Abstract
Celiac disease is an autoimmune disorder of the small bowel, classically associated with diarrhea, abdominal pain, and malabsorption. The diagnosis of celiac disease is made when there are compatible clinical features, supportive serologic markers, representative histology from the small bowel, and response to a gluten-free diet. Histologic findings associated with celiac disease include intraepithelial lymphocytosis, crypt hyperplasia, villous atrophy, and a chronic inflammatory cell infiltrate in the lamina propria. It is important to recognize and diagnose celiac disease, as strict adherence to a gluten-free diet can lead to resolution of clinical and histologic manifestations of the disease. However, many other entities can present with clinical and/or histologic features of celiac disease. In this review article, we highlight key clinical and histologic mimickers of celiac disease. The evaluation of a patient with serologically negative enteropathy necessitates a carefully elicited history and detailed review by a pathologist. Medications can mimic celiac disease and should be considered in all patients with a serologically negative enteropathy. Many mimickers of celiac disease have clues to the underlying diagnosis, and many have a targeted therapy. It is necessary to provide patients with a correct diagnosis rather than subject them to a lifetime of an unnecessary gluten-free diet.
Celiac disease
Celiac disease (CD) is an autoimmune condition characterized by sensitivity to gluten, a protein found in wheat, barley, and rye.1 In the United States, 0.71% of the population has CD, with highest prevalence in whites and females.2 Although 0.63% of the American population follows a gluten-free diet, the majority of these individuals do not have CD.2 In fact, most cases of CD are undiagnosed.2 The prevalence of CD is increasing in developing countries.3
A combination of immune, genetic, and environmental factors play a role in the development of the disease. In CD, gliadin, a component of gluten, reaches the small intestine and triggers an inflammatory response characterized by villous atrophy and inflammatory cell infiltration of the epithelium and lamina propria.1 In patients that develop CD, human leukocyte antigen (HLA)-DQ2 (~95% of cases) or HLA-DQ8 (~5% of cases) genetic alleles must be present; however, these alleles are also present in 30–40% of the general population.1 Environmental factors such as breastfeeding may be protective, while rotavirus infection may promote disease development.4, 5
Clinical features of CD vary by age group. Young children may manifest with diarrhea and abdominal distention; older children can present with short stature, neurological symptoms, and anemia; adults classically have diarrhea and abdominal pain, but may present with extra-intestinal features, such as iron-deficiency anemia and premature metabolic bone disease.1 Diarrhea is becoming less common as a presenting feature, and the diagnosis is being increasingly made in asymptomatic or at-risk populations.6 The diagnosis of CD is made using a combination of clinical, serologic, and histologic findings. Serological testing is often performed in patients with unexplained abdominal discomfort, chronic diarrhea, laboratory test abnormalities (vitamin or mineral deficiencies), premature metabolic bone disease, family history of CD, or a personal history of other autoimmune disorders.1 The initial screening test for CD is immunoglobulin A (IgA) anti-tissue transglutaminase (TTG) antibody, except in patients with known or suspected IgA deficiency, in which case an IgG-based serology is required.3 Upper endoscopy with duodenal biopsies remains the gold standard for diagnosis, and should be performed in all adult patients when serological testing is positive, and in patients with strong features of CD despite negative serologies.1 Duodenal biopsies classically demonstrate intraepithelial lymphocytosis, crypt hyperplasia, villous atrophy, and a chronic inflammatory cell infiltrate in the lamina propria (Figure 1a).1 Patients with CD should exhibit resolution of symptoms when started on a gluten-free diet.1 As outlined above, the absence of HLA-DQ2 and HLA-DQ8 alleles effectively rules out the disease.
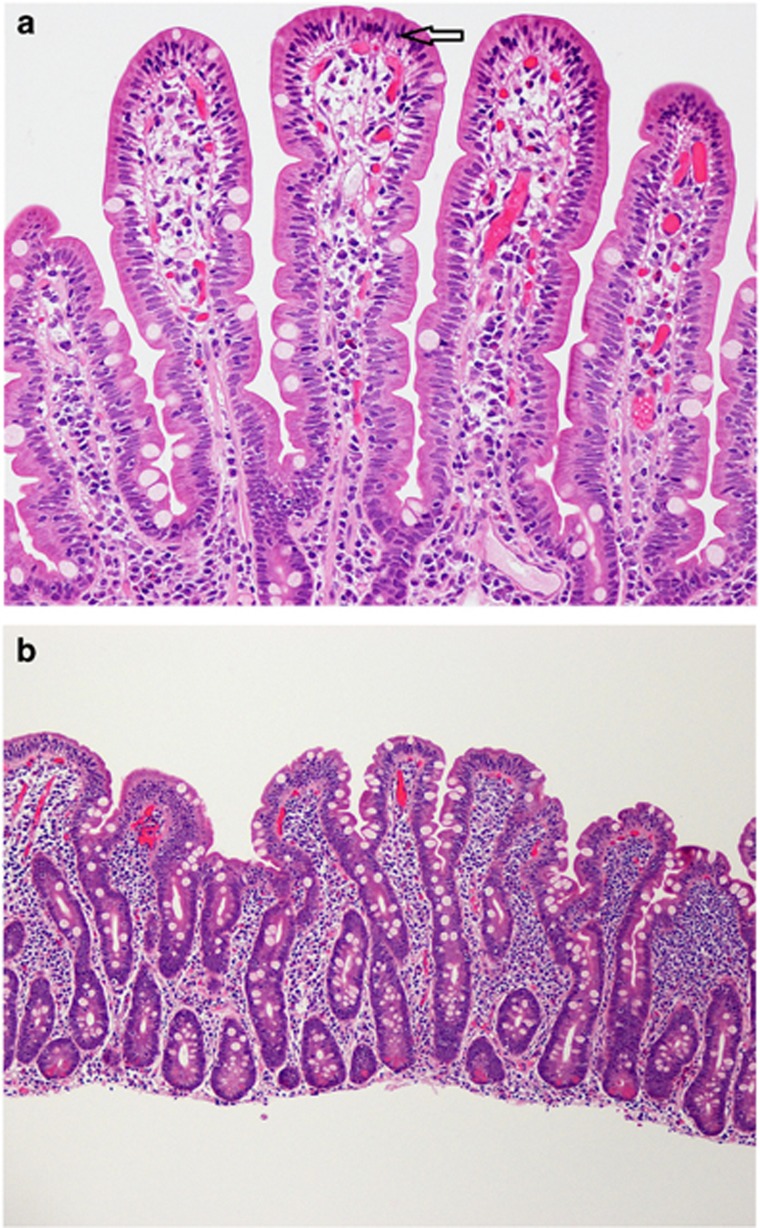
Figure 1.
(a and b) Histologic features associated with celiac disease. Histologic features associated with: (a) an early phase of celiac disease, characterized by a tip-predominant intraepithelial lymphocytosis alone (see arrow); and (b) a later phase of celiac disease, characterized by intraepithelial lymphocytosis, crypt hyperplasia, villous atrophy (partial in this case with a villous:crypt ratio of 1:2), and a chronic inflammatory cell infiltrate in the lamina propria. (a at 200 ×, b 100 × ; Haemotoxylin and Eosin stain).
A gluten-free diet forms the cornerstone of management in patients with CD, with elimination of wheat, barley, and rye.1 Oats are generally safe in patients with CD, although there may be a small risk of oat cross-contamination or contact with gluten-containing food products; most patients with CD are allowed to ingest oat products unless they present with severe clinical features, in which case oats may be held initially. After a new diagnosis of CD is made, all patients should be referred to a skilled dietician for counseling, and testing should be performed to assess for vitamin and mineral deficiencies.1 Bone densitometry should also be considered in adult patients. Clinical improvement should occur soon after implementation of a gluten-free diet, while histologic response may take months to years.1 In patients that fail to improve on a gluten-free diet, the diagnosis of CD should be verified, dietary compliance should assessed, and histologic mimickers considered, particularly in patients with negative celiac serologies at the time of the diagnosis.1 Complications of long-standing CD include small intestinal adenocarcinoma,7 enteropathy-associated T-cell lymphoma,8 and refractory celiac sprue.9
Clinical mimickers of cd
CD is known for its protean manifestations, and while CD classically has been associated with diarrhea, bloating, and weight loss, which can be seen in many gastrointestinal conditions, the extra-intestinal features of CD broadens the number of conditions that may clinically mimic CD.
Irritable bowel syndrome (IBS) is the most commonly diagnosed gastrointestinal disorder, and has features that mimic CD.10 Symptoms include abdominal pain along with altered bowel form and/or frequency.10 IBS is often associated with other disorders including somatic comorbidities.11, 12, 13, 14 The ROME IV criteria is often used to diagnose IBS and requires recurrent abdominal pain on average at least one day per week for the last 3 months associated with two or more of the following: related to defecation, change in frequency of stool, and change in form or consistency of stool.15 Symptoms need to be present for at least 6 months.15 Before a diagnosis of IBS is made, alarm features need to be assessed, and consideration should be given to test for CD in those with a diarrhea-predominant or mixed-type pattern. Functional diarrhea is similar to IBS, yet without abdominal pain, and may also clinically mimic CD.16
Small intestinal bacterial overgrowth (SIBO) is known to cause diarrhea, bloating, and weight loss, which may mirror symptoms of classic CD; SIBO may also be a cause of recurrent or refractory symptoms in a patient with known CD.17 Patients may have carbohydrate, protein, or fat malabsorption, and deficiencies in iron, vitamin B12, and fat-soluble vitamins,17 which may occur, in part, due to intraluminal damage from proliferating bacteria.18 In healthy individuals, several physiological mechanisms exist that serve to limit bacterial growth17 and SIBO occurs when these protective mechanisms fail.18 While no universal definition of SIBO exists, the gold standard remains a small bowel aspirate demonstrating 105 or more colony forming units per milliliter of bacteria grown.19 The treatment of SIBO consists of managing any underlying diseases, correcting nutritional deficiencies, and use of antibiotics.17
Autoimmune and/or inflammatory conditions such as inflammatory bowel disease (IBD), microscopic colitis, thyroid dysregulation, and adrenal insufficiency may all cause clinical features that mimic CD, or be concurrently present in patient known to have CD. IBD occurs when the intestinal microbiome triggers an inappropriate inflammatory response, and while there is a genetic susceptibility to IBD, the pathogenesis is multifaceted.20 While individuals can be affected at any point in life, the disease typically occurs in those 15 to 30 years of age.20 Although Crohn’s disease can affect any part of the gastrointestinal tract, it most commonly involves the terminal ileum.20 Given the small bowel involvement with Crohn’s disease, clinical features may mimic CD with abdominal pain, diarrhea, weight loss, and features of malabsorption. The treatment for Crohn’s disease is complex and individualized, and involves lifestyle modifications (smoking discontinuation, if applicable, and NSAID avoidance), medical management with varying degrees of immunomodulatory or immunosuppression therapy, and occasionally surgical intervention.20, 21
Infectious mimickers include giardiasis and both viral and bacterial gastroenteritis, although most viral and bacterial infections are self-limited and do not cause the chronic symptoms that can be seen with Giardia infection, unless post-infectious IBS ensues. Other chronic parasitic infections may also cause symptoms that mimic CD. Other less common clinical mimickers include tropical sprue, autoimmune enteropathy, drug-induced enteropathy, Whipple’s disease, and others that will be discussed in more detail below.
While there are some patients who have celiac-like symptomatology, and others who have celiac-like histology on small bowel biopsies, some conditions can mimic both clinical and histologic features of CD, leading to diagnostic challenges in this group of patients. Given the large number of clinical celiac mimickers, the remainder of the review will focus on the histologic mimickers of CD.
Histologic mimickers of cd
The characteristic histologic features of CD include partial or total villous atrophy, crypt hyperplasia, increased tip-predominant intraepithelial lymphocytes (IELs), lymphocyte and plasma cell infiltrate in lamina propria, and normal CD3+/CD8+ infiltrates. The modified Marsh-Oberhuber classification (Table 1) can be used to classify the histologic features associated with CD.22 In this scheme, IELs, crypt architecture, and the degree of villous blunting are used to categorize lesions into types 0–4.22 Type 3 and higher is associated with increased IELs, hyperplastic crypts, and villous blunting, and is more often seen in symptomatic CD. Histologic features such as mucosal erosions, neutrophilic infiltrates, non-tip-predominant IELs, loss of goblet or plasma cells, crypt abscesses, and loss of CD8 expression are uncharacteristic of CD.
Table 1. Modified Marsh-Oberhuber classification for diagnosis of celiac disease.
| Type | Intraepithelial lymphocytes | Crypts | Villous blunting |
|---|---|---|---|
| 0 | Normal | Normal | None |
| 1 | Increased | Normal | None |
| 2 | Increased | Hyperplastic | None |
| 3a | Increased | Hyperplastic | Mild |
| 3b | Increased | Hyperplastic | Moderate |
| 3c | Increased | Hyperplastic | Severe (flat) |
| 4 | Increased | Atrophic | Severe (flat) |
Early histologic mimickers
Early histologic mimickers of CD can be defined as those conditions causing increased IELs with no villous atrophy, and crypts that are normal or have minimal hyperplasia (Figure 2a, Table 2). Prior to 2005, the “normal” number of IELs in the small bowel was defined as 6–40 lymphocytes per 100 epithelial cells.23 However, recent studies highlight that the upper limit of normal for lymphocyte count from duodenal biopsies is 25 per 100 epithelial cells, and from 2005 onward, many pathologists define greater than 25–30 lymphocytes per 100 epithelial cells as abnormal.24, 25 The difference in the upper limit of normal IELs over time may be secondary to the use of jejunal biopsies in older studies, and the fact that some studies have based counts on anti-CD-3 staining, which is not used routinely in clinical practice.
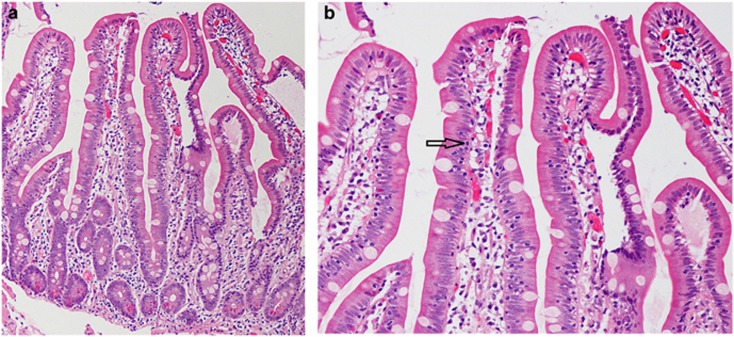
Figure 2.
(a and b) Histologic features associated with early mimickers of celiac disease. Histologic features associated with early mimickers of celiac disease (in this case, NSAID use). Early histologic mimickers demonstrate increased intraepithelial lymphocytes, often in a non-tip-predominant pattern (see arrow), no villous atrophy, and crypts that are either normal or have minimal hyperplasia (a at 100 ×, b at 200 × ; Haemotoxylin and Eosin stain).
Table 2. Early and late histologic mimickers of celiac disease.
| Early histologic mimickersa | Late histologic mimickersb |
|---|---|
| Non-steroidal anti-inflammatory drugs | Medications (olmesartan, ipilimumab, colchicine, mycophenolate mofetil, methotrexate, and azathioprine) |
| Inflammatory bowel disease | Common variable immunodeficiency |
| Small intestine bacterial overgrowth | Giardia |
| Helicobacter pylori | Small intestine bacterial overgrowth |
| Self-limited gastroenteritis | Crohn’s disease |
| Autoimmune conditions | Autoimmune enteropathy |
| Unexplained | Collagenous sprue |
| Tropical sprue | |
| Whipple’s disease | |
| Enteropathy-associated T-cell lymphoma | |
| CD4+ T-cell lymphoma | |
| Unclassified sprue |
Early histologic mimickers are characterized by increased intraepithelial lymphocytes with no villous atrophy, and crypts that are either normal or have minimal hyperplasia.
Late histologic mimickers are characterized by increased intraepithelial lymphocytes, partial or total villous atrophy, crypt hyperplasia, and chronic inflammation in the lamina propria.
The histologic finding of isolated IELs (also referred to as lymphocytic duodenitis) has been associated with a number clinical conditions,26, 27, 28, 29 and is becoming increasingly noted. In one large single-center study, an analysis of 15,839 duodenal biopsies from 2000 to 2010 in adult patients revealed that 1,105 (7%) cases reported the finding of isolated IELs with normal architecture.28 While the finding of isolated IELs was present in 3% of cases in 2000, it was noted in nearly 11% of cases by 2010.28 Moreover, during this time period, the odds of a CD diagnosis decreased by 0.9 annually, while the odds of a non-CD diagnosis increased by 1.1 annually.28 CD was found in only 6.8% of patients with isolated increased IELs in the duodenum and normal villi architecture, when patients with known CD were excluded.28 In addition to this large study, three other smaller studies (N=14–100 patients) similarly reported the etiologies for isolated IELs in the duodenum to be from known or newly diagnosed CD (9–21%) or IBD (2–12%), non-steroidal anti-inflammatory drug (NSAID) use (14–21%), SIBO (5–9%), autoimmune conditions (4–14%), or “other” (9–43%), with 7–34% of cases of the IELs being unexplained.26, 27, 29
Increased lymphocytic duodenitis has also been reported in children. The etiologies for this histologic finding in children do not differ significantly from those found in adults, with CD, IBD, Helicobacter pylori (H. pylori), NSAID use, and autoimmune conditions being reported.30 However, approximately 40% of children have no apparent explanation for the increased IELs.30
Non-steroidal anti-inflammatory drugs
NSAID use has been shown to cause duodenal histopathology nearly identical to what is found in early CD. The histopathology will typically resolve with drug discontinuation, but can recur with re-introduction of the drug.31, 32 Possible etiologies for these findings are may be due to the direct toxic effects of NSAIDs or their metabolites, which are excreted in bile, or due to a hypersensitivity reaction.32 The most susceptible region to the effects of NSAIDs is the duodenal bulb and the presence of a neutrophilic infiltration in the lamina propria may be a potential clue to NSAID use. However, these histologic findings are non-specific, and may also be seen in those with H. pylori infection, which may also lead to chronic active gastritis on gastric biopsies.
In a large retrospective study, nearly 14% of patients with isolated IELs in the duodenum reported NSAID use, a percentage that may be inaccurately low due to underreporting by patients.28 Other studies reported NSAID use in over 20% of those with duodenal IELs.29 In addition, a number of those with documented CD, SIBO, H. pylori, IBD, and microscopic colitis were concurrently using NSAIDs, making it challenging to know the etiology of the histologic finding given the overlap in risk factors or exposures.28
Inflammatory bowel disease
IBD has also been associated with lymphocytic duodenitis. Histologic clues to IBD in those with lymphocytic duodenitis can include endoscopic erosions, neutrophilic infiltration, crypt abscesses, edema, and granulomas, which are rarely seen. A non-tip predominance of IELs has also been reported.
In a study that reviewed all duodenal biopsies over a 10-year period, IBD was present in 74 patients who had increased IELs and normal architecture, making up 7.2% of patients with this histologic finding.33 Among these patients, 13 had ulcerative colitis (UC), 54 had Crohn’s disease, and 3 had indeterminate-type IBD.33 The mean age of adult patients with UC and Crohn’s disease at the time of this histologic finding was 40 and 39 years, respectively,33 while mean duodenal IEL count for in UC and Crohn’s disease was 45 and 44 IELs per 100 epithelial cells, respectively.33 The IELs were evenly distributed along edges of the villi in the majority with IBD (n=62), and only three patients had a tip-predominant pattern of IELs.33 All three of the latter patients were negative for CD. Of the 54 patients with Crohn’s disease, gastric biopsies were obtained in 34 patients, and nearly half (n=16) had focal lymphocytic gastritis.33
Late histologic mimickers
Late histologic mimickers of CD are characterized by having duodenal biopsies that demonstrate increased IELs, partial or total villous atrophy, and crypt hyperplasia (Figure 3a,Table 2). Given the more advanced enteropathy compared to early histologic mimickers, clinical features are more likely to be present. While the differential diagnosis becomes narrower with progressive degrees of enteropathy, the etiology remains elusive in a large number of patients with this finding.
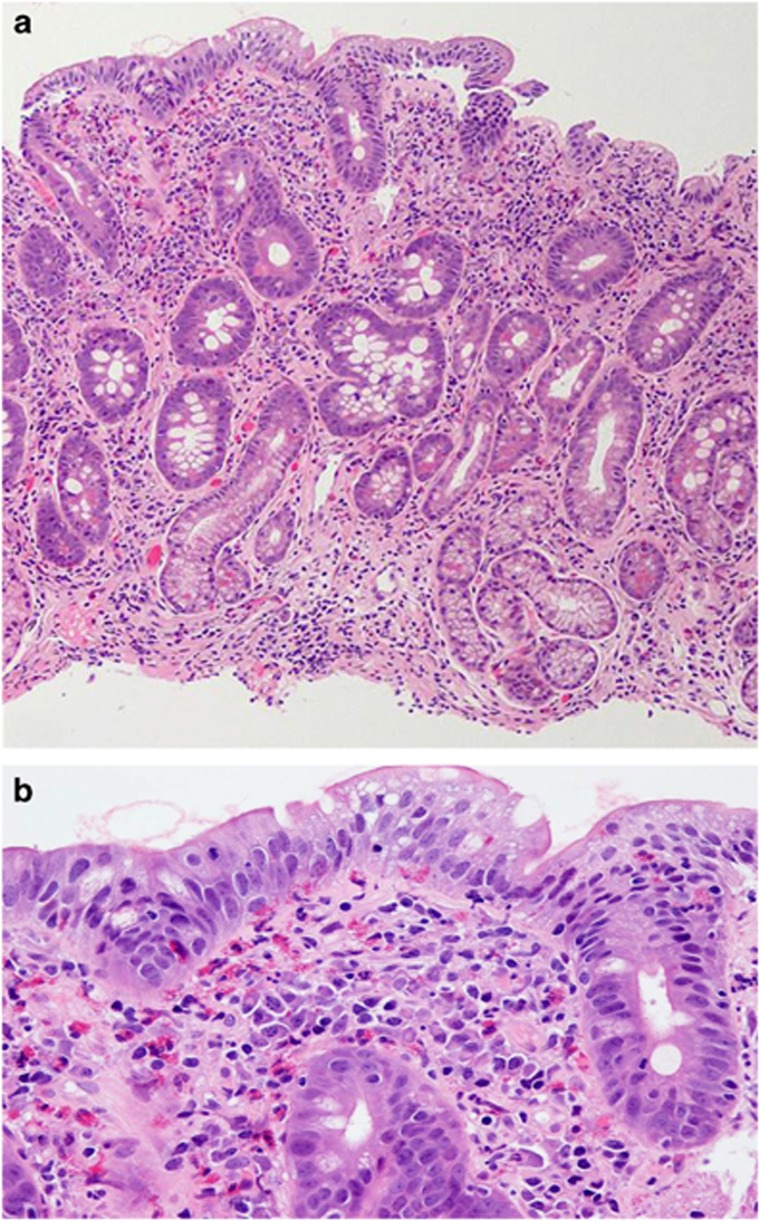
Figure 3.
(a and b) Histologic features associated with late mimickers of celiac disease. Histologic features associated with late mimickers of celiac disease (in this case, drug-induced from olmesartan). Late histologic mimickers are characterized by increased intraepithelial lymphocytosis, partial or total villous atrophy, crypt hyperplasia, and chronic inflammation in the lamina propria. This figure also demonstrates a prominent collagen band that can be seen in cases of drug-induced enteropathy (a at 100 ×, b at 200 × ; Haemotoxylin and Eosin stain).
In one study, adults with villous atrophy in the duodenum and negative CD serologies were recorded over a 10-year period.34 A total of 72 patients were identified who subsequently underwent a comprehensive testing, including HLA haplotyping, anti-enterocyte antibodies, Giardia stool antigen, human immunodeficiency virus testing, immunoglobulin levels, breath testing for SIBO, T-cell gene rearrangement studies, and detailed travel and medication review.34 Etiologies for the enteropathy were as follows: seronegative CD in 28% medication-related villous atrophy in 26% “unclassified sprue” in 14% common variable immunodeficiency (CVID) in 16% autoimmune enteropathy, giardiasis, tropical sprue, CD4+ small intestinal T-cell lymphoma, and SIBO each in 4% collagenous sprue in 3% and enteropathy-associated T-cell lymphoma, Crohn’s disease, and extensive gastric metaplasia each in 1%.34 Seronegative CD was defined as patients with negative TTG, deamidated gliadin peptide, and antiendomysial antibodies, positive HLA-DQ2 or DQ8, histology consistent with CD, clinical and/or histologic response to a gluten-free diet, and negative testing for other entities.34 Among 19 patients with medication-related villous atrophy, 16 were later linked to olmesartan, two to mycophenolate mofetil, and one to methotrexate.34
Olmesartan
Olmesartan medoxomil is the prodrug of olmesartan, an angiotensin II receptor blocker, used to treat hypertension. This drug was approved in the United States in 2002 and in Europe in 2003. Twenty-two patients referred to a tertiary care center for chronic diarrhea and/or weight loss who were found to have a serologically negative enteropathy were noted to be taking olmesartan.35 Intestinal biopsies showed villous atrophy in all 22 patients, while 15 of the 22 additionally had acute neutrophilic inflammation and 7 had the presence of excess collagen deposition.35 Discontinuation of olmesartan led to clinical response in all patients, with an average weight gain of 12.2 kg after drug withdrawal, and histologic recovery in all patients that underwent follow-up biopsies.35 Consequently, the US Food and Drug Administration released a warning in July 2013 that olmesartan can cause a sprue-like enteropathy.36
Subsequently, two separate studies, one in French and one in Spanish populations, demonstrated olmesartan-associated enteropathy in 36 and 11 patients, respectively.37, 38 Among these three studies, some interesting observations were noted. The mean age for patients with olmesartan-induced enteropathy ranged from 70–72 years. The patients were on a mean dose of 40 mg daily and had been taking the drug for a mean of 2.3–3.1 years before the diagnosis was made.35, 37, 38 Diarrhea was universal in all patients while abdominal pain was present in 45–75%, and profound weight loss was noted in many. Positivity for HLA-DQ2 or DQ8 was noted in 61–100% of those tested, a percentage much higher than found in the general population.35, 37, 38 In the previously described study with 16 cases of olmesartan-induced enteropathy, 11 had prominent collagen deposition.34 Therefore, older age at onset, prominent collagen deposition, and neutrophilic infiltration in a patient with a serologically negative enteropathy should always prompt a careful medication review, even if the drug was started years previously; the presence of permissive HLA haplotyping may confer increased risk in those patients taking olmesartan.34
Other medications
Ipilimumab is a humanized monoclonal antibody against cytotoxic T-lymphocyte antigen 4 used to treat metastatic melanoma and advanced prostate cancer that has been associated with features of CD, autoimmune enteropathy, and IBD.39, 40 Colchicine is a microtubule polymerization inhibitor that causes mitotic arrest. At low doses, colchicine is associated with minimal mucosal changes; however, at high doses, it can cause severe villous flattening, leading to a clinical enteropathy.41 Mycophenolate mofetil, an immunosuppressive agent used commonly in transplant patients, is associated with crypt and villi architectural changes and marked lamina propria inflammation that can mimic CD.42 Additionally, it can result in epithelial cell apoptosis similar to that noted in graft-versus-host disease.42 These patients can be difficult to manage, especially if alternative immunosuppressive agents are contraindicated or suboptimal. Methotrexate is an antimetabolite, antineoplastic agent that inhibits DNA and RNA synthesis and prevents crypt mitotic activity and leads to villous diminution.43 Finally, azathioprine is an immune modulator commonly used for autoimmune hepatitis and IBD, that has been reported to cause a small bowel enteropathy, which is reversible with drug discontinuation.44
Collagenous sprue
Collagenous sprue (CS), first described in 1970, results in malabsorption and diarrhea.45 While CS shares most of the histologic features found with CD, it also characteristically demonstrates an irregularly thickened layer of type I collagen adjacent to the surface epithelium.45, 46 Normal collagen band thickness in the duodenum should be five microns or less; in CS, the collagen band is thick, irregular, often wraps around capillaries, and may result in surface epithelial detachment. The link between CS and gluten is not well-defined; management often begins with a gluten-free diet, although most patients do not fully respond.45 Patients with refractory CS may require treatment with corticosteroids or immunosuppresants, and histologic changes may persist following treatment.46 As noted previously, the finding of CS should prompt a careful medication review.
Combined variable immunodeficiency
CVID can both cause clinical and/or histologic features of a small bowel enteropathy.47 A diagnosis of CVID is made using the following criteria: (1) IgG is reduced at least two standard deviations below normal levels; (2) at least one other immunoglobulin level is reduced (IgA and/or IgM); (3) there is a poor response to vaccines; and (4) other immunodeficiency states have been excluded.48 CVID can occur at any age, although it is most common in children and young adults less than 30 years of age. Due to impaired antibody response, respiratory and gastrointestinal tract infections are common, although the absence of recurrent infections should not rule out the disease.
Histologic findings commonly seen with CVID include reduced or absent plasma cells, increased IELs, glandular apoptosis, villous blunting, and lymphoid aggregates.47 However, approximately 30% of CVID patients have a normal number of plasma cells on intestinal biospies.47 These patients should have their management directed towards their CVID, given a gluten-free diet is unlikely to be helpful once CD has been ruled out, which can be challenging to do in a CVID patient with permissive celiac haplotyping, as serologies may be difficult to interpret if both IgA and IgG levels are low.
Small intestinal bacterial overgrowth
Several studies have been performed examining the impact of SIBO on small bowel histology, with results that make it challenging to interpret the true range of impact that SIBO has. In a study of 52 subjects without a known history of SIBO, duodenal aspirates and biopsies were performed and 26 (50%) subjects were found to have SIBO.49 Twenty of the 26 were noted to have colonic-type bacteria on cultures. A comparison of patients with and without SIBO found no difference in villous height, crypt depth, villous/crypt ratio, and lamina propria cell counts.49 There was a statistical difference in lamina propria IgA plasma cell counts as well as IEL counts in the setting of colonic-type bacteria, both higher among patients with SIBO, yet with values within normal limits.49 One study found a significant, inverse correlation between advancing age and IEL counts after adjusting for SIBO.49 Another compared histologic findings in patients with SIBO to controls.50 Decreased villous/crypt ratio (<3:1) was statistically more common in patients with SIBO (24%) compared to controls (7%).50 However, there were no significant differences between patients with SIBO and controls regarding IELs, crypt apoptosis, basal plasmacytosis, cryptitis/villitis, peptic duodenitis, erosions/ulcers, eosinophilia, and absence of goblet or Paneth cells.50 The degree of association between serologically negative enteropathy and SIBO needs to needs to be further defined.
Tropical sprue
Tropical sprue (TS) is condition endemic to certain parts of the world, notably South Asia, Caribbean, Central, and South America.51 Symptoms include diarrhea and weight loss. Although there are no laboratory tests to diagnose TS, work-up classically reveals negative CD serologies, deficiencies in folate and vitamin B12, and low serum protein. Small bowel histology looks identical to that seen in CD, and imparts the importance of travel history in diagnosing this condition. Management of TS includes folate and B12 replacement along with antibiotics, such as a tetracycline derivative.51 Complete recovery is typically expected in travelers who develop TS; however, in endemic sprue, up to 50% of the population may have clinical relapse.51
Autoimmune enteropathy
Autoimmune enteropathy is a rare cause of intractable diarrhea characterized by autoantibodies in the serum and small bowel enteropathy.52, 53 While the condition commonly affects infants, it is being increasingly recognized in adults.52, 53 A diagnosis of autoimmune enteropathy is made using the following criteria: (1) chronic diarrhea (>6 weeks); (2) features of malabsorption; (3) representative small bowel histology (partial/total villous blunting, deep crypt lymphocytosis, increased crypt apoptotic bodies, and minimal intraepithelial lymphocytosis); (4) exclusion of other causes of villous atrophy; and (5) the presence of anti-enterocyte or anti-goblet cell antibodies.54 While these autoantibodies may be found in ~85% of those with autoimmune enteropathy, they tend to be non-specific. Contrary to other celiac mimickers, histology for autoimmune enteropathy typically reveals absence of goblet and Paneth cells, less prominent surface IELs, and a lymphoplasmacytic infiltrate. Many of these patients have refractory diarrhea and nutritional deficiencies, requiring immunosuppressive treatment.52 A particularly severe form of autoimmune enteropathy is known as the immunodysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX) syndrome, which results from mutations in the FOXP3 gene that controls regulatory T-cells.55
Whipple’s disease
While Whipple’s disease is often listed among the disorders that can mimic CD both clinically and histologically, there are some notable differences. Whipple’s disease occurs predominantly in men with presentation often at 30–50 years of age. Patients present with multi-system complaints of diarrhea, weight loss, arthralgias, fever, lymphadenopathy, cardiac abnormalities, and neurologic features.56 While the duodenal villi are often described as blunted in patients with Whipple’s disease, they are typically broad, with the lamina propria expanded with macrophages; dilated lacteals and lipid deposition may also be noted. Periodic acid-Schiff staining of the small bowel tissue is typically positive in cases of Whipple’s disease, although this is non-specific and can also be seen in those with Mycobacterium avium intracellulare. As a result, polymerase chain reaction testing can be used for confirmation.56
Conclusion
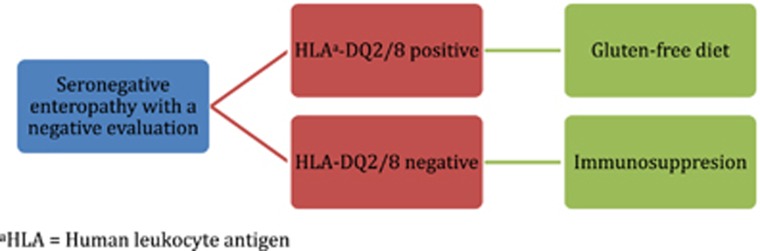
An algorithm for the work-up (Figure 4) and management (Figure 5) of patients with a serologically negative enteropathy can be used to guide clinicians. The evaluation of a patient with a serologically negative enteropathy necessitates a detailed evaluation by an expert gastrointestinal pathologist to ensure that adequate tissue has been obtained, and to be sure histologic clues favoring another diagnosis have not been overlooked. The histologic finding of duodenal IELs with normal villous architecture is being seen with increased frequency; while up to one-third of such cases have no known cause identified, NSAID use is a strong contributing factor. Several prescription medications can mimic CD and should be considered in all patients with a serologically negative enteropathy. Many mimickers of CD have clues to the underlying diagnosis (Table 3), and many have a targeted therapy. It is necessary to provide patients with a correct diagnosis and appropriate therapy rather than subject them to a lifetime of an unnecessary gluten-free diet.
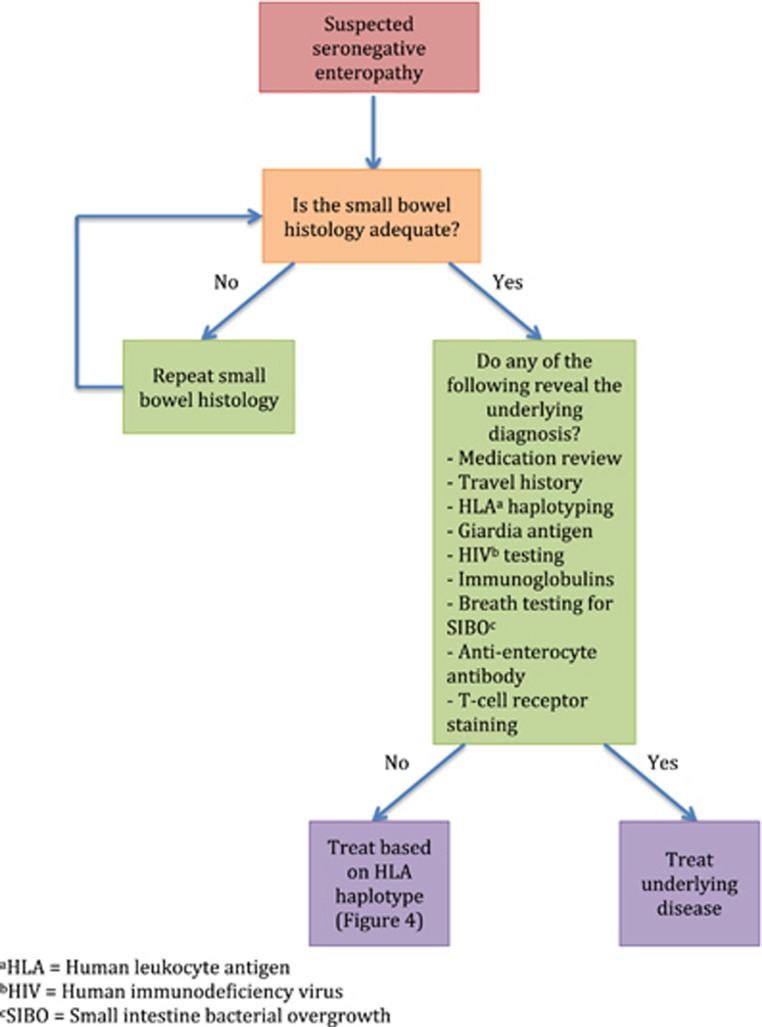
Figure 4.
Proposed algorithm for work-up of seronegative enteropathies.
Figure 5.
Proposed algorithm for management of seronegative enteropathies after other etiologies have been excluded.
Table 3. Clinical pearls on celiac disease mimickers and clues to their diagnosis.
| Celiac disease (CD) mimicker | Histology | Diagnostic clues |
|---|---|---|
| Autoimmune enteropathy | Loss of goblet and Paneth cells, more neutrophils, apoptosis | Can check anti-enterocyte and anti-goblet cell antibodies |
| Bacterial overgrowth | May not have any change other than mildly decreased villous:crypt ratio | Look for other causes of enteropathy |
| Collagenous sprue | CD-like with thickened collagen band>5 microns, detached surface | Review medication list |
| Common variable immunodeficiency | Loss of plasma cells; lamina propria appears somewhat empty | Up to 30% with normal plasma cells; check immunoglobulin levels |
| Helicobacterpyloriinfection | May have increased IELs, but also neutrophils, gastric metaplasia | May have chronic active gastritis as a clue |
| Inflammatory bowel disease | May have increased IELs as a sole duodenal feature | IELs more on sides or evenly distributed (non-tip predominant) |
| Medication effect | May range from IELs only to total villous atrophy, +/−collagen | NSAIDs, olmesartan, mycophenolate |
| Tropical sprue | Identical to CD | Low B12 and folate Notable travel |
| Whipple’s disease | Broad rather than flat villi, filled macrophages, lipid deposits | PAS staining and PCR testing |
IELs, intraepithelial lymphocytes; NSAIDs, non-steroidal anti-inflammatory drugs; PAS, periodic acid-Schiff.
Footnotes
Guarantor of the article: Amy S. Oxentenko, MD.
Specific author contributions: Amrit K. Kamboj: Drafting the manuscript and approved the final draft; Amy S. Oxentenko: Drafting and editing the manuscript and approved the final draft.
Financial support: None.
Potential competing interests: None.
References
- Green PHR, Cellier C. Celiac disease. N Engl J Med 2007; 357: 1731–1743. [DOI] [PubMed] [Google Scholar]
- Rubio-Tapia A, Ludvigsson JF, Brantner TL et al. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012; 107: 1538–1544. [DOI] [PubMed] [Google Scholar]
- Fasano A, Catassi C. Celiac disease. N Engl J Med 2012; 367: 2419–2426. [DOI] [PubMed] [Google Scholar]
- Ivarsson A, Hernell O, Stenlund H et al. Breast-feeding protects against celiac disease. Am J Clin Nutr 2002; 75: 914–921. [DOI] [PubMed] [Google Scholar]
- Stene LC, Honeyman MC, Hoffenberg EJ et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol 2006; 101: 2333–2340. [DOI] [PubMed] [Google Scholar]
- Rampertab SD, Pooran N, Brar P et al. Trends in the presentation of celiac disease. Am J Med 2006; 119355: e9–355.e14. [DOI] [PubMed] [Google Scholar]
- Green PHR, Fleischauer AT, Bhagat G et al. Risk of malignancy in patients with celiac disease. Am J Med 2003; 115: 191–195. [DOI] [PubMed] [Google Scholar]
- Cellier C, Delabesse E, Helmer C et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet 2000; 356: 203–208. [DOI] [PubMed] [Google Scholar]
- Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut 2010; 59: 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome. JAMA 2015; 313: 949–958. [DOI] [PubMed] [Google Scholar]
- Riedl A, Schmidtmann M, Stengel A et al. Somatic comorbidities of irritable bowel syndrome: A systematic analysis. J Psychosom Res 2008; 64: 573–582. [DOI] [PubMed] [Google Scholar]
- Lovell RM, Ford AC. Prevalence of gastro-esophageal reflux-type symptoms in individuals with irritable bowel syndrome in the community: a meta-analysis. Am J Gastroenterol 2012; 107: 1793–1801. [DOI] [PubMed] [Google Scholar]
- Ford AC, Marwaha A, Lim A et al. Systematic review and meta-analysis of the prevalence of irritable bowel syndrome in individuals with dyspepsia. Clin Gastroenterol Hepatol 2010; 8: 401–409. [DOI] [PubMed] [Google Scholar]
- Fond G, Loundou A, Hamdani N et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2014; 264: 651–660. [DOI] [PubMed] [Google Scholar]
- Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016; 150: 1262–1279.e2. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- Grace E, Shaw C, Whelan K et al. Review article: small intestinal bacterial overgrowth prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment Pharmacol Ther 2013; 38: 674–688. [DOI] [PubMed] [Google Scholar]
- Singh VV, Toskes PP. Small bowel bacterial overgrowth: presentation, diagnosis, and treatment. Curr Gastroenterol Rep 2003; 5: 365–372. [DOI] [PubMed] [Google Scholar]
- Khoshini R, Dai S-C, Lezcano S et al. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci 2008; 53: 1443–1454. [DOI] [PubMed] [Google Scholar]
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009; 361: 2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN. Treatment of IBD: where we are and where we are going. Am J Gastroenterol 2015; 110: 114–126. [DOI] [PubMed] [Google Scholar]
- Oberhuber G, Granditsch G, Vogelsand H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999; 11: 1185–1194. [DOI] [PubMed] [Google Scholar]
- Ferguson A, Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut 1971; 12: 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veress B, Franzén L, Bodin L et al. Duodenal intraepithelial lymphocyte-count revisited. Scand J Gastroenterol 2004; 39: 138–144. [DOI] [PubMed] [Google Scholar]
- Hayat M, Cairns A, Dixon MF et al. Quantitation of intraepithelial lymphocytes in human duodenum: what is normal? J Clin Pathol 2002; 55: 393–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar S, Nehra V, Murray JA et al. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am J Gastroenterol 2003; 98: 2027–2033. [DOI] [PubMed] [Google Scholar]
- Mahadeva S, Wyatt JI, Howdle PD. Is a raised intraepithelial lymphocyte count with normal duodenal villous architecture clinically relevant? J Clin Pathol 2002; 55: 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmidt E, Smyrk TC, Boswell CL et al. Increasing duodenal intraepithelial lymphocytosis found at upper endoscopy: time trends and associations. Gastrointest Endosc 2014; 80: 105–111. [DOI] [PubMed] [Google Scholar]
- Aziz I, Evans KE, Hopper AD et al. A prospective study into the aetiology of lymphocytic duodenosis. Aliment Pharmacol Ther 2010; 32: 1392–1397. [DOI] [PubMed] [Google Scholar]
- Shmidt E, Smyrk TC, Faubion WA et al. Duodenal intraepithelial lymphocytosis with normal villous architecture in pediatric patients. J Pediatr Gastroenterol Nutr 2013; 56: 51–55. [DOI] [PubMed] [Google Scholar]
- Freeman HJ. Sulindac-associated small bowel lesion. J Clin Gastroenterol 1986; 8: 569–571. [DOI] [PubMed] [Google Scholar]
- James Freeman H. Drug-induced sprue-like intestinal disease. Int J Celiac Dis 2016; 2: 49–53. [Google Scholar]
- Patterson ER, Shmidt E, Oxentenko AS et al. Normal villous architecture with increased intraepithelial lymphocytes a duodenal manifestation of Crohn disease. J Clin Pathol Am J Clin Pathol March 2015; 143143: 445–450. [DOI] [PubMed] [Google Scholar]
- Green PHR, Degaetani M, Tennyson CA et al. Villous atrophy and negative celiac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol 2013; 108: 647–65345. [DOI] [PubMed] [Google Scholar]
- Rubio-Tapia A, Herman ML, Ludvigsson JF et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc 2012; 87: 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes to include intestinal problems (sprue-like enteropathy) linked to blood pressure medicine olmesartan medoxomil, 2013.
- Marthey L, Cadiot G, Seksik P et al. Olmesartan-associated enteropathy: results of a national survey. Aliment Pharmacol Ther 2014; 40: 1103–1109. [DOI] [PubMed] [Google Scholar]
- Marco-Marqués A, Sanahuja-Martinez A, Bosca-Watts MM et al. Could HLA-DQ suggest why some patients have olmesartan-related diarrhea and others don’t? Am J Gastroenterol 2015; 110: 1507–1508. [DOI] [PubMed] [Google Scholar]
- Gentile NM, D’souza A, Fujii LL et al. Association between ipilimumab and celiac disease. Mayo Clin Proc 2013; 88: 414–417. [DOI] [PubMed] [Google Scholar]
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012; 30: 2691–2697. [DOI] [PubMed] [Google Scholar]
- Race TF, Paes IC, Faloon WW. Intestinal malabsorption induced by oral colchicine. Comparison with neomycin and cathartic agents. Am J Med Sci 1970; 259: 32–41. [DOI] [PubMed] [Google Scholar]
- Parfitt JR, Jayakumar S, Driman DK. Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol 2008; 32: 1367–1372. [DOI] [PubMed] [Google Scholar]
- Altmann GG. Changes in the mucosa of the intestine following methotrexate administration or abdominal X-irradiation. Am J Anat 1974; 140: 263–279. [DOI] [PubMed] [Google Scholar]
- Ziegler TR, Fernández-Estívariz C, Gu LH et al. Severe villus atrophy and chronic malabsorption induced by azathioprine. Gastroenterology 2003; 124: 1950–1957. [DOI] [PubMed] [Google Scholar]
- Weinstein WM, Saunders DR, Tytgat GN et al. Collagenous sprue — an unrecognized type of malabsorption. N Engl J Med 1970; 283: 1297–1301. [DOI] [PubMed] [Google Scholar]
- James HJ. Collagenous sprue. Can J Gastroenterol 2011; 25: 189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JA, Lederman HM, Maitra A et al. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID). Am J Surg Pathol 2007; 31: 1800–1812. [DOI] [PubMed] [Google Scholar]
- Conley ME, Notarangelo LD, Etzioni A Diagnostic criteria for primary immunodeficiencies. [DOI] [PubMed]
- Riordan SM, McIver CJ, Wakefield D et al. Small intestinal mucosal immunity and morphometry in luminal overgrowth of indigenous gut flora. Am J Gastroenterol 2001; 96: 494–500. [DOI] [PubMed] [Google Scholar]
- Lappinga PJ, Abraham SC, Murray JA et al. Small intestinal bacterial overgrowth: histopathologic features and clinical correlates in an underrecognized entity. Arch Pathol Lab Med 2010; 134: 264–270. [DOI] [PubMed] [Google Scholar]
- Ramakrishna BS, Venkataraman S, Mukhopadhya A. Tropical malabsorption. Postgr Med J 2006; 82: 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile NM, Murray JA, Pardi DS. Autoimmune enteropathy: a review and update of clinical management. Curr Gastroenterol Rep 2012; 14: 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco Quirós A, Arranz Sanz E, Bernardo Ordiz D et al. From autoimmune enteropathy to the IPEX (immune dysfunction, polyendocrinopathy, enteropathy, X-linked) syndrome. Allergol Immunopathol 2009; 37: 208–215. [DOI] [PubMed] [Google Scholar]
- Akram S, Murray JA, Pardi DS et al. Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol 2007; 5: 1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patey-Mariaud de Serre N, Canioni D, Ganousse S et al. Digestive histopathological presentation of IPEX syndrome. Mod Pathol 2009; 22: 95–102. [DOI] [PubMed] [Google Scholar]
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med 2007; 356: 55–66. [DOI] [PubMed] [Google Scholar]