a high mobility group (HMG) transcription factor gene flanked by genes encoding a triose phosphate transporter homolog (TPT) and an RNA helicase21–23. The HMG domain proteins are designated as SexP for the (+) and SexM for the (−) mating types respectively. The sequences of the genes encoding SexP and SexM are divergent but bi-allelic at the MAT loci, in contrast to the idiomorphic nature of MAT loci in many ascomycetes and basidiomycetes that encoding entirely divergent proteins24.
The process of sexual reproduction in the fungi has been linked to the virulence and the outbreak of infectious diseases. For instance, the mating type has been found to be a source of variation in virulence in some opportunistic fungal pathogens, such as A. fumigatus 25. Studies have demonstrated a predominance of MAT1-1 isolates among invasive aspergillosis cases, as well MAT1-1 isolates in the larvae of Galleria mellonella were more virulent than MAT1-2 isolates25, 26. While in Cryptococcus neoformans, the α mating-type isolates have greater abilities to cause virulence than a mating type27. Even the MAT locus genes themselves have been associated with the pathogenesis of Fusarium graminearum, as demonstrated by the reduced virulence of mat1-1-1 and mat1-2-1 mutants in corn stalk rot assays28.
In Mucorales, increased pathogenicity in (+) mating types has been observed in pathogenic fungi. For example, when spore suspensions of plant pathogenic Mucor piriformis were used as inoculum, the (+) mating type produced significantly larger lesions than those produced by the (−) type29. Similarly Stewart and Munday30 found that the (+) mating type of M. amphibiorum may be capable of causing greater rates and severe infection in toads than the (−) mating type. The (+) mating type produced spherules more rapidly with daughter cells, leading to a more rapid dissemination of infective propagules and a more severe infection30. In contrast, the (−) mating type isolates of Mucor circinelloides f. lusitanicus (Mcl) were more virulent in the wax moth host compared to (+) isolates; however, the loss of virulence in (−) mating type was associated with the sporangiospore size dimorphism, not related directly to the MAT locus31, 32. Nevertheless, a sexual cycle has never been studied in M. irregularis, although genetic variation has been reported among isolates15. The relationship between genetic/genomic variation and the level of virulence is also poorly investigated.
In this study, we hypothesized the virulence of M. irregularis can vary with its mating types. First, we characterized the MAT locus and the SexM/P genes of M. irregularis using genomic and morphological methods. Second, we analyzed the mating type of 17 clinical isolates that were collected from China. All isolates, but the standard strain CBS103.93 (−) have not been characterized in previous investigation12. Third, we investigated the impact of mating type on fungal virulence by using a subcutaneous injection mice model; we studied the virulence of three M. irregularis isolates with different mating types: B50k (+), B50n (+/0), and CBS103.93 (−) in vivo. Through the comparison of pathological alternation under microscopy, serum cytokine profiles and the percentages of T helper 1 (Th-1), Th-2 and Th-17 cells among three isolates in the murine model, we found that the severities of inflammation and immunoreaction were more prevalent in M. irregularis (+) mating type. The findings from in vivo study suggested the two mating types of M. irregularis may have different impact on virulence phenotypes and clinical manifestations.
Results
Morphological and physiological features
All the M. irregularis isolates (CBS103.93 and CMFCCC B50e~B50t, Table S1) produced whitish to yellowish colonies that were yellow on the reverse when grown on MEA. The sporangiospores were hyaline, smooth walled (Fig. 1A,B), variable in size and shape, sub-spherical to ellipsoidal or reniform, ca. 3.7–13.3 × 2.0–10.3 μm. We found the growth rates and the optimal growth temperatures of clinical isolates were similar to the standard strain on MEA at room temperature (~21 °C), 24 °C, 28 °C, 32 °C, 35 °C, and 38 °C. After 4 days, colonies had grown over the plates at 24 °C, 28 °C, 32 °C, except at 35 °C. No growth was observed at 38 °C after 3 weeks in any isolates. The inability to grow at temperatures >37 °C was consistent to previous reports2.
Figure 1.
Zygospores formed in the intraspecific cross of Mucor irregularis CBS103.93 (−) × B50k (+). (A,B) SEM images of CBS103.93 and B50k spores. The surface of two mating type isolates are both smooth. (C) Line of zygospores formed on MEA 10 days after CBS103.93 × B50k were paired. (D) Electron micrograph of a cross between CBS103.93 × B50k showing zygospores; bar, 50 μm. (E) SEM image of mature zygospores with stellate ornamentation; bar, 50 μm.
Mating type determination through crossing experiment
After 10 days incubation at 25 °C in the dark on MEA, zygospores was typically formed in a prominent dark band at the contact zone between M. irregularis CBS 103.93 (−) and (+) isolates such as B50k and B50r as shown in Fig. 1C. The typical zygospores of M. irregularis were medium to dark brown (Fig. 1D), up to about 60 μm in diameter, with stellate ornamentations (Fig. 1E) and opposed suspensors. No sexual reactivity was observed when CBS 103.93 were pairing with 14 M. irregularis isolates, which in contrast can form zygospores while crossing with B50k (+). Therefore, these 14 isolates were presumably to be (−) type, the same mating type as CBS103.93.
Dissimilarity between SexP and SexM at the MAT locus
The MAT locus in M. irregularis B50p genome (accession No. AZYI00000000.1.) was identified by using the top hits from TBLASTN queried with the known MAT locus sequences in from other members of Mucorales. The gene organization at the locus was predicted using FGENESH pipeline (http://www.softberry.com/berry.phtml) and was searched against the genome sequences of the R. oryzae, M. circinelloides, and P. blakesleeanus genome databases in the GenBank. Genomic analysis showed that M. irregularis isolate B50p contained a single SexM gene with HMG domain, flanked by a predicted triose phosphate transporter (tpt) and RNA helicase (rnhA) genes (Fig. 2A).
Figure 2.
Sequence analysis of the MAT locus in Mucor irregularis. (A) Arrangement of MAT locus for the SexM (−) or SexP (+) alleles with their adjacent genes in M. irregularis. The SexM and SexP in M. irregularis are flanked by genes for RNA helicase and a triosephosphate transporter. The orientations of SexP and SexM genes are the same. The dissimilar DNA sequences of the sexM/P (from the TSS to stop codon) of M. irregularis are highlighted by a light grey box, gene orientation are show in the arrows. (B) Mating-type determination of M. irregularis isolates by PCR. DNA amplified with the MAT specific primer designed from5’ of tpt to the 5’ of RNA helicase. Lane 1: molecular ladder; Lane 2: M. irregularis CBS103.93; lane 3: no template control; lane 4: ddH2O (as the negative control); lane 5: Rhizopus oryzae B81a (as the negative control); lanes e-t: M. irregularis isolates B50e- B50t. (C) Dot plot comparison between the homeodomain region of M. irregularis isolates CMFCCC B50k (x-axis) and CBS103.93 (y-axis).
To examine whether the MAT locus was identical in sequences among all the SexP (+) and SexM (−) mating types of M. irregularis isolates, PCR was performed using a specific primer pair targeting from the 5′ of tpt to the 5’ of rnhA of B50p. A single band was amplified from all isolates, and the size of fragments was either 1660 or 1960 bp (Fig. 2B). The sequenced results demonstrated that the (+) type had longer fragment, and the (−) type isolates had shorter fragment with the exception of B50n, which had the same longer band as the (+) type. Every isolate contains a single gene, either SexM or SexP, at the locus flanked by the genes tpt and rnhA, respectively (Fig. 2A). The overall ratio of SexM (−): SexP (+) in China was 14:3.
DNA dot-plot analysis between the homeodomain region of CBS103.93 (−) and the corresponding region from B50k (+) showed that there was a highly dissimilar core corresponding to SexM and SexP genes (only 32.4% similarity in nucleotide); in contrast the tpt and rnhA were highly similar between two mating types (99.4% and 97.3% similarities, respectively) (Fig. 2C).
The SexP gene was 933 bp long and had no intron. A transcription start site (TSS) was located 157 bp upstream of the start codon. Comparison of the promoter region (264 bp upstream of the start codon) of SexP from M. irregularis with sequences of other Mucorales revealed a conserved transcription factor binding motif with a CCAAT-box (GATCxxxxAxCCAAT) located at 73 bp upstream of the start codon. SexM (579 bp long) was shorter than SexP, no intron and shared only 32.4% DNA sequence identity with SexP. Also a TSS was found at location of 195 bp upstream of the start codon. Comparing the promoter regions in SexM (359 bp upstream of the start codon) between M. irregularis and other Mucorales did not reveal any conserved sequences. Similarly, there was no conserved sequence between the promoter regions of SexM and SexP in M. irregularis, indicating that both genes may be regulated by different mechanisms and processes.
The amino acid sequences similarities of SexP and SexM to other Mucoralean fungi ranged from 25 to 60%, and from 26 to 36%, respectively. The SexP in M. irregularis had the greatest similarity to SexP in M. mucedo, and phylogenetic analysis showed that the SexP proteins of M. irregularis and M. mucedo clustered together (see Supplementary Fig. S2). Unexpectedly, although the M. irregularis SexM shared the highest similarity with SexM of R. delemar, phylogenetic analysis indicated that SexM of M. irregularis was grouped within the SexP subgroup (see Supplementary Fig. S2), and distinct from all other SexM proteins, suggesting the SexM/P of M. irregularis may be evolved before speciation within the Mucorales.
Comparative analysis of these MAT locus sequences indicated that SexP (+) type was differentiated from SexM (−) type, however the intraspecific variation between SexM/P sequences was quite low among each type. Moreover, the phylogenetic tree showed that the distribution of mating type, at least the SexM (−) type, might have little correlation to the source of isolates in China (see Supplementary Fig. S3 and Table S1).
Of all the isolates, B50n appeared to be a natural mutant and was labeled as (+/0). First, although the size of its MAT locus belonged to the (+) type, it could not mate with CBS103.93 (−). Second, there was a 2 bp deletion (at the upstream of TSS) and 4 mutations at the upstream region of SexP start codon in this isolate in comparison to those of B50k and B50r (Fig. 3). Third, when B50n was paired with other 16 isolates, only 5 (−) isolates (B50f, o, p, q and t) presented mating responses; it failed to form zygospores when crossed with other (+) or (−) types isolates, indicating that B50n was partially defect in sexual reproduction.
Figure 3.
Alignments of the promoter region of SexP among three SexP (+) type Mucor irregularis isolates. The multiple alignments were performed using the ClustalW program implemented in MacVector 11.
Mice were susceptible to M. irregularis infection
To elucidate the possible variation in the pathogenicity among the different mating types of M. irregularis, a mice model was employed. Subcutaneous injection model in the back of BALB/c mice was used as an indicator of the degree of inflammation. Three isolates of various mating types and clinical manifestation profiles were selected: the standard strain CBS103.93 (−), the natural SexP mutant isolate B50n (+/0) and B50k (+), which was isolated from a patient who developed severe clinical symptoms (unpublished data, Fig. S1). R. oryze B81a was used as a positive control due to its aggressive pathogenicity and the saline (0.9% NaCl) was used as the negative control.
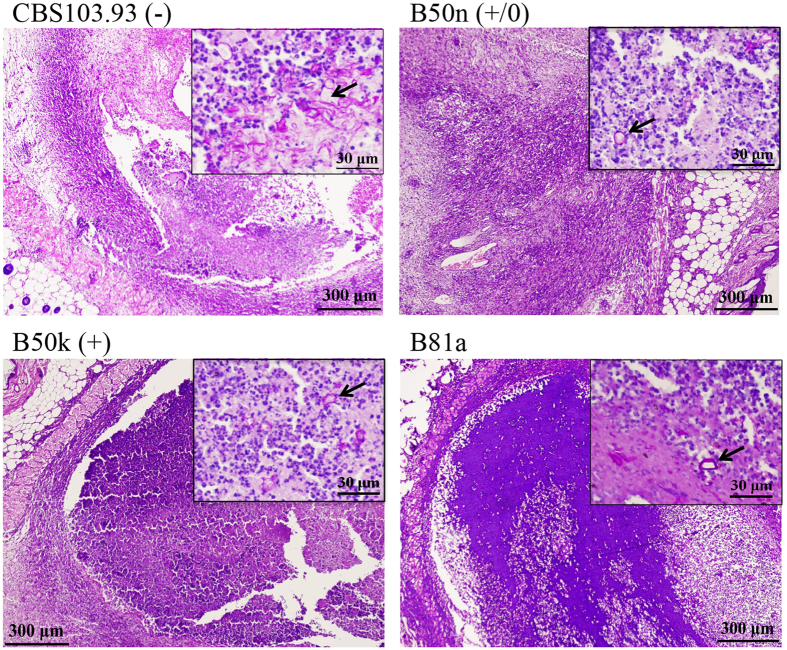
Seven days after the inoculation, a small lump in BALB/c mice was observed (Fig. 4C). After 14 days, B50k (+)-injected mice presented much larger lump (p = 0.0258) than CBS103.93 (−) (Fig. 4A,C) and more serious splenomegaly (Fig. 4B,D), indicating a more serious immunoreaction. The lump in B50n (+/0)-injected group was also larger than CBS103.03 group, but smaller than the B50k (+) group. In general, the severest manifestation of the lesions in the model mice was observed at about 15 days (Fig. 4C). To further analyze the degree of inflammation and pathological characteristics in BALB/c mice, the lesion was biopsied at 15 day after infection (DAI). Under the microscopic examination, a main feature of the lesion including inflammation, necrosis, and granulation was evident, along with a non-specific pattern of inflammatory response infiltrated with neutrophil granulocytes, lymphocytes, plasma cells, and sometimes multinucleated macrophages (Fig. 5). Consistent with these general observation, B50k (+)-injected group presented more serious inflammatory cell infiltration and necrosis than CBS103.93 (−) -injected group (Fig. 5), suggesting an enhanced immune response in B50k (+) group. B50n (+/0)-injected group had mediate inflammatory reaction between those of B50k (+) and CBS103.93 (−) (Fig. 5).
Figure 4.
Clinical progression of mice model induced by intradermal injection of Mucor irregularis CBS103.93, B50n and B50k; Rhizopus oryzae B81a was used as a positive control and the saline was the negative control. (A) Representative clinical presentation at 15 days post infection (lumps in circles). (B) Demonstration of splenomegaly in mice injected with different isolates. Groups of BALB/c mice were injected with each isolate, and after 15 days their spleens were sacrificed for observation. (C) The lump development following intradermal infection. Data are shown with mean ± SD (n = 20). (D) Effects of injection from four Mucorales on organ coefficients of the mice spleens. Organ coefficient was calculated as the percentage of the ratio of wet weight of the spleens to the body weight. The data represents means ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001 was considered statistically significant.
Figure 5.
Photomicrography of the dermis and cutis of BALB/c mice experimentally infected with Mucor irregularis CBS103.93, B50n, B50k and Rhizopus oryzae B81a at 15 days after the injection. HE staining of dermis sections (Low-power sections, 100X, bars = 300 μm) revealed the presence of an accentuated inflammatory infiltrate. PAS staining (upper right corner of each graph, high power magnification, 400X, bars = 30 μm) revealed the presence of hypha (arrow).
Differential Th-1, Th-2 and Th-17 cytokine profiles after M. irregularis challenge
The host defense mechanisms against fungi usually range from an early non-specific immune response to activation and induction of specific adaptive immunoreaction by the production of Th-1, Th-2 and Th-17 cytokines. To determine the levels of Th-1, Th-2 and Th-17 cytokines induced in mice infected with M. irregularis, and the association between their levels and disease prognosis, numerous cytokines in serum was examined by Luminex. Higher levels of the Th-1 cytokines (IFN-γ) were found in B50k-injected mice than CBS103.93-injected ones (Fig. 6A), while R. oryze B81a group (positive control) had the highest level among all groups. Again, B50n group still displayed higher level of cytokines than CBS103.93. However, the Th-2 cytokines IL-4 (Fig. 6B) showed no significant difference among groups. The levels of Th-17 cytokines (IL-17, IL-22) showed minor difference (Fig. 6C,D), even though the average level of B50k group was higher than that in B50n and CBS103.93 groups. However, other cytokines such as IL-1α, IL-1β, MCP-1, M-CSF (CSF-1), MCP-3, MIP-1α, MIP-1β, MIP-2, TNFα were not significantly different among tested groups.
Figure 6.
Effects of injection from four Mucorales on cytokine levels in serum. Serum IFN-γ (A), IL-4 (B), IL-17 (C) and IL-22 (D) concentrations were measured at 15 days after the injection of Mucor irregularis CBS103.93, B50n, B50k and Rhizopus oryzae B81a. Saline-treated mice served as control.
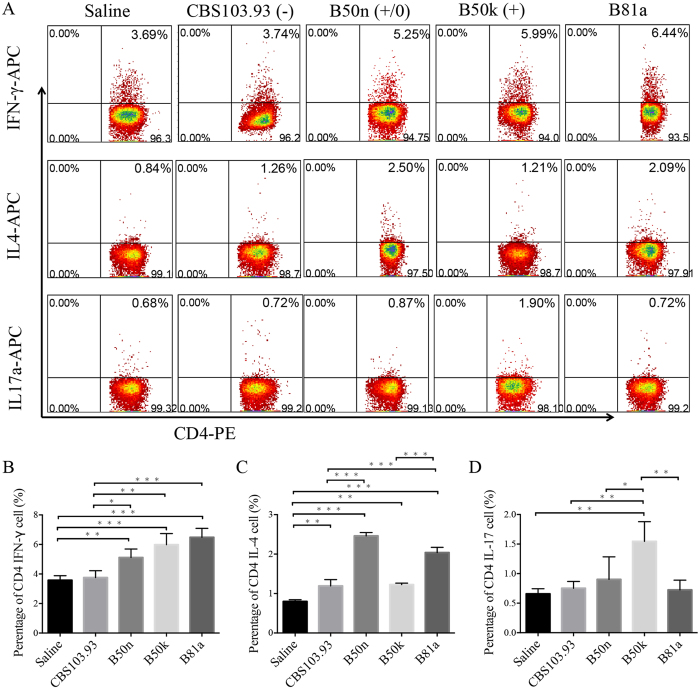
Guided by the cytokine profiles, Th-1, Th-2 and Th-17 cells numbers in mice of each group was evaluated by flow cytometry. Percentage of Th-1 cell (CD4+IFN-γ+) in B50k group (5.98 ± 0.70%) was significantly higher than that in CBS103.93 group (3.58 ± 0.34%) (Fig. 7A,B), but lower than that of R. oryze B81a group (6.48 ± 0.58%). The Th-1 cell percentage of B50n group (5.11 ± 0.57%) was also higher than CBS103.93 but lower than B50k (Fig. 7A,B). Similar to the serum cytokine profiles, percentage of Th-2 cells (CD4+IL-4+) in B50k group (1.22 ± 0.04%) did not differ from those in CBS103.93 group (1.19 ± 0.16%), while B50n mutant group (2.46 ± 0.09%) showed significantly higher percentage (~1 fold increase) than CBS103.93 and B50k (Fig. 7A,C). Notably, B50k group showed highest percentage of Th-17 (CD4+IL-17+) (1.55 ± 0.33%) when compared to control (0.67 ± 0.09%), CBS103.93 (0.75 ± 0.11%), and B50n (0.90 ± 0.38%) (Fig. 7A,D).
Figure 7.
Effects of injection from four Mucoralean fungi on the proportion of Th cells in the spleen. Original dot plots from FACS analysis of CD4+ IFN-γ+ cells (A,B), CD4+ IL4+ cells (A,C), and CD4+ IL17+ cells (A,D) isolated from spleens. The numbers in the dot plots indicated the percentage of cells whining each quadrant. B, C and D showed the percentage of CD4+ IFN-γ+ cells, CD4+ IL4+ cells, and CD4+ IL17+ cells of the splenocytes. Data are mean ± SD of five mice, in two separate experiments. *p < 0.05, **p < 0.01, and ***p < 0.001.
Discussion
Mucor irregularis has emerged as a lethal infectious disease to the human hosts10–15. From the clinical point view, immunodeficiency or immunosuppression may not account for all the etiology and pathogenesis of the M. irregularis infection since M. irregularis infections are always occur in the patients without any apparent immune impairment, of which is different with other pathogenic fungi in Mucorales. Although, the pathogenic mechanism of M. irregularis remains largely unknown, increasing signs/evidence suggest the invasiveness or virulence of M. irregularis is closely related to the pathogenic process, independent to the biological variation of patients15. The genomic data of M. irregularis therefore becomes an important resource to seek for specific pathogenic mechanisms of M. irregularis.
The mating type has previously been linked to increased virulence in fungi25–31. In order to understand the possible correlation between mating type and virulence in M. irregularis, we characterized the mating type genes of 17 clinical isolates of M. irregularis that have been mostly collected from individual infected patients in China. The sequence data, coupled with the mating crosses results test showed that 14 clinical isolates are (−) type. The sequence data also demonstrated that each isolate possesses a mating type locus that is homologous to those of other Mucorales. Either SexM or SexP domain gene was identified in the MAT locus, demonstrating that M. irregularis is heterothallic. Although the SexP or SexM contained specific sequences that did not exist in the complementary mating type, both genes’ cluster consisted of an HMG domain gene flanked by tpt and RNA helicase genes, which located at the same chromosomal positions. Similar architecture of the putative mating type locus has also been found in other heterothallic Mucoralean fungi such as M. circinelloides 21 and R. oryzae 23. Together, the data suggested the MAT gene organization in Mucorales was ancient and conserved. However, unlike the MAT locus of R. oryzae which contained all genes in the MAT locus positioned at the same orientation, TPT and SexM/P genes in M. irregularis were in inverted orientations, which was the same as P. blakesleeanus and M. circinelloides 21–23.
Moreover, one isolate B50n with deleted/mutated SexP homolog at SexP mating locus, was identified in our study - the kind of natural mutation has never been reported previously in other heterothallic Mucorales. When the SexP has mutations, only 5 of the 14 SexM (−) isoates could mate with it. The partial defect in mating abilities in B50n mutant provided evidence that this MAT locus was involved in the regulation of mating process/sexual reproduction in M. irregularis. Similar observations have been reported in other fungi, for example, disrupting the SexM gene at the (−) mating type caused sterility in the (−) mating type of M. circinelloides 32; the SsΔMAT-1b S. scitamineum mutants were defective in mating when mixed with the opposite mating type33. Generally, the MAT locus has been corroborated across Mucorales species such as R. oryzae, M. mucedo, M. circinelloides, P. blakesleeanus and M. irregularis, indicating that the MAT locus governs sexual reproduction of the Mucorales.
We investigated the influence of mating type on the virulence of M. irregularis. Three M. irregularis isolates, ex-type CBS103.93 (−), B50n (+/0) and B50k (+) were used to compare their virulence through the in vivo murine model. Macroscopic and microcosmic analyses of the infected mice showed disease progression among the mating types; B50k (+) produced greater lesion and caused more damage to murine host than CBS103.93 (−). The murine data was consistent with the severity of clinical symptoms in patients (see Supplementary Fig. S1). Our findings indicated an association between mating type and virulence in M. irregularis; the (+) mating type appeared to be more virulent than the (−) mating type.
Contrasting virulence level between opposite mating types has been reported in other human fungal pathogens such as Candida parapsilosis and Cryptococcus neoformans 34–38. For example, Kwon-Chung39 demonstrated that the α mating type was more virulent than the a mating type, using congenic strains of Cryptococcus neoformans var. neoformans. Although, no difference in virulence between mating types has been found with Cryptococcus neoformans var. grubii, cells of α mating type have been shown to have an enhanced predilection to penetrate the central nervous system during co-infection experiments in mice40. Also in M. circinelloides, another member of Mucorales, (−) mating type isolates produced larger asexual sporangiospores that were more virulent in the wax moth host compared to (+) isolates that produce smaller less virulent sporangiospores32, and the greater virulence was related with the sporangiospore size for the larger spores could start invasive hyphal growth immediately upon phagocytosis by host immune cells, whereas smaller spores have a long period of isotropic growth32. Moreover, SEM analyses revealed that the larger (−) spores are decorated with ‘bumps’ on the surface, and the surface of the smaller (+) spores tended to be smooth in M. circinelloides 32. Unlike M. circinelloides, the surfaces of spores from both two mating types of M. irregularis were smooth, and there was no significant variation in the size of M. irregularis spores between two mating types, suggesting that spore size may be unrelated to the virulence in M. irregularis.
Apart from the variation in virulence between the two mating types of M. irregularis, the host immune responses were also different during the M. irregularis infection. At 15 DPI, B50k−injected BALB/c mice presented a significantly larger lump when compared to CBS103.93 mice, indicating more serious inflammation. Previous analysis of fungal infection has revealed that immunoreactions in the fungi−infected human or animal models were closely correlated with the invasiveness or virulence of fungi, and the immune mechanisms of defense against fungal infections are numerous, ranging from innate immunity to sophisticated adaptive mechanisms41. The Th-1, Th-2, and Th-17 cells (three major T helper cell subsets differentiated from CD4+ T cells) are crucial component of the adaptive immune response to fungal pathogens and the essential role in protecting body from being infected with diverse fungal pathogens42. Increased IFN-γ and IL-17 level in the serum, as well as an enhanced percent of CD4+IFN-γ+ (Th-1) and CD4+IL-17+ (Th-17) cells in the spleen of infected M. irregularis mice, suggested that cell-mediated immunity can play an important role in the pathogenesis of M. irregularis infection. In agreement of severity of inflammatory response, both cytokine profiles and Th-1 and Th-17 cell populations were highly responded in B50k-injected BALB/c mice. On the other hand, B50n (+/0), with a defective mating ability showed an intermediate inflammatory response and immune response between CBS103.93 and B50k, also suggested a can’t-be-ignored correlation between mating type and virulent level. From the results, we may conclude 3 points: first, cell-mediated immunity is playing an important role to control the cutaneous M. irregularis infection, especially the Th-1 and Th-17 pathway, consistent with our previous study that IL-17 and IFN-γ mRNA expression was significantly increased over time in M. irregularis infected skin lesion43; second, the mating type may have a potential correlation with the severity of inflammation; third, although the mating type gene is not a direct virulence factor, but link to virulent progression through unknown mechanisms.
Until recently, little is known about the relationship of MAT locus to virulence in fungi. The mating process in Cryptococcus neoformans is accomplished by several pheromone response pathways that require genes such as GPA1, STE4, STE20, STE12, STE2 besides pheromone genes37, 44. Studies to establish mating events to virulence in C. neoformans demonstrated that the disruption of GPA1 led to a sterile and avirulent phenotype due to the deficiency of two major virulence factors, melanin production and capsule formation44. In addition, the MATα allele, STE12α of C. neoformans, was found to affect both capsule and melanin production, and further was required for monokaryotic fruiting45. Moreover, while the major cytoplasmic 5′ → 3′ exonuclease Xrn1p in C. neoformans is responsible for uni- and bisexual mating, it has been linked to virulence associated phenotypes such as growth at 37 °C, capsule and melanin46.
In our study, M. irregularis isolates of opposite mating types, though based on limited isolate from each mating type, had elicited different levels of immune response, suggested that the virulence between them could be different. How could these mating events influence the virulence in M. irregularis remain to be unraveled.
Hitherto, the innate sensing of Mucorales spp. by immune and/or nonimmune (e.g. endothelial) cells and the role of adaptive immunity in patients with mucormycosis are still poorly understood. Blakeslee reported that in many species of Mucorales the (+) mating type regularly showed greater vigor than its (−) homolog, and he hypothesized that an unidentified factor associated with the (+) mating type probably exists during the process of zygospore germination, which asserts a dominance over the (−) components47. Of interest, β-glucan exposure during germinating growth of Rhizopus triggered dectin-1 signaling in human dendritic cells and resulted in robust induction of the IL-23/Th-17 responses48. Also the mannan could inhibit the macrophage-mediated phagocytosis of conidia and inhibit the cell-mediated immune response49. These finding led us to investigate that whether the cell wall of different mating type isolates possessed varying contents of polysaccharide such as β-glucan or mannan which might elicit different levels of immune responses. In addition, we observed that the colonies and sporangiospores of M. irregularis B50k /r (+) were more grayish than CBS103.93 (−) (see Supplementary Fig. S4), but the mutant B50n was more white than the latter (see Supplementary Fig. S4), and the colonies of (−) type isolates had color variation. Therefore, the pigment which has long been recognized as virulence factor in many fungi45, maybe not a case for M. irregularis. The dissimilarity between SexP and SexM, suggested that these genes might have evolved to regulate different set of genes including virulent factors or being regulated differently. Since the virulence of a pathogen is associated polygenic traits, these mating coordinated cellular events are needed to be studied in M. irregularis, and to test whether other mating associated genes such as the homologue of Xrn1p in M. irregularis could also affect the virulence.
Although (+) mating type appeared to be more virulent, (−) mating type was more prevalent in our collection; 14 out of 17 clinical isolates had (−) mating type. This skewed mating ratio raised questions on the disease transmission mechanism and the role of the SexM on M. irregularis virulence as well. We would not have a good explanation until additional sampling and thorough investigation on the favourable environment and growth requirement for each mating type are determined. Up date, only ~30 cases so far have been reported worldwide, and both isolates outside of Asia, which reported by Schell’s group were happened to be SexP12 (as shown in Supplementary Table S1). Thus, the mechanisms of pathogenesis of M. irregularis require more in-depth investigation. Nevertheless, our conclusion are also reasonable as the mating type bias is not a phenomenon confined solely to M. irregularis isolates, although equal frequency of mating types could be the norm across the majority of studies50. And the mating type skew had already been found in another member of Mucorales. In M. piriformis, which causes pear rot, the two mating types occurred in approximately equal proportions in one half of the orchards sampled, but the (+) mating type predominated in the other half29. Besides, Kano51 suggested that human pathogenic fungi were biased toward single mating type. For example, the ratio of MAT1-1: MAT1-2 of Sporothrix globosa which isolated from different area in Japan was 1:351. Moreover, clinical and environmental isolates of C. neoformans display a severe bias of the MATα mating-type over MATa (the ratio was ca. 45:1 in environmental isolates and ca. 30:1 in clinical isolates)39. Nieuwenhuis concluded that such a skew is consistent with rare sexual reproduction and/or strong selection on pleiotropic effects of a mating-type allele50.
Other than the possible geographic preference, this mating type bias in our study may be related to virulence itself. As demonstrated in the murine model, mating type (+) induced a stronger inflammatory and cytokine response than (−) mating type strain. For control of fungal infection, during immune responses, IL-17 is responsible for recruiting neutrophils and to synthesize antimicrobial peptides and proinflammatory cytokines, and IL-17 producing T cells can shift to produce IFN-γ and induce Th-1 responses52. Also, natural killer (NK) cells exhibited anti-infective activity against R. oryzae, which is the most virulent mucormycete53. In addition, a previous study showed that Pseudomonas aeruginosa could escapes cytotoxic T lymphocytes response during chronic infection54. Thus, this facts led us to speculate that in M. irregularis, SexP (+) isolates with higher virulence might be destroyed by these immunocompetent cells, in contrast, the SexM (−) isolates which induced a weak immune response might partial escape from CD4+ cytotoxic T lymphocytes but persistently present as antigen, eventually appearing as chronic infection. This may explain the preference of a chronic infected model of M. irregularis on the skin and a longer duration of infection in clinical cases, but we cannot rule out other mechanisms, of which (−) mating type may contribute to virulence on the skin. To answer this question, more isolates from China and the world are required to understand their genetic diversity, mating type, and virulence activities.
In conclusion, we have characterized, for the first time, the two different mating types (SexM and SexP) in M. irregulairs, and confirmed that the clinical isolates from China were predominantly SexM in MAT locus. We discovered a natural mutant with multiple deletion/mutation in MAT locus and lost certain capacity to mating, indicating the role of MAT locus in the regulation of mating. Also, from murine model, we showed that M. irregularis infection caused lesion and disease proliferation in spleen and induced production of inflammasome-dependent cytokines at 15 DPI. Moreover, there were differential responses of cytokines towards the two mating types in M. irregularis. The SexP (+) type appeared to be more virulent than the SexM (−) mating type. The future is to further understand the genetic variability of the isolates, the molecular mechanism of sex and its gene regulation in depth to gain further insights into their impact of infection and virulence.
Materials and Methods
Fungal isolates and growth conditions
A total of 17 M. irregularis clinical isolates collected from different regions of China (CBS103.93 and CMFCCC B50e~B50t, see Supplementary Table S1) were ex situ conserved at Centre of Medical Fungi, China Committee for Culture Collection of Microorganisms (CMFCCC), Nanjing, China. Specifically, M. irregularis CBS103.93 was the first reported isolate in China from a primary cutaneous infection in a 34-year-old farmer suffered from a skin lesion for about 17 years2. B50k was isolated from a 64-year-old man who was diagnosed recently by our team (unpublished data), based upon a 3-year-history of a cutaneous lesion on right limb (see Supplementary Fig. S1). For sporangiospore production, all isolates were grown on malt extract agar (MEA) (Oxoid, UK) at 28 °C. DNA extraction was performed from each MEA culture that was inoculated with 1000 spores onto a sheet of cellophane.
Mating type crosses
The lengths and widths of 20 randomly chosen sporangiospores in each isolate were measured. Crossing combination was performed by pairing the mycelial agar plug (5 mm × 5 mm) of reference strain CBS103.93 (−) with each of other M. irregularis isolates on MEA, followed by incubation in the dark at 25 °C. The presence of zygospores suggests the pairing isolate having the opposite mating type to the strain CBS103.93 (−). Additional crosses experiments were performed by pairing B50k, which was characterized to be (+) type, and B50n, which was determined to be (+/0) type with each of other isolates as mentioned above. Each pairing had three replicates, and the whole crossing combination experiment was repeated twice.
Morphological analyses
The lengths and widths of 20 randomly chosen sporangiospores in each isolate were measured. For scanning electron microscopy (SEM), the sporangiospores and zygospores of M. irregularis were collected and suspended in 0.1 M sodium cacodylate, then immobilized on Millipore Nitrocellulose filters (Millipore HAWP, 0.46 μm), which were immediately fixed in 2% glutaraldehyde, 0.05% malachite green oxalate in 0.1 M sodium cacodylate buffer. The fixation buffer was then removed and the membrane was washed in 0.1 M sodium cacodylate prior to being subjected to an ethanol dehydration series (2 times for 10–15 min in 25%, 50%, 75%, 95%, and 3 times in 100% ethanol). Samples were then critical point dried, sputter coated, and imaged with the Hitachi S3000N (Hitachi Company, Japan) at Nanjing agricultural University.
Characterization and molecular analysis of MAT in M. irregularis
To obtain the putative MAT locus (sex) genes in M. irregularis, the published sequences of the SexM/P genes in Phycomyces blakesleeanus (EU009461, EU009462), M. circinelloides (FJ009106, FJ009107 and HM565940), Rhizopus oryzae (HQ450316) and M. mucedo (JN587498) from NCBI were used as query sequences to search against the assembled M. irregularis genome (B50p, accession No. AZYI00000000.1) by BLASTP and TBLASTN programs (e-value < 1e-10). Then the MAT locus sequence derived from the genomic analysis of B50p was used as a template for primer design to target the SexM/P gene loci in 17 tested isolates. Specific primers were designed targeting between the 5’ of tpt and the 5’ of RNA helicase using MacVector 11 (Accelrys, USA) (listed in Supplementary Table S2).
The genomic DNA of the 17 isolates’ was extracted as previously described15. The PCR mix contained (25 μl total volume): 12.5 ng genomic DNA, 10 pmol primers, 12.5 μl 2 × Vazyme LAmp Master Mix (P312, Vazyme, China). The temperature profile was: 94 °C for 3 min, 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 3 min. The amplified fragments were isolated from an gel purified and sequenced. FGENESH55 was used to predict the putative transcription start site (TSS) and number of exons. The sense of genes in the MAT locus was determined in ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) and displayed with a dot plot diagram. Finally, the ORF of SexM/P was cloned using gene specific primers (listed in Supplementary Table S2), sequenced and verified in each tested isolate.
The sequences of MAT genes from 17 M. irregularis isolates were aligned using MacVector 11 with manual adjustment. The phylogenetic relationship among these isolations was inferred with MEGA 6.056 using neighbor-joining (NJ) algorithms with the following parameters: P distance, pairwise deletion and bootstrap (1,000 replicates). Also the amino acid sequences of SexM/P were aligned with SexM/P sequences reported from other Mucorales species (listed in Supplementary Table S3). A phylogenetic tree was constructed by NJ with 500 bootstrap replications using MEGA 6.0 as described above. These newly determined MAT sequences of M. irregularis isolates have been deposited in GenBank under accession numbers KY434081 to KY434097.
M. irregularis mouse infection model
Seven-week old BALB/c female mice were housed at 22 °C under a 12-h light-dark cycle and with ad libitum access to food and water. This study was carried out in strict accordance with the recommendations in the guide for the care and use of laboratory animals by the authority of the People’s Republic of China. The experimental protocols were reviewed and approved by the Medical Ethics Committee in the Chinese Academy of Medical Sciences and Peking Union Medical College (Permit Number: 2016-018). The mice were assigned to five groups (n = 20/per group) which were infected by CBS103.93 (−), B50k (+), B50n (+/0), Rhizopus oryzae B81a (as the positive control), and the saline (0.9% NaCl as the negative control), respectively. R. oryzae B81a was also obtained from CMFCCC and cultured on MEA for seven days at 28 °C before the experiment. There was no significant difference in mice gender distribution and weight among groups.
Spores of the respective isolates were harvested by washing the agar surface with sterile saline containing 0.05% Tween 80. The spore suspension was filtered through nylon filters (11 μm pore size), counted on a hemocytometer and re-suspended with saline. An aliquot of 106 spores/0.1 mL was injected into each mouse via intradermal inoculation into the side near a flank of the back.
Mice were monitored twice daily for any signs of illness for 15 days, and the body weight was monitored. The size of lesion occurrence was observed in four infected groups of mice respectively. Fifteen days after the injection, the mice were sacrificed and the spleens, kidneys and livers were excised from each mouse. The organs were weighed after excluding the adipose tissue. Organ coefficient was calculated as the percent of the ratio of the wet weight of the organ to the body weight.
Hematoxylin-eosin (HE) and periodic acid-Schiff (PAS) staining
Biopsies were obtained from infected mice on 15 days post-infection (DPI). Tissues were fixed in 10% buffered formalin and embedded in paraffin. The slides were stained with HE and PAS to identify fungal elements to observe the changes in the skin layers and subcutaneous tissues.
Serum cytokine measurement
Blood samples were also collected from mice at 15 DPI. Serum samples were obtained after centrifugation (2000 g for 10 min) of nonheparinized whole blood to remove the blood clots. Thirty-six cytokines including interferon-γ (IFN-γ), IL-17, IL-22, IL-4 were measured in the serum with Luminex using ProcartaPlex multiplex luminex immunoassays panels, Affymetrix (eBioscience, Waltham, USA) according to the manufacturer’s instructions in a Luminex 100/200 system (Luminex, Austin, USA). Results from technical duplicates were averaged and indicated as Mean ± SD.
Flow cytometric analysis
Fresh spleens were harvested from each group, then placed in 35-mm dishes containing RPMI 1640 medium with 10% Foetal bovine serum (FBS), and homogenized by smashing with the plunger from a 10-ml syringe. The homogenate was passed through a 70 μm cell strainer, and the erythrocytes were lysed with RBC Lysis Buffer (BioGems international, Westlake Village, USA). The remaining cells were counted using TC20 Automated Cell Counter (Bio-Rad, Hercules, USA) and centrifuged (1200 g for 8 min), and then were stained with PE-conjugated CD4 antibodies (eBiosciences) for 30 min at 4 °C. Cells stained with the appropriate isotype-matched immunoglobulin were used as negative controls. The cells were fixed and permeabilized using a Cytofix/Cytoperm kit (BD Biosciences, Sparks, MD) according to the manufacturer’s instructions. Intracellular IL-17A, IL-4 and IFN-γ were detected using APC-conjugated antibodies (eBiosciences) in a permeation buffer. The cells were analyzed using flow cytometer (Attune NxT acoustic focusing cytometer, Thermo Fisher Scientific, Waltham, USA).
Statistics
All experiments were repeated three times. To analyze the differences among the groups, one-way ANOVA followed by Tukey’s multiple comparison test was utilized using statistical software (GraphPad Prism Software, version 4.03, USA). The data in the graphs were expressed as the mean values ± SD; *p < 0.05, **p < 0.01, and ***p < 0.001 was considered statistically significant.
Electronic supplementary material
Acknowledgements
We thank the editor and three anonymous reviewers for their constructive comments, which helped us to improve the manuscript. We are grateful to Siwei Zhu and Huilin Zhi for technical assistance in the murine model experiment. We also acknowledge the support and assistance from Jun Chen in performing the HE/PAS analyses. This work was funded by the National Natural Science Foundation of China (grant No. 81471905), and the Postdoctoral Science Foundation of Chinese Academy of Medical Sciences and Peking Union Medical College (2015).
Author Contributions
W.X., G.L. and W.L. designed the experiments. W.X., J.P., G.L, Y.S., G.L. and M.F. performed the experiments. W.X., Z.L., Q.W., H.M. C.K.M.T. and D.L. analyzed the data. W.X., G.L. and C.K.M.T. wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Wenqi Xu and Guanzhao Liang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10954-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vinay K, et al. Primary cutaneous mucormycosis presenting as a giant plaque: uncommon presentation of a rare mycosis. Mycopathologia. 2014;178:97–101. doi: 10.1007/s11046-014-9752-6. [DOI] [PubMed] [Google Scholar]
- 2.Zheng R, Chen G. A non-thermophilic Rhizomucor causing human primary cutaneous mucormycosis. Mycosystema. 1991;4:45–57. [Google Scholar]
- 3.Zhao Y, et al. Primary cutaneous mucormycosis caused by Rhizomucor variabilis in an immunocompetent patient. Mycopathologia. 2009;168:243–7. doi: 10.1007/s11046-009-9219-3. [DOI] [PubMed] [Google Scholar]
- 4.Lu XL, et al. Primary cutaneous zygomycosis caused by Rhizomucor variabilis: a new endemic zygomycosis? A case report and review of 6 cases reported from China. Clin Infect Dis. 2009;1(49(3)):e39–43. doi: 10.1086/600817. [DOI] [PubMed] [Google Scholar]
- 5.Li DM, Lun LD. Mucor irregularis infection and lethal midline granuloma: a case report and review of published literature. Mycopathologia. 2012;174:429–39. doi: 10.1007/s11046-012-9559-2. [DOI] [PubMed] [Google Scholar]
- 6.Xia XJ, Shen H, Liu ZH. Primary cutaneous mucormycosis caused by Mucor irregularis. Clin Exp Dermatol. 2015;40:875–8. doi: 10.1111/ced.12642. [DOI] [PubMed] [Google Scholar]
- 7.Hemashettar BM, et al. Chronic rhinofacial mucormycosis caused by Mucor irregularis (Rhizomucor variabilis) in India. J. Clin Microbiol. 2011;49:2372–5. doi: 10.1128/JCM.02326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patil AB, et al. Rhizomucor variabilis: a rare causative agent of primary cutaneous zygomycosis. Indian J Med Microbiol. 2013;31:302–5. doi: 10.4103/0255-0857.115662. [DOI] [PubMed] [Google Scholar]
- 9.Tomita H, et al. Rhizomucor variabilis infection in human cutaneous mucormycosis. Clin Exp Dermatol. 2011;36:312–4. doi: 10.1111/j.1365-2230.2010.03956.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi S, et al. Primary cutaneous mucormycosis caused by Mucor irregularis in an elderly person. J. Dermatol. 2015;42:210–4. doi: 10.1111/1346-8138.12736. [DOI] [PubMed] [Google Scholar]
- 11.Abuali MM, et al. Rhizomucor variabilis var. regularior and Hormographiella aspergillata infections in a leukemic bone marrow transplant recipient with refractory neutropenia. J. Clin Microbiol. 2009;47:4176–9. doi: 10.1128/JCM.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schell WA, O’Donnell K, Alspaugh JA. Heterothallic mating in Mucor irregularis and first isolate of the species outside of Asia. Med Mycol. 2011;49:714–23. doi: 10.3109/13693786.2011.568975. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro NF, et al. Burn wounds infected by contaminated water: case reports, review of the literature and recommendations for treatment. Burns. 2010;36:9–22. doi: 10.1016/j.burns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Rammaert B, et al. Mucor irregularis-associated cutaneous mucormycosis: Case report and review. Med Mycol Case Rep. 2014;6:62–5. doi: 10.1016/j.mmcr.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu XL, et al. Taxonomy and epidemiology of Mucor irregularis, agent of chronic cutaneous mucormycosis. Persoonia. 2013;30:48–56. doi: 10.3767/003158513X665539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heitman J, Carter DA, Dyer PS, Soll DR. Sexual reproduction of human fungal pathogens. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppin E, Debuchy R, Arnaise S. & PicardM. Mating types and sexual development in filamentous ascomycetes. Microbio Mol Biol Rev. 1997;61:411–428. doi: 10.1128/mmbr.61.4.411-428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schipper, M.A. & Stalpers, J.A. Zygomycetes: the Order Mucorales. (ed. Howard, D.H.). Pathogenic Fungi in Humans and Animals 2 edn. 67–125 (Marcel Dekker, 2003).
- 19.Schipper MAA. A study of variability in Mucor hiemalis and related species. Stud Mycol. 1973;4:1–40. [Google Scholar]
- 20.Schipper MAA. On certain species of Mucor with a key to all accepted species. 2. On the genera Rhizomucor and Parasitella. Stud Mycol. 1978;17:52–71. [Google Scholar]
- 21.Lee SC, et al. Microsporidia evolved from ancestral sexual fungi. Curr Biol. 2008;18:1675–9. doi: 10.1016/j.cub.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Idnurm A, Walton FJ, Floyd A, Heitman J. Identification of the sex genes in an early diverged fungus. Nature. 2008;451:193–6. doi: 10.1038/nature06453. [DOI] [PubMed] [Google Scholar]
- 23.Gryganskyi AP, et al. Structure, function, and phylogeny of the mating locus in the Rhizopus oryzae complex. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casselton LA. Fungal sex genes-searching for the ancestors. Bioessays. 2008;30:711–4. doi: 10.1002/bies.20782. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Perez S, Blanco JL, Alba P, Garcia ME. Mating type and invasiveness are significantly associated in Aspergillus fumigatus. Med Mycol. 2010;48:273–7. doi: 10.3109/13693780903095414. [DOI] [PubMed] [Google Scholar]
- 26.Cheema MS, Christians JK. Virulence in an insect model differs between mating types in Aspergillus fumigatus. Med Mycol. 2011;49:202–7. doi: 10.3109/13693786.2010.512301. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen K, et al. Interaction between genetic background and the mating-type locus in Cryptococcus neoformans virulence potential. Genetics. 2005;171:975–83. doi: 10.1534/genetics.105.045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Q, et al. The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michailides TJ, Spotts RA. Mating types of Mucor piriformis isolated from soil and pear fruit in Oregon orchards. Mycologia. 1986;78:766–770. doi: 10.2307/3807521. [DOI] [Google Scholar]
- 30.Stewart NJ, Munday BL. Possible differences in pathogenicity between cane toad-, frog- and platypus-derived isolates of Mucor amphibiorum, and a platypus-derived isolate of Mucor circinelloides. Med Mycol. 2005;43:127–32. doi: 10.1080/13693780410001731538. [DOI] [PubMed] [Google Scholar]
- 31.Idnurm A. Sex determination in the first-described sexual fungus. Eukaryot Cell. 2011;10:1485–1491. doi: 10.1128/EC.05149-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li CH, et al. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan M, et al. The mating-type locus b of the sugarcane smut Sporisorium scitamineum is essential for mating, filamentous growth and pathogenicity. Fungal Genet Biol. 2016;86:1–8. doi: 10.1016/j.fgb.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Kwon-Chung KJ, Edman JC, Wickes BL. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect Immun. 1992;60:602–5. doi: 10.1128/iai.60.2.602-605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sai S, et al. Evolution of Mating within the Candida parapsilosis Species Group. Eukaryot Cell. 2011;10:578–87. doi: 10.1128/EC.00276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idnurm A, et al. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat Rev Microbiol. 2005;3:753–64. doi: 10.1038/nrmicro1245. [DOI] [PubMed] [Google Scholar]
- 37.Hsueh YP, Heitman J. Orchestration of sexual reproduction and virulence by the fungal mating-type locus. Curr Opin Microbiol. 2008;11:517–24. doi: 10.1016/j.mib.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metin B, Findley K, Heitman J. The mating type locus (MAT) and sexual reproduction of Cryptococcus heveanensis: insights into the evolution of sex and sex-determining chromosomal regions in fungi. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon-Chung KJ, Bennett JE. Distribution of α and a mating types of Cryptococcus neoformans among natural and clinical isolates. Am J Epidemiol. 1978;108:337–40. doi: 10.1093/oxfordjournals.aje.a112628. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen K, et al. Cryptococcus neoformans α strains preferentially disseminate to the central nervous system during coinfection. Infect Immun. 2005;73:4922–33. doi: 10.1128/IAI.73.8.4922-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanco JL, Garcia ME. Immune response to fungal infections. Vet Immunol Immunopathol. 2008;125:47–70. doi: 10.1016/j.vetimm.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Espinosa V, Rivera A. Cytokines and the regulation of fungus-specific CD4 T cell differentiation. Cytokine. 2012;58:100–6. doi: 10.1016/j.cyto.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bao W, et al. Interleukin-22 mediates early host defense against Rhizomucor pusilluscan pathogens. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lengeler KB, et al. Mating-type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryot Cell. 2002;1:704–18. doi: 10.1128/EC.1.5.704-718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang YC, et al. Cryptococcus neoformans STE12α regulates virulence but is not essential for mating. J Exp Med. 2000;191:871–82. doi: 10.1084/jem.191.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wollschlaeger C, et al. Distinct and redundant roles of exonucleases in Cryptococcus neoformans: implications for virulence and mating. Fungal Genet Biol. 2014;73:20–8. doi: 10.1016/j.fgb.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blakeslee AF. Sexual reaction between hermaphroditic and dioecious mucors. Biol Bull. 1915;29:87–100. doi: 10.2307/1536300. [DOI] [Google Scholar]
- 48.Chamilos G, et al. Generation of IL-23 producing dendritic cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of T(H)-17 responses. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Indranil Samanta. Cutaneous, subcutaneous and systemic mycology. Veterinary Mycology. Springer India, chapter 4, 11-153 (2015).
- 50.Nieuwenhuis B, James T. The frequency of sex in fungi. Philos Trans R Soc Lond B Biol Sci. 2016;371 doi: 10.1098/rstb.2015.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kano R, et al. The MAT1-1: MAT1-2 ratio of Sporothrix globosa isolates in Japan. Mycopathologia. 2015;170:81–6. doi: 10.1007/s11046-014-9808-7. [DOI] [PubMed] [Google Scholar]
- 52.Martinez-Martinez L, et al. A novel gain-of-function STAT1 mutation resulting in basal phosphorylation of STAT1 and increased distal IFN-γ-mediated responses in chronic mucocutaneous candidiasis. Mol Immunol. 2015;68:597–605. doi: 10.1016/j.molimm.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt S, et al. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology. 2013;218:939–44. doi: 10.1016/j.imbio.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Rieber N, et al. Flagellin induces myeloid-derived suppressor cells: implications for Pseudomonas aeruginosa infection in cystic fibrosis lung disease. J Immunol. 2013;190:1276–84. doi: 10.4049/jimmunol.1202144. [DOI] [PubMed] [Google Scholar]
- 55.Salamov AA, Solovyev VV. Ab initio gene finding in Drosophila genomic DNA. Genome Res. 2000;10:516–522. doi: 10.1101/gr.10.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.