Abstract
Human epididymis protein 4 (HE4) has been suggested as a useful new biomarker of lung cancer; however, few relevant large-scale studies have been published. In this study, we evaluated the utility of serum HE4 for lung cancer detection. HE4 levels were measured in serum samples from 100 lung cancer patients, 57 patients with benign lung diseases, and 274 healthy controls by using a chemiluminescent immunoassay, and variations in HE4 levels were analyzed by clinical status such as lung cancer, benign lung disease, and healthy condition, Tumor, Lymph Nodes, Metastasis (TNM) stage, tumor score, and histological cancer type. Lung cancer patients had significantly higher serum HE4 levels than patients with benign lung diseases and healthy controls (P<0.0001). The area under the ROC curve for HE4 was 0.84 (95% confidence interval, 0.78–0.89; P<0.0001) between lung cancer patients and healthy controls. Serum HE4 levels were significantly higher in patients with advanced disease (according to TNM stage) than in healthy controls (P<0.0001). HE4 levels were significantly elevated in patients with tumors of all types, those of different histological subgroups, and those with the smallest tumors (P=0.002). This report supports the potential of serum HE4 as an ancillary diagnostic marker for lung cancer detection.
Keywords: HE4, Lung cancer, Tumor marker
Lung cancer exhibits the highest mortality rate of all cancers [1]. As lung cancers do not exhibit specific early symptoms, it is difficult to use routine clinical procedures to screen for and diagnose early disease; early detection is crucial for improving survival. Several lung cancer-screening methods are currently available including annual low-dose computed tomography combined with an annual chest X ray and sputum cytology. However, current evidence does not support screening for lung cancer using these methods [2]. In contrast, cancer detection using a serum marker affords many advantages, including technical simplicity, a relatively lower cost, non invasiveness, no radiological damage, and the possibility of continuous monitoring. Several serum biomarkers, including carcinoembryonic antigen, serum cytokeratin 19 fragment, and progastrin-releasing peptide, are elevated in the serum lung cancer patients [3,4]. However, they are not recommended for the diagnosis of early-stage lung cancer because their sensitivities and specificities are relatively low.
Human epididymis protein 4 (HE4) is one of the most intensively studied novel biomarkers for early diagnosis and monitoring of ovarian cancer [5,6]. Abnormal HE4 immunoreactivity was first detected in tissue microarrays from lung cancer patients [7], and HE4 levels were closely associated with the occurrence, development, and prognosis of lung cancer [8]. We focused on HE4 levels, as opposed to other tumor markers, because it is important to identify new, sensitive, and specific biomarkers beneficial for cancer patient detection. There were several studies on the association of serum HE4 with lung cancer in patients who were confirmed pathologically as lung cancer and those who were diagnosed as benign lung disease. However, this is the first study of patients who have not identified the nature of lung mass or their respiratory symptoms. We compared whether HE4 could classify lung cancer and benign lung disease among patients suspected of having lung cancer and healthy control and lung cancer patients. In this way, we evaluated the clinical usefulness of serum HE4 levels for lung cancer detection.
The study adhered to all tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Korea (approval no. SCHBC 2015-07-014-004). All patients provided written informed consent. Because healthy control groups were retrospectively analyzed through the medical record review, consent acquisition about them was omitted.
Serum samples were collected at the time of diagnosis from 157 newly admitted patients with lung masses or solitary pulmonary nodules (100 cases of lung cancer and 57 cases of pathologically confirmed benign lung diseases [e.g., pneumonia and tuberculosis]) between August 1, 2015 and January 21, 2016. Thus, all samples were collected before any form of treatment was administered. Moreover, samples were collected from 274 healthy donors who visited the healthcare center of our hospital between July 5, 2015 and January 21, 2016. The gender distribution of each group was as follows: lung cancer, 80 men and 20 women; benign lung disease, 34 men and 23 women; and healthy controls, 81 men and 193 women. The median and range of age of each group was as follows; 60 yr (range 34–91 yr), 60 yr (40–87 yr), and 59 yr (30–88 yr), respectively.
Samples were held for 1 hr at room temperature and then sera were isolated by centrifugation (1,500g) and stored at −20℃. Tumors were classified by using the criteria of the 7th edition of Tumor, Lymph Nodes, Metastasis (TNM) Classification of Malignant Tumors [9]. Cancer histological types were determined according to the criteria of the WHO and the International Union against Cancer TNM staging system [10].
HE4 levels were measured by using an Abbott Architect i2000 analyzer running the Architect HE4 assay (Abbott Diagnostics, Chicago, IL, USA). This is a two-step immunoassay that quantitatively measures HE4 levels in human serum using chemiluminescent microparticle technology. During the first step, HE4 in a specimen binds to anti-HE4 coated microparticles. Following washing, an anti-HE4 acridinium-labeled conjugate is added to the reaction mixture. Following incubation, the microparticles are washed and a trigger solution is added. The resulting chemiluminescent reaction is measured in relative light units (RLUs). A direct relationship is evident between the HE4 level of a sample and the RLUs detected; HE4 level is defined as pmol/L. The analytical measurement range of HE4 in our institution has been established as 20–1,499 pmol/L and the coefficient of variation of total imprecision is 8.0%.
The study population was divided into lung cancer patients, patients with benign lung diseases, and healthy controls. The lung cancer group was categorized by TNM staging system, tumor (T) score, and histological type.
Because the serum HE4 levels in the populations were not normally distributed according to the Shapiro-Wilk test, non-parametric statistical analyses were used to analyze tumor marker distributions. Differences between two independent groups were compared by using the Mann-Whitney U test, and comparisons between more than two groups were conducted by employing the Kruskal-Wallis H test of variance. ROC curves were constructed, and the area under the ROC curve (AUC) with a 95% confidence interval (CI) was calculated. Sensitivity and specificity were calculated between lung cancer patients and patients with benign lung diseases or healthy controls, respectively. All statistical analyses were performed with Analyse-it (Analyse-it-software, Leeds, UK). P<0.05 was considered statistically significant.
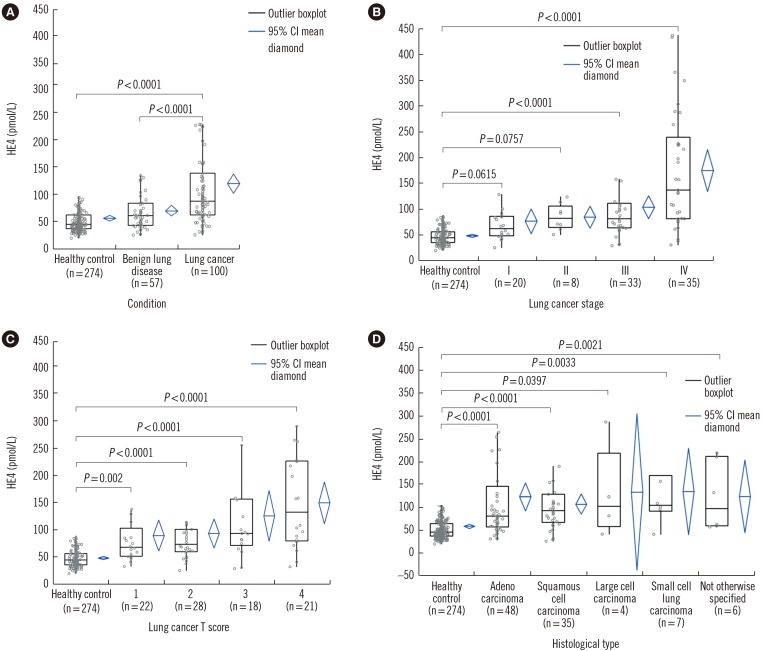
HE4 levels were significantly higher in patients with lung cancer (median 87.6 pmol/L, range 25.5–437.1 pmol/L; P<0.0001) and patients with benign lung diseases (60.4 pmol/L, range 4.9–177.5 pmol/L; P<0.0001) compared with healthy controls (44.6 pmol/L, range 20.0–381.9 pmol/L) (Fig. 1A).
Fig. 1. Distribution of human epididymis protein 4 (HE4) levels in different groups. (A) The serum HE4 levels of 100 lung cancer patients were significantly higher than those of 57 patients with benign lung diseases and 274 healthy controls (P<0.0001). (B) Serum HE4 levels were significantly higher in TNM stage 3 and 4 patients than in healthy controls (P<0.0001). (C) HE4 levels were significantly higher in patients with all tumor scores (T1, T2, T3, and T4) than in healthy controls. (D) HE4 levels were significantly higher in patients with any histological subtype of lung cancer than in healthy controls.
Abbreviation: CI, confidence interval.
The AUC for the discrimination of lung cancer patients from healthy controls was 0.84 (95% CI 0.78–0.89), significantly different from the non-discriminant bisector (z test: P<0.0001). The estimated sensitivity and specificity of HE4 level for lung cancer diagnosis at different cut-offs are shown in Table 1. The AUC value for the discrimination of lung cancer from benign lung disease was 0.70 (95% CI 0.62–0.78).
Table 1. Optimal cut-off values of HE4 for diagnosis between lung cancer and healthy control and benign lung disease (sensitivity, specificity, AUC, and 95% CI).
| AUC | 95% CI | Cut-off (pmol/L) | Sensitivity | Specificity | Youden's index J | SE | P | |
|---|---|---|---|---|---|---|---|---|
| Healthy control | 0.84 | 0.78–0.89 | 41.1 | 0.908 | 0.449 | 0.353 | 0.026 | < 0.0001 |
| 57.9 | 0.806 | 0.770 | 0.576 | |||||
| 74.4 | 0.633 | 0.908 | 0.541 | |||||
| Benign lung disease | 0.71 | 0.62–0.79 | 46.2 | 0.890 | 0.333 | 0.223 | 0.042 | < 0.0001 |
| 70.0 | 0.660 | 0.684 | 0.362 | |||||
| 134.3 | 0.260 | 0.930 | 0.207 |
Abbreviations: HE4, human epididymis protein 4; AUC, area under the curve; CI, confidence interval; SE, standard error of AUC.
When comparing HE4 levels in lung cancer patients according to TNM stages, HE4 was elevated in TNM stage 1 and 2 (not statistically significant), as well as in stage 3 and 4 (statistically significant) compared with healthy controls. The median values by histological type are detailed in Fig. 1B.
In terms of T scores, serum HE4 levels were significantly higher in lung cancer patients with any T score than in healthy controls. The median values by T score are detailed in Fig. 1C.
Significant differences in HE4 levels were evident comparing lung cancer patients (all histological types) with healthy controls. The median values by histological type are detailed in Fig. 1D.
We found that serum HE4 levels in patients with any histological type of lung cancer were significantly elevated than in healthy controls and patients with benign lung diseases. Moreover, even patients with T scores of 1 could be distinguished from healthy controls.
HE4 is a member of the whey-acidic-protein (WAP) domain family, which shares 50 well-conserved amino acids. Two WAP family genes encode leukocyte protease inhibitors: secretory leukocyte protease inhibitor (SLPI) and elafin [11,12]. SLPI and elafin are expressed in various carcinomas, including lung cancer; thus, these genes and proteins may play roles in cancer development or progression [13,14].
In this study, the serum HE4 levels in patients with T score 1 were significantly different from those in healthy controls, although the Stage 1 and 2 results were not statistically significant. This may be due to patients with low T scores having nodal involvement or distal metastasis. However, we hypothesize that serum HE4 can be used as an ancillary detection tool for small-size lung cancer tumors. Interestingly, the metastasis (M) scores showed a significant difference, while the node (N) scores did not, in contrast to the report of Liu et al [15]. Patients with any histological type of lung cancer exhibited higher serum HE4 levels than healthy controls. Small-cell lung cancer was associated with the highest median HE4 level, although the differences were not statistically significant. Nagy et al [16] reported that the highest HE4 levels were exhibited by patients with large-cell carcinomas but other histological types yielded similar values. However, a number of studies have found that non-small-cell lung cancer is associated with higher HE4 levels than small-cell lung cancer [15,17,18]. These discrepancies may be attributable to the fact that the small-cell lung cancer subgroups were comprised of fewer patients. Although no significant difference was detected among histological types, additional studies involving a greater number of small-cell lung cancer cases are needed to enable a better understanding of the relationship between HE4 levels and histological subtypes of lung cancer.
Our study has certain limitations. First, the work was performed at a single center, which may limit the generalizability of our findings. Moreover, relatively few patients with large-cell carcinoma, small-cell lung cancer, and early stage lung cancer were included. Second, several factors, including sex, age, menopausal status, glomerular filtration rate, caffeine consumption, and smoking, which influence serum HE4 levels, were not considered [19]. Third, evaluation of other lung cancer detection markers was not performed because they are not recommended for the diagnosis of early-stage lung cancer [3,4].
We found that serum HE4 was overexpressed in patients with any histological type of lung cancer compared with those with benign lung diseases and healthy controls, even when the tumor size was small (T score 1). Thus, serum HE4 testing may have potential as an ancillary lung cancer detection tool, thus improving clinical outcomes by facilitating timely treatment.
Acknowledgments
This work was supported by Abbott Diagnostics, Korea and the Soonchunhyang University Research Fund.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article are reported.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al., editors. SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute; [Updated on Apr 2015]. http://seer.cancer.gov/csr/1975_2012. [Google Scholar]
- 2.Manser R, Lethaby A, et al., editors. Screening for lung cancer. Cochrane database of systematic reviews 2013, Issue 6. Art. No.:CD001991. John Wiley & Sons, Ltd; 2013. pp. 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tas F, Aydiner A, Topuz E, Yasasever V, Karadeniz A, Saip P. Utility of the serum tumor markers: CYFRA 21.1, carcinoembryonic antigen (CEA), and squamous cell carcinoma antigen (SCC) in squamous cell lung cancer. J Exp Clin Cancer Res. 2000;19:477–481. [PubMed] [Google Scholar]
- 4.Brower V. Biomarker studies abound for early detection of lung cancer. J Natl Cancer Inst. 2009;101:11–13. doi: 10.1093/jnci/djn483. [DOI] [PubMed] [Google Scholar]
- 5.Hellström I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695–3700. [PubMed] [Google Scholar]
- 6.Kong SY, Han MH, Yoo HJ, Hwang JH, Lim MC, Seo SS, et al. Serum HE4 level is an independent prognostic factor in epithelial ovarian cancer. Ann Surg Oncol. 2012;19:1707–1712. doi: 10.1245/s10434-011-1943-5. [DOI] [PubMed] [Google Scholar]
- 7.Galgano MT, Hampton GM, Frierson HF., Jr Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006;19:847–853. doi: 10.1038/modpathol.3800612. [DOI] [PubMed] [Google Scholar]
- 8.Iwahori K, Suzuki H, Kishi Y, Fujii Y, Uehara R, Okamoto N, et al. Serum HE4 as a diagnostic and prognostic marker for lung cancer. Tumour Biol. 2012;33:1141–1149. doi: 10.1007/s13277-012-0356-9. [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Gospodarowicz MK, et al., editors. TNM classification of malignant tumors. 7th ed. Oxford, UK: Wiley-Blackwell; 2009. [Google Scholar]
- 10.Galateau-Salle F, Churg A, Roggli V, Travis WD World Health Organization Committee for Tumors of the Pleura. The 2015 World Health Organization classification of tumors of the pleura: Advances since the 2004 classification. J Thorac Oncol. 2016;11:142–154. doi: 10.1016/j.jtho.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Thompson RC, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986;83:6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiedow O, Schröder JM, Gregory H, Young JA, Christophers E. Elafin: an elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence. J Biol Chem. 1990;265:14791–14795. [PubMed] [Google Scholar]
- 13.Devoogdt N, Revets H, Ghassabeh GH, De Baetselier P. Secretory leukocyte protease inhibitor in cancer development. Ann N Y Acad Sci. 2004;1028:380–389. doi: 10.1196/annals.1322.044. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Zou Z, Maass N, Sager R. Differential expression of elafin in human normal mammary epithelial cells and carcinomas is regulated at the transcriptional level. Cancer Res. 1995;55:2537–2541. [PubMed] [Google Scholar]
- 15.Liu W, Yang J, Chi PD, Zheng X, Dai SQ, Chen H, et al. Evaluating the clinical significance of serum HE4 levels in lung cancer and pulmonary tuberculosis. Int J Tuberc Lung Dis. 2013;17:1346–1353. doi: 10.5588/ijtld.13.0058. [DOI] [PubMed] [Google Scholar]
- 16.Nagy B, Jr, Krasznai ZT, Balla H, Csobán M, Antal-Szalmás P, Hernádi Z, et al. Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann Clin Biochem. 2012;49:377–380. doi: 10.1258/acb.2011.011258. [DOI] [PubMed] [Google Scholar]
- 17.Tang QF, Zhou ZW, Ji HB, Pan WH, Sun MZ. Value of serum marker HE4 in pulmonary carcinoma diagnosis. Int J Clin Exp Med. 2015;8:19014–19021. [PMC free article] [PubMed] [Google Scholar]
- 18.Escudero JM, Auge JM, Filella X, Torne A, Pahisa J, Molina R. Comparison of serum human epididymis protein 4 with cancer antigen 125 as a tumor marker in patients with malignant and nonmalignant diseases. Clin Chem. 2011;57:1534–1544. doi: 10.1373/clinchem.2010.157073. [DOI] [PubMed] [Google Scholar]
- 19.Ferraro S, Braga F, Lanzoni M, Boracchi P, Biganzoli EM, Panteghini M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic review. J Clin Pathol. 2013;66:273–281. doi: 10.1136/jclinpath-2012-201031. [DOI] [PubMed] [Google Scholar]