Abstract
Objectives:
The reported 1- and 3-year overall survival rates after esophagectomy for stage I superficial esophageal squamous cell carcinoma (SESCC) are 95–97% and 86%, and those after definitive chemoradiotherapy (CRT) are 98% and 89%, respectively. This study was performed to elucidate the efficacy and safety of another treatment option for SESCC: endoscopic resection (ER) followed by CRT.
Methods:
We retrospectively reviewed the overall survival, recurrence, and grade ≥3 adverse events of consecutive patients who refused esophagectomy and underwent ER followed by CRT for SESCC from 1 January 2006 to 31 December 2012.
Results:
In total, 66 patients with SESCC underwent ER followed by CRT during the study period, and complete follow-up data were available for all patients. The median age was 67 (range, 45–82) years, and the median observation period was 51 (range, 7–103) months. Local and metastatic recurrences occurred in 2 (3%) and 6 (9%) patients, respectively, and 17 (26%) patients died. The 1-, 3-, and 5-year overall survival rates were 98%, 87%, and 75%, respectively. One of the 23 patients with mucosal cancer and 5 of 43 with submucosal cancer developed metastatic recurrences (P=0.65). Five of the 61 patients with negative vertical resection margin and 1 of 5 with positive vertical resection margin developed metastatic recurrences (P=0.39). None of the 30 patients without lymphovascular involvement developed metastatic recurrences; however, 6 of 36 patients with lymphovascular involvement developed metastatic recurrences (P=0.0098). Grade ≥3 adverse events occurred in 21 (32%) patients and all adverse events were associated with CRT, hematological adverse events in 13 (20%), and non-hematological adverse events in 9 (14%).
Conclusions:
ER followed by CRT provides survival comparable with that of esophagectomy or definitive CRT and has a low local recurrence rate. A particularly favorable outcome is expected for cancers without lymphovascular involvement.
Introduction
In Japan, >90% of esophageal malignancies are squamous cell carcinomas, and superficial carcinomas account for about 40% of all esophageal squamous cell carcinomas.1 Esophagectomy with lymph node dissection was historically the standard treatment for patients with superficial esophageal squamous cell carcinoma (SESCC). However, esophagectomy is highly invasive and associated with increased morbidity and mortality.2 Endoscopic resection (ER), such as endoscopic mucosal resection or endoscopic submucosal dissection, is a minimally invasive treatment with high curability for esophageal lesions. This treatment was recently established as the standard treatment for esophageal cancers confined to the mucosa without lymphovascular involvement,3 which are known to have a low risk of lymph node metastasis.1 However, ER is not indicated for submucosal cancers because such cancers have a relatively high risk of metastasis (8–64%).1, 4, 5, 6
Chemoradiotherapy (CRT), which is less invasive than esophagectomy, is an alternative treatment for submucosal cancers. Some studies have demonstrated that definitive CRT can eliminate cancers in the esophagus and surrounding lymph nodes.7, 8, 9 Although the overall survival after definitive CRT is comparable with that after esophagectomy9, 10 the main limitations of definitive CRT are local failure9, 10 and cardiopulmonary adverse events.11, 12 ER followed by CRT is another option for submucosal cancer. In this treatment, ER is conducted to completely remove or reduce the size of the submucosal cancer, thus reducing the risk of local recurrence. When esophageal cancer is completely removed by ER, CRT is conducted to prevent lymph node recurrence without boost radiotherapy to the primary field. The reduction in the radiation dose may obviate radiation-related adverse events. Although this treatment is theoretically associated with high local curability and fewer cardiopulmonary adverse events, limited data regarding its efficacy and safety are available.12, 13 The present study was performed to retrospectively evaluate the long-term outcome after ER followed by CRT for SESCC with a substantial risk of metastasis (i.e., submucosal cancer or cancer with lymphovascular involvement).
Methods
This retrospective study was performed at a referral cancer center in Japan. Consecutive patients who refused esophagectomy and instead underwent ER followed by CRT for SESCC at Osaka Medical Center for Cancer and Cardiovascular Diseases from 1 January 2006 to 31 December 2012 were included. Patients were excluded if any evidence of nodal involvement was detected using contrast-enhanced CT scan of the neck, chest, and abdomen before treatment. Written informed consent to conduct ER and CRT was obtained from all patients. The study protocol was approved by the institutional review board of Osaka Medical Center for Cancer and Cardiovascular Diseases.
Treatments
ER
ER was mainly performed with either endoscopic mucosal resection using a cap-fitted panendoscope 14 or endoscopic submucosal dissection (Figure 1). Endoscopic mucosal resection was used only for small lesions and endoscopic submucosal dissection was performed for larger lesions. Endoscopic mucosal resection was performed using a gastroscope (GIF-Q260J or GIF-Q240Z; Olympus Optical Co., Tokyo, Japan) with a disposable transparent attachment (D-206-04; Olympus) mounted on the tip of the endoscope. A specialized crescent-shaped snare (SD-221L-25 or SD-7P; Olympus) was used. Endoscopic submucosal dissection was performed using a gastroscope (GIF-Q260J or GIF-Q240Z; Olympus) with a disposable transparent attachment (D-201-11804; Olympus) mounted on the tip of the endoscope. Either a Hook Knife (Olympus) or a Flush Knife (Fuji Film Medical, Tokyo, Japan) was mainly used. Details of the ER procedures are described in previous reports.15, 16
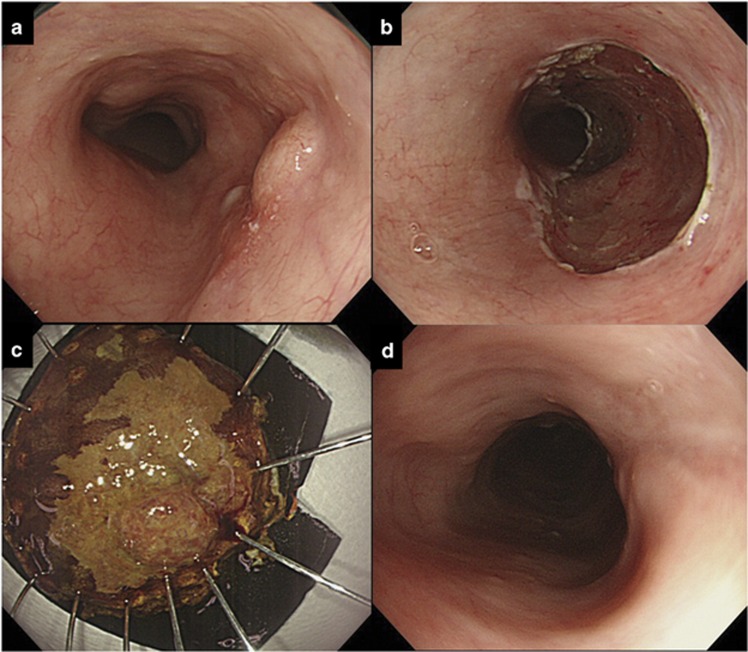
Figure 1.
Endoscopic resection for superficial esophageal squamous cell carcinoma (SESCC). (a) SESCC immediately before endoscopic submucosal dissection (ESD). (b) Resection wound immediately after ESD. (c) Resected specimen pinned flat to a hard Styrofoam plate. (d) Follow-up endoscopy performed to check the healing of an ESD-induced ulcer before chemoradiotherapy.
CRT
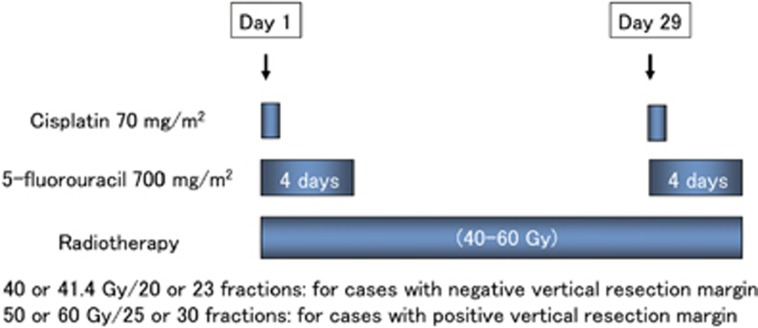
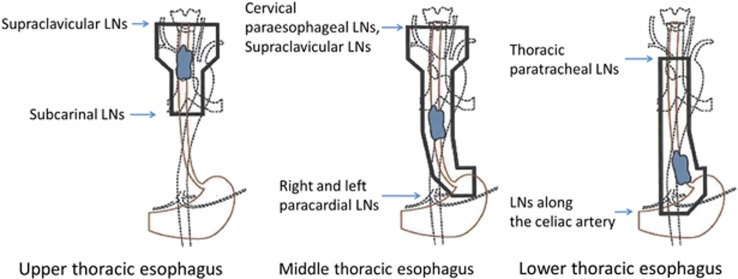
In principle, a follow-up endoscopy was routinely performed 1–2 months after ER. After confirming that ER-induced ulcers had been mostly replaced by scar tissue, chemoradiotherapy was started. The most frequently used treatment protocol consisted of cisplatin (70 mg/m2 as a rapid intravenous infusion after prehydration on days 1 and 29) and 5-fluorouracil (700 mg/m2 per 24 h in a 96-h continuous infusion on days 1–4 and 29–32) (Figure 2). Doses were reduced according to each patient’s creatinine clearance. Concurrent radiotherapy by external beam radiation using 10-MV X-rays was delivered at a dose of 2.0 or 1.8 Gy per day, 5 days a week. A total dose of 40.0 or 41.4 Gy was administered to the surrounding area of the esophagus in patients with a negative vertical resection margin to prevent lymph node recurrence, based on previous reports 17, 18 (Figures 2 and 3). In patients with a positive vertical resection margin, boost radiation was applied to the primary field (usually a boost dose of 10.0 or 9.0 Gy and a total dose of 50.0–50.4 Gy). The dose was prescribed to the isocenter in the middle of the planned target volume. The planned target volume was defined by adding 3-cm margins in a longitudinal direction and a 1-cm margin radially to each side of the primary esophageal tumor.
Figure 2.
Standard protocol of chemoradiotherapy for superficial esophageal squamous cell carcinoma in this study.
Figure 3.
Irradiation fields for thoracic esophageal squamous cell carcinoma according to tumor location. LNs, lymph nodes.
Follow-up
After ER followed by CRT, all patients were included in a follow-up program. As our study included many patients carrying high metastatic risk, a strict follow-up schedule was applied, especially during the 2-year period after CRT.19, 20 Blood tests, upper gastrointestinal endoscopy with iodine staining, and computed tomography of the neck, chest, and abdomen were usually performed every 4 months up to 2 years post-treatment and every 6 months thereafter. Follow-up patient data were obtained from the medical records. For patients who moved away from our hospital, we attempted to obtain outcome details by questionnaire or telephone conversation with the patient, patient’s family, or referring physician.
Measured outcomes and statistical analysis
All outcomes were evaluated based on the data from 1 January 2015. The primary outcome was overall survival. The secondary outcomes were local recurrence, metastatic recurrence, metastatic recurrence according to the lymphovascular involvement status, metachronous esophageal lesions, and grade ≥3 adverse events. A subgroup analysis was conducted to identify risk factors for metastatic recurrence. Adverse events were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 throughout the study period. All outcomes with the exception of adverse events and the subgroup analysis were evaluated using the Kaplan–Meier method, and the log-rank test was used to analyze the difference between two curves. The subgroup analysis was performed using the chi-square test or Fisher’s exact test for categorical outcomes. The computer software JMP version 8 (SAS Institute, Cary, NC, USA) was used for data analysis. A P value of <0.05 was considered statistically significant.
Results
Patients, lesions, and treatments
In total, 66 patients with SESCC were treated with ER followed by CRT during the study period and retrospectively analyzed. The patient and lesion characteristics and treatment details are shown in Table 1. The rate of complete follow-up was 100%, and the median observation period was 51 (range, 7–103) months. The median age was 67 (range, 45–82) years, 61 (92%) patients were male, and 13 (20%) patients had synchronous double cancers. Fifty-four (82%) lesions were removed by endoscopic submucosal dissection. No lesions had a positive lateral resection margin. Five (8%) lesions had a positive vertical resection margin. Forty-three (65%) lesions were pathological T1b or deeper invasive cancers, and 36 (55%) lesions had lymphovascular involvement. All of five lamina propria mucosae tumors had lymphovascular involvement. CRT was completed in all patients. With respect to radiotherapy, a total dose of 40.0 or 41.4 Gy was irradiated to the surrounding area of the esophagus in all patients, and additional boost radiation was applied in six patients (10.0 Gy in four patients and 20.0 Gy in two patients; five of these patients had a positive vertical resection margin and one had a lymph node for which the possibility of metastasis could not be ruled out). With respect to chemotherapy, the doses in the second cycle were reduced in 15 (23%) patients, and the second cycle was not administered to 4 (6%) patients because of adverse events that had occurred in the first cycle.
Table 1. Patient and lesion characteristics and treatment details.
| Complete follow-up rate: 100% | N=66 |
|---|---|
| Observation period, months | 51 (7–103) |
| Age, years | 67 (45–82) |
| Sex | |
| Male | 61 (92) |
| Female | 5 (8) |
| Synchronous double cancers | 13 (20) |
| Head and neck cancer | 8 (12) |
| Gastric cancer | 5 (8) |
| Others | 3 (5) |
| Tumor location | |
| Cervix | 3 (5) |
| Upper thorax | 12 (18) |
| Middle thorax | 35 (52) |
| Lower thorax | 15 (23) |
| Abdomen | 1 (2) |
| Endoscopic resection | |
| EMRC | 12 (18) |
| ESD | 54 (82) |
| Vertical resection margin | |
| Positive | 5 (8) |
| Negative | 61 (92) |
| Pathological tumor depth | |
| Lamina propria mucosae | 5 (8) |
| Muscularis mucosae | 18 (27) |
| SM1 | 8 (12) |
| SM2 or deeper | 35 (53) |
| Lymphovascular involvement | |
| Positive | 36 (55) |
| Negative | 30 (45) |
| Chemotherapy | |
| Standard-dose CF | 58 (88) |
| Low-dose CF | 6 (9) |
| Others | 2 (3) |
| Radiation dose, Gy | |
| 40.0 | 55 (83) |
| 41.4 | 5 (8) |
| 50.0 | 4 (6) |
| 60.0 | 2 (3) |
CF, cisplatin and 5-fluorouracil; EMRC, endoscopic esophageal mucosal resection using a cap-fitted panendoscope; ESD: endoscopic submucosal dissection; SM1, submucosa up to 0.2 mm from muscularis mucosae; SM2, submucosa deeper than 0.2 mm from muscularis mucosae.
Data are presented as median (range) or n (%).
Outcomes
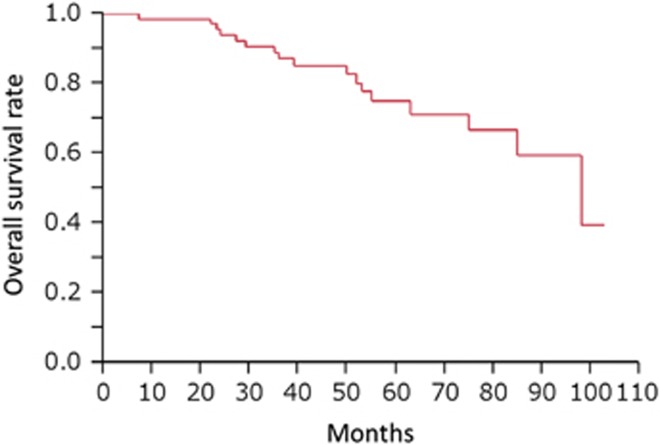
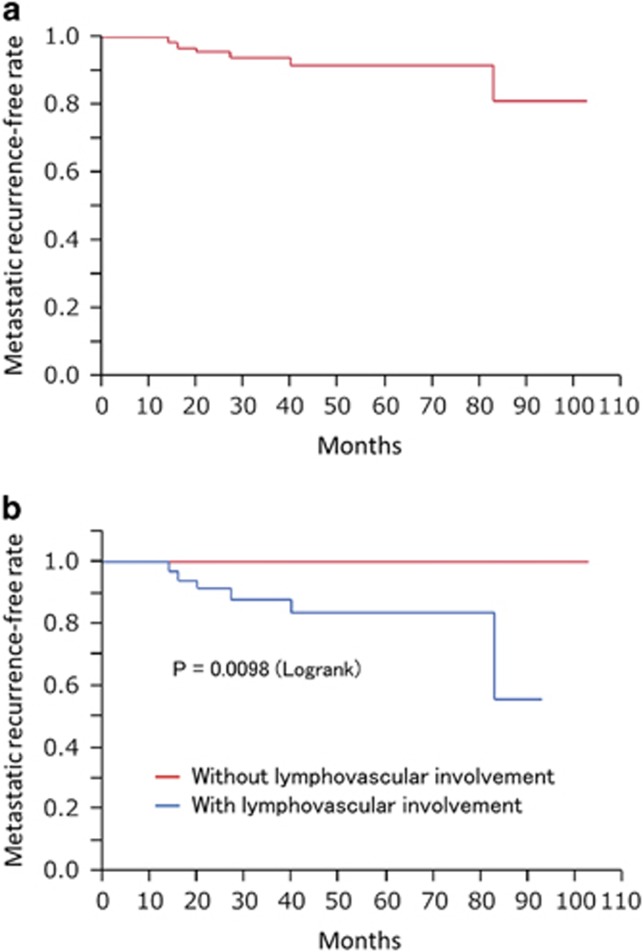
Two (3%) patients died of esophageal cancer and 15 (23%) patients died of other causes throughout the study period. The 1-, 2-, 3-, 4-, and 5-year overall survival rates were 98%, 94%, 87%, 85%, and 75%, respectively (Figure 4). Local recurrences occurred in two (3%) patients throughout the study period, and the 1-, 3-, and 5-year local recurrence rates were 0.0%, 1.5%, and 1.5%, respectively. Metastatic recurrences occurred in six (9%) patients throughout the study period. Hematological recurrence occurred in one patient and lymph node recurrences occurred in five patients. Metastatic recurrences occurred outside the irradiation field in three patients, inside in two patients, and on both sides in one patient. The 1-, 3-, and 5-year metastatic recurrence-free rates were 100%, 94%, and 92%, respectively (Figure 5a). Metachronous esophageal lesions occurred in nine (14%) patients throughout the study period, and the 1-, 3-, and 5-year incidences of metachronous esophageal lesions were 1.5%, 10.0%, and 12.0%, respectively. All metachronous esophageal lesions were successfully treated by endoscopic resection. Grade ≥3 adverse events occurred in 27 (41%) patients, and all adverse events were associated with CRT. Hematological toxicity occurred in 22 (33%) patients, non-hematological toxicity occurred in 9 (14%) patients, and 1 (1.5%) patient died potentially because of radiation pneumonitis after irradiation of 50.0 Gy (Table 2).
Figure 4.
Overall survival rate after endoscopic resection followed by chemoradiotherapy for superficial esophageal squamous cell carcinoma.
Figure 5.
Metastatic recurrence-free rate after endoscopic resection followed by chemoradiotherapy for superficial esophageal squamous cell carcinoma. (a) Metastatic recurrence-free rate in all patients. (b) Metastatic recurrence-free rate in patients with and without lymphovascular involvement.
Table 2. Details of grade ≥3 adverse events.
| Grade (NCI-CTCAE version 4) | 3 | 4 | 5 | Total |
|---|---|---|---|---|
| Hematological toxicities | 22 (33) | |||
| Leukopenia | 19 | 19 | ||
| Neutropenia | 14 | 2 | 16 | |
| Thrombocytopenia | 3 | 3 | ||
| Anemia | 2 | 2 | ||
| Non-hematological toxicities | 9 (14) | |||
| Anorexia | 7 | 7 | ||
| Nausea | 4 | 4 | ||
| Esophagitis | 2 | 2 | ||
| Gastric hemorrhage | 1 | 1 | ||
| Thromboembolic event | 1 | 1 | ||
| Radiation pneumonitis | 1 | 1 |
NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
Data are presented as n (%) or n.
Subgroup analysis of risk factors for metastatic recurrence
In the subgroup analysis, lymphovascular involvement was identified as a risk factor for metastatic recurrence (Table 3). None of the 30 patients without lymphovascular involvement had metastatic recurrence; however, 6 of 36 patients with lymphovascular involvement had metastatic recurrences (P=0.0098) (Figure 5b).
Table 3. Incidence of metastatic recurrence after endoscopic resection followed by chemoradiotherapy for each variable.
| Incidence of metastatic recurrence | P value | |
| Age, years | 0.20 | |
| ≤65 | 1/30 (3%) | |
| >65 | 5/36 (14%) | |
| Tumor location | 0.15 | |
| Ce+Ut | 2/15 (13%) | |
| Mt | 1/35 (3%) | |
| Lt+Ae | 3/16 (19%) | |
| Pathological tumor depth | 0.65 | |
| Mucosa | 1/23 (4%) | |
| Submucosa | 5/43 (12%) | |
| Vertical resection margin | 0.39 | |
| Positive | 1/5 (20%) | |
| Negative | 5/61 (8.2%) | |
| Lymphovascular involvement | 0.028 | |
| Positive | 6/36 (17%) | |
| Negative | 0/30 (0%) |
Ae, abdominal esophagus; Ce, cervical esophagus; Lt, lower thoracic esophagus; Mt, middle thoracic esophagus; Ut, upper thoracic esophagus.
Discussion
This retrospective study demonstrated that the 1-, 3-, and 5-year survival rates after ER followed by CRT were 98%, 87%, and 75%, respectively. Local and metastatic recurrences occurred in two (3%) and six (9%) patients, respectively. Grade ≥3 adverse events occurred in 21 (32%) patients, hematological adverse events in 13 (20%), and non-hematological adverse events in 9 (14%).
Esophagectomy is the standard treatment for stage I esophageal squamous cell carcinoma, and CRT is considered the best alternative treatment of esophagectomy. The reported 1- and 3-year overall survival rates after esophagectomy for stage I SESCC are 95–97% and 86%, respectively.9, 21 The reported 2- and 4-year overall survival rates after definitive CRT for stage I SESCC are 93% and 81%, respectively,8 and the reported 1- and 3-year overall survival rates are 98% and 89%, respectively.9 When we compare these data with the results of the present study, the 1-year survival rate of 98%, 2-year survival rate of 94%, 3-year survival rate of 87%, and 4-year survival rate of 85% after ER followed by CRT were comparable outcomes to esophagectomy or definitive CRT.
ER followed by CRT is mainly indicated for submucosal cancers or cancers with lymphovascular involvement because these factors are strong predictors of metastasis.4, 5 In such patients, ER is both a diagnostic and therapeutic tool. CRT can be conducted after confirming the histologic findings of a cancer and estimating the risk of metastasis by examination of the resected specimen. When the cancer has a low risk of metastasis (i.e., mucosal cancer without lymphovascular involvement), treatment can be completed without CRT.
ER followed by CRT was developed to enhance local curability and reduce the adverse events of CRT. Previous studies of definitive CRT showed local recurrence in 19–29% of patients.8, 12 Local recurrence may sometimes require esophagectomy, which is associated with high morbidity and mortality.22, 23, 24 In the present study, local recurrence developed in only two patients (3%), which is much fewer than after definitive CRT. Complete removal or the reduction in size of the esophageal cancer by ER probably contributed to the lower local recurrence after the treatment. Hematological and non-hematological adverse events of definitive CRT have been reported in 26% and 32% of patients, respectively.9 Hematological adverse events are mainly caused by chemotherapy, and non-hematological adverse events are mainly caused by radiotherapy. In the present study, the rate of hematological adverse events (33%) was similar to that of definitive CRT, and the rate of non-hematological adverse events (14%) was lower than that of definitive CRT. The lower radiation dose of ER followed by CRT may have inhibited the occurrence of non-hematological adverse events.
Two studies investigated the efficacy of ER followed by CRT for stage I esophageal squamous cell carcinoma. Both studies reported favorable outcomes after the treatment.12, 13 However, the number of patients in each study was only 16, which is too small to evaluate the efficacy and adverse events of the treatment. In addition, the follow-up periods were 39 and 43 months, respectively. Longer follow-up periods are required considering that the target of this treatment is early-stage cancer. Our study included 66 patients with a median observation period of 51 (range, 7–103) months. The complete follow-up rate of this study was high (100%) by an intensive survey. A larger number of patients and a longer complete follow-up period would increase the reliability of this study.
Predicting the response to ER followed by CRT is very important, especially when alternative treatments such as esophagectomy are available. Favorable outcomes of this treatment are expected for submucosal cancers without lymphovascular involvement as indicated by the lack of recurrence of such cancers in this study. However, metastatic recurrence was observed in 17% of cancers with lymphovascular involvement. Akutsu et al. 5 reported that the risk of metastasis in cancers invading the lamina propria mucosae or deeper with lymphovascular involvement (49%) is much higher than that in cancers invading the muscularis mucosae or deeper without lymphovascular involvement (15%). When we compare the rate of metastasis (49%) with the rate of recurrence (17%) after ER followed by CRT, CRT may have eliminated some of the metastasis. However, some technical modification is desired to improve the efficacy of ER followed by CRT for cancers with lymphovascular involvement.
The results of this study may be biased because it was a single-center retrospective study. To reduce bias, we included all patients who were treated during the study period and identified the outcome of all patients. Another limitation is the selection of patients. This study included 13 (20%) patients with synchronous double cancers and 6 (9%) patients aged >75 years. Although inclusion of such patients may underestimate the efficacy of ER followed by CRT, we can at least conclude that this treatment has efficacy comparable with that of esophagectomy or definitive CRT.
In conclusion, ER followed by CRT for SESCC has survival comparable with that of definitive CRT or esophagectomy. Especially favorable outcomes are expected for cancers without lymphovascular involvement.
Study Highlights
Footnotes
Guarantor of the article: Ryu Ishihara, MD.
Specific author contributions: Concept and design: K.H. and R.I.; analysis and interpretation of data: K.H., R.I., and Y.Y.; drafting of the article: K.H. and R.I.; critical revision of the article: R.I., H.I., and Y.K.; final approval of the article: K.H., R.I., Y.Y., N.H., S.Y., M.A., S.S., T.I., M.K., Y.T., S.S., N.M., H.N., T.K., T.A., Y.T., K.H., N.U., H.I., N.K., T.H., Y.K., K.K., and T.T.
Financial support: None.
Potential competing interests: None.
References
- Takubo K, Aida J, Sawabe M et al. Early squamous cell carcinoma of the oesophagus: the Japanese viewpoint. Histopathology 2007; 51: 733–742. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Kinugasa S, Yoshimura H et al. Clinical outcomes of extended esophagectomy with three-field lymph node dissection for esophageal squamous cell carcinoma. Am J Surg 2005; 189: 98–109. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Takahashi M, Yoshida T et al. Endoscopic resection (endoscopic mucosal resection/ endoscopic submucosal dissection) for superficial esophageal squamous cell carcinoma: current status of various techniques. Dig Endosc 2013; 25 (Suppl 1): 13–19. [DOI] [PubMed] [Google Scholar]
- Yamashina T, Ishihara R, Nagai K et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol 2013; 108: 544–551. [DOI] [PubMed] [Google Scholar]
- Akutsu Y, Uesato M, Shuto K et al. The overall prevalence of metastasis in T1 esophageal squamous cell carcinoma: a retrospective analysis of 295 patients. Ann Surg 2013; 257: 1032–1038. [DOI] [PubMed] [Google Scholar]
- Li B, Chen H, Xiang J et al. Prevalence of lymph node metastases in superficial esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2013; 146: 1198–1203. [DOI] [PubMed] [Google Scholar]
- Bidoli P, Bajetta E, Stani SC et al. Ten-year survival with chemotherapy and radiotherapy in patients with squamous cell carcinoma of the esophagus. Cancer 2002; 94: 352–361. [DOI] [PubMed] [Google Scholar]
- Kato H, Sato A, Fukuda H et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn J Clin Oncol 2009; 39: 638–643. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Ishihara R, Motoori M et al. Comparison between definitive chemoradiotherapy and esophagectomy in patients with clinical stage I esophageal squamous cell carcinoma. Am J Gastroenterol 2011; 106: 1048–1054. [DOI] [PubMed] [Google Scholar]
- Motoori M, Yano M, Ishihara R et al. Comparison between radical esophagectomy and definitive chemoradiotherapy in patients with clinical T1bN0M0 esophageal cancer. Ann Surg Oncol 2012; 19: 2135–2141. [DOI] [PubMed] [Google Scholar]
- Ishikura S, Nihei K, Ohtsu A et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol 2003; 21: 2697–2702. [DOI] [PubMed] [Google Scholar]
- Kawaguchi G, Sasamoto R, Abe E et al. The effectiveness of endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer. Radiat Oncol 2015; 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Kato M, Yamamoto J et al. EMR combined with chemoradiotherapy: a novel treatment for superficial esophageal squamous-cell carcinoma. Gastrointest Endosc 2004; 59: 199–204. [DOI] [PubMed] [Google Scholar]
- Inoue H, Endo M, Takeshita K et al. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC). Surg Endosc 1992; 6: 264–265. [DOI] [PubMed] [Google Scholar]
- Ishihara R, Iishi H, Uedo N et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008; 68: 1066–1072. [DOI] [PubMed] [Google Scholar]
- Kanzaki H, Ishihara R, Ohta T et al. Randomized study of two endo-knives for endoscopic submucosal dissection of esophageal cancer. Am J Gastroenterol 2013; 108: 1293–1298. [DOI] [PubMed] [Google Scholar]
- Kato K, Muro K, Minashi K et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys 2011; 81: 684–690. [DOI] [PubMed] [Google Scholar]
- Onozawa M, Nihei K, Ishikura S et al. Elective nodal irradiation (ENI) in definitive chemoradiotherapy (CRT) for squamous cell carcinoma of the thoracic esophagus. Radiother Oncol 2009; 92: 266–269. [DOI] [PubMed] [Google Scholar]
- The Japan Esophageal Society. Esophageal cancer diagnosis and treatment guideline [in Japanese] 3rd edn. Kanehara-Shuppan: Tokyo, 2012. [Google Scholar]
- Ono S, Fujishiro M, Niimi K et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70: 860–866. [DOI] [PubMed] [Google Scholar]
- Igaki H, Kato H, Tachimori Y et al. Clinicopathologic characteristics and survival of patients with clinical Stage I squamous cell carcinomas of the thoracic esophagus treated with three-field lymph node dissection. Eur J Cardiothorac Surg 2001; 20: 1089–1094. [DOI] [PubMed] [Google Scholar]
- Tachimori Y, Kanamori N, Uemura N et al. Salvage esophagectomy after high-dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2009; 137: 49–54. [DOI] [PubMed] [Google Scholar]
- Miyata H, Yamasaki M, Takiguchi S et al. Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol 2009; 100: 442–446. [DOI] [PubMed] [Google Scholar]
- Morita M, Kumashiro R, Hisamatsu Y et al. Clinical significance of salvage esophagectomy for remnant or recurrent cancer following definitive chemoradiotherapy. J Gastroenterol 2011; 46: 1284–1291. [DOI] [PubMed] [Google Scholar]