Abstract
Objectives:
Studies on the epidemiology of primary biliary cholangitis (PBC) in the Chinese population are lacking. We aimed to determine the epidemiology of PBC in Hong Kong (HK) with a population of 7.3 million.
Methods:
We retrieved data from the electronic database of the HK Hospital Authority, the only public healthcare provider in Hong Kong. PBC cases between 2000 and 2015 were identified by International Classification of Diseases (ICD)-9 code. We estimated the age-/sex-adjusted incidence rate and prevalence of PBC, and analyzed the adverse outcomes (hepatocellular carcinoma (HCC), liver transplantation, and death).
Results:
One thousand and sixteen PBC patients aged ≥20 years were identified (female-to-male ratio 4:1; median age 60.6 years, interquartile range (IQR) 51.8–72.6 years; median follow-up 5.6 years, IQR 1.6–8.7 years). The average age/sex-adjusted annual incidence rate and prevalence were 8.4 per million person-years and 56.4 per million, respectively. Between 2000 and 2015, the age/sex-adjusted annual incidence rate increased from 6.7 to 8.1 per million person-years (Poisson P=0.002), while age/sex-adjusted prevalence increased from 31.1 to 82.3 per million (Poisson P<0.001). Fifty patients developed HCC, and 49 underwent liver transplantation. Case fatality risk decreased from 10.8 to 6.4% (Poisson P=0.003). The 5- and 10-year overall survival rates were 81.5 and 78.3%, whereas the transplant-free survival rates were 78.0% and 74.3%, respectively. Increasing age, cirrhosis and being treatment-naïve were associated with lower transplant-free survival.
Conclusions:
There is a considerable increase in the incidence and prevalence of PBC in the Chinese population over the past 16 years, with significant morbidity and mortality.
Introduction
Primary biliary cholangitis (PBC) is a chronic cholestatic liver disease due to immune-mediated attack on the small-sized biliary ducts leading to progressive ductal destruction and loss.1 It is typically characterized by the presence of anti-mitochondrial antibodies (AMA) serologically. The major complications include cirrhosis leading to hepatic decompensation as well as hepatocellular carcinoma (HCC).1 Recently, a change of terminology, from primary biliary cirrhosis to primary biliary cholangitis, has been advocated to reflect more accurately the natural history and characteristics of this disease.2
The epidemiology of PBC has been well described in the western population, with incidence rate and prevalence ranging from 3.3 to 58 per million person-years and 19 to 402 per million, respectively.3 PBC is most prevalent in North America and northern Europe, especially in the Scandinavian regions and Britain.4 The disease prevalence has been increasing in the West, which could be attributed to several factors: improved survival with ursodeoxycholic acid (UDCA), increased disease awareness, advances in diagnosis, and better retrieval of data by electronic medical systems.5
However, population-based studies are lacking in the Asian countries including China,3 except South Korea, in which the age-/sex-adjusted incidence rate and prevalence were estimated to be 8.6 per million person-years and 47.5 per million, respectively.6 Epidemiological data are important to ascertain the disease burden, to help in resource planning, and to provide basis for further researches regarding disease etiology such as genetic and environmental factors.
The aim of the present study was to determine the epidemiology of PBC in Hong Kong. Adverse outcomes including HCC, need of liver transplantation, and death would also be investigated.
Methods
Data sources and study population
Data were retrieved from the Clinical Data Analysis and Reporting System (CDARS) and the Clinical Management System (CMS) of the Hong Kong Hospital Authority. The Hospital Authority is the only public healthcare provider which covers 87–94% of all secondary and tertiary care in Hong Kong with a population of around 7.3 million.7 In 2012, the outpatient attendances reached 6.88 million persons. Under this healthcare system, there are altogether 42 public hospitals and institutions, 47 Specialist Outpatient Clinics, and 73 General Outpatient Clinics.8 Essential clinical data including demographics, date of hospitalizations and consultations, diagnoses, investigation results, management (both procedures and medications), and mortality are recorded electronically in the CMS for easy access to patient data to facilitate daily clinical practice of physicians.9 Data in the CMS are also transferred to the CDARS for both administrative and research purposes. A number of high-quality, population-based studies have been conducted based on the data retrieved from CDARS.10, 11, 12, 13, 14 Local studies showed that the coding system in CDARS was accurate with high positive and negative predictive values of more than 90%.12, 15
In the present study, no personal identifiable data were retrieved from the database system. The study protocol was approved by the Institutional Review Board, The University of Hong Kong and West Cluster of Hospital Authority, Hong Kong.
Case ascertainment
The study period ranged from year 2000 to year 2015. PBC cases with age ≥20 years between 1999 and 2015 were identified from CDARS with the International Classification of Diseases (ICD)-9 code of 571.6. PBC cases in 1999 were also identified so that the number of new cases in 2000 could be determined.
Demographics of these cases (including age at diagnosis, gender, date of diagnosis, and date of death) were obtained. Cases with HCC, cirrhosis, and cirrhotic complications including esophageal varices, gastric varices, ascites, portal hypertension, hepatic encephalopathy, and hepatorenal syndrome were identified with the ICD-9 codes as shown in Supplementary Table 1.
These epidemiological data allowed the calculation of age- and sex-adjusted incidence rate and prevalence of PBC. Adverse outcomes including HCC, need of liver transplantation, and death were also analyzed.
Data validation
To validate the coding accuracy, we reviewed the medical records from both the CMS and case notes of the patients from the Hong Kong West (HKW) cluster, which is one of the seven hospital clusters of the Hospital Authority. Queen Mary Hospital is the only acute hospital in the HKW cluster, and is also a tertiary referral center which provides liver transplantation services.
PBC was diagnosed in our unit if two out of the three criteria were fulfilled: (1) cholestatic liver function pattern with alkaline phosphatase (ALP) raised to at least 1.5 times the upper limit of normal; (2) presence of anti-mitochondrial antibody (AMA); (3) histology showing “nonsuppurative destructive cholangitis with destruction of interlobular biliary ducts”.16
Positive predictive value (PPV) was calculated by dividing the number of patients who truely have PBC by the number of patients identified to have PBC by the CDARS (n=276). To calculate the negative predictive value (NPV), we randomly sampled 276 patients from a group of non-PBC patients identified by the CDARS (92 patients for each of the three periods: 2000–2005, 2006–2010, and 2011–2015), and cross validated with the records of PBC patients who followed up in our hepatology unit. The validity of the coding over the periods of 2000–2005, 2006–2010, and 2011–2015 were also assessed.
Statistical analyses
All statistical analyses were performed using R version 3.2.3 (A language and environment for statistical computing, Vienna, Austria, ISBN 3-900051-07-0, URL http://www.R-project.org) statistical software. Continuous variables were expressed as median and interquartile range (IQR). Mann–Whitney U-test was used to compare continuous variables between two groups. The χ2-test or Fisher’s exact test when appropriate, was applied for comparing categorical variables. The incidence rate and prevalence of PBC between 2000 and 2015 were calculated. These two estimates were age/sex-adjusted to the population of Hong Kong in year 2000.17 Evaluation of the count data and temporal trends was assessed by Poisson regression model.
The Cox proportional hazards model was used to identify variables that were associated with adverse outcomes. The Kaplan-Meier method was used to analyze the adverse outcomes, and statistical significance was determined by means of log-rank test. A P-value of <0.05 was used to define statistical significance.
Results
Data validation
Between 2000 and 2015, a total of 1,016 PBC patients aged at least 20 years were identified. Among them, 276 patients (27.2%) were from the HKW cluster. Of these, 240 were confirmed to have PBC after cross validating with the medical records, yielding a PPV of 87%. None was found to have PBC from a random sample of non-PBC patients identified by CDARS (n=276), yielding a NPV of 100%. Between 2000 and 2005, 118 PBC cases out of 127 (93%) were correctly coded. Between 2006 and 2010, the coding was correct for 77 PBC cases out of 95 (81%). Between 2011 and 2015, 45 PBC cases out of 54 were rightly coded (83%).
Patient characteristics
Of the 1,016 PBC patients identified, 79% of cases being female (female cases 802; male cases 214). Among these cases, 892 were newly diagnosed (female cases 704; male cases 188). The median duration of follow-up was 5.6 years (interquartile range (IQR) 1.6–8.7 years). The median age at diagnosis was 60.6 years (IQR 51.8–72.6 years). Women were diagnosed with PBC earlier than men. The median age at diagnosis was 59.8 years (IQR 51.4–71.8 years) for women, compared with 65.2 years (IQR 54.6–75.2 years) for men (P=0.008). The proportion of patients with cirrhosis did not change significantly between 2000 and 2015 (29.1% in 2000 and 24.1% in 2015; Poisson P=0.456; Table 1a). The proportion of patients receiving UCDA increased from 68.4 to 86.0% between 2000 and 2015 (Poisson P<0.001; Table 1b).
Table 1a. Cirrhosis in PBC patients between 2000 and 2015.
| Year (no. of cases) | 2000 (n=158) | 2001 (n=178) | 2002 (n=209) | 2003 (n=226) | 2004 (n=252) | 2005 (n=269) | 2006 (n=288) | 2007 (n=317) |
|---|---|---|---|---|---|---|---|---|
| No. of patients with cirrhosis | 46 (29.1%) | 50 (28.1%) | 53 (25.4%) | 57 (25.2%) | 60 (23.8%) | 63 (23.4%) | 68 (23.6%) | 78 (24.6%) |
| Year (no. of cases) | 2008 (n=358) | 2009 (n=421) | 2010 (n=501) | 2011 (n=530) | 2012 (n=560) | 2013 (n=584) | 2014 (n=625) | 2015 (n=656) |
| No. of patients with cirrhosis | 86 (24.0%) | 110 (26.1%) | 121 (24.2%) | 126 (23.8%) | 134 (23.9%) | 151 (25.9%) | 152 (24.3%) | 158 (24.1%) |
PBC, primary biliary cholangitis.
Table 1b. UCDA use in PBC patients between 2000 and 2015.
| Year (no. of cases) | 2000 (n=158) | 2001 (n=178) | 2002 (n=209) | 2003 (n=226) | 2004 (n=252) | 2005 (n=269) | 2006 (n=288) | 2007 (n=317) |
|---|---|---|---|---|---|---|---|---|
| No. of patients receiving UDCA | 108 (68.4%) | 131 (73.6%) | 152 (72.7%) | 160 (70.8%) | 187 (74.2%) | 205 (76.2%) | 210 (72.9%) | 225 (71.0%) |
| Year (no. of cases) | 2008 (n=358) | 2009 (n=421) | 2010 (n=501) | 2011 (n=530) | 2012 (n=560) | 2013 (n=584) | 2014 (n=625) | 2015 (n=656) |
| No. of patients receiving UDCA | 267 (74.6%) | 331 (78.6%) | 404 (80.6%) | 436 (82.3%) | 463 (82.7%) | 475 (81.3%) | 531 (85.0%) | 564 (86.0%) |
PBC, primary biliary cholangitis; UCDA, ursodeoxycholic acid.
Incidence rate and prevalence
The crude incidence rate of PBC in 2015 was 8.9 per million person-years.
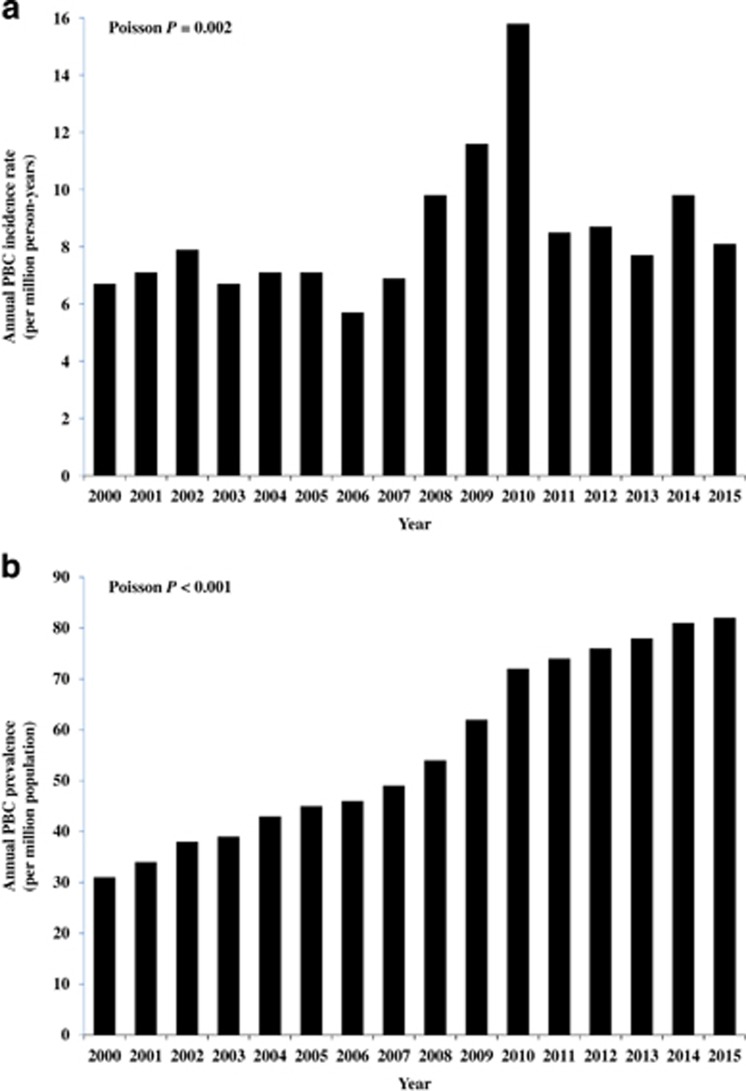
The average age-/sex-adjusted annual incidence rate of PBC was 8.4 per million person-years between 2000 and 2015. Age-/sex-adjusted annual incidence rate of PBC increased from 6.7 to 8.1 per million person-years between 2000 and 2015 (Poisson P=0.002; Figure 1a).
Figure 1.
(a) Annual age-/sex-adjusted incidence rate of PBC between 2000 and 2015. (b) Annual age-/sex-adjusted prevalence of PBC between 2000 and 2015. PBC, primary biliary cholangitis.
Increasing age was associated with increasing incidence of PBC (Table 2a). For women, the average annual incidence rate was highest among 60- to 79-year-old patients (33.6 per million person-years), compared with the youngest age group (20–39 years) (1.1 per million person-years). For men, the average annual incidence rate was highest among patients aged 80 years or above (15.7 per million person-years), compared with the youngest age group (0.8 per million person-years). The incidence rate was also dependent on gender, with PBC more frequently to be diagnosed in women. The age-adjusted incidence rate was 12.8 per million person-years in women compared with 3.8 per million person-years in men.
Table 2a. Average age/sex-adjusted annual Incidence rate of PBC (per million person-years) according to age and gender between 2000 and 2015.
| Age (years) | Women | Men | Total | Incidence rate ratio (95% CI) |
|---|---|---|---|---|
| 20–39 | 1.1 | 0.8 | 2.1 | Reference |
| 40–59 | 17.1 | 3.4 | 10.1 | 4.82 (3.39–6.86) |
| 60–79 | 33.6 | 10.7 | 22.2 | 10.61 (7.47–15.09) |
| ≥80 | 31.5 | 15.7 | 25.9 | 12.40 (8.12–18.92) |
| Overall | 12.8 | 3.8 | 8.4 | — |
CI, confidence interval; PBC, primary biliary cholangitis.
The average age-/sex-adjusted prevalence was 56.4 per million. Age-/sex-adjusted prevalence of PBC increased from 31.1 per million to 82.3 per million between 2000 and 2015 (Poisson P<0.001; Figure 1b).
In 2015, the crude prevalence of PBC was 109.0 per million in the entire population (Table 2b). PBC was most prevalent among 60- to 79-year-old patients (300.2 per million compared with 10.9 per million among patients aged 20–39 years; prevalence ratio of 27.5; 95% confidence interval (CI) 18.1–41.9). The prevalence of PBC was higher among women, with a female to male ratio of 6.3 (238.2 per million vs. 37.8 per million).
Table 2b. Crude prevalence of PBC (per million population) according to age and gender in 2015.
| Age (years) | Women | Men | Total | Incidence rate ratio (95% CI) |
|---|---|---|---|---|
| 20–39 | 75.3 | 8.8 | 10.9 | Reference |
| 40–59 | 148.3 | 23.9 | 92.2 | 8.45 (5.50–13.0) |
| 60–79 | 508.5 | 89.3 | 300.2 | 27.50 (18.06–41.90) |
| ≥80 | 353.2 | 108.6 | 257.7 | 23.60 (14.89–37.41) |
| Overall | 238.2 | 37.8 | 109.0 | — |
CI, confidence interval; PBC, primary biliary cholangitis.
Adverse outcomes
The development of various adverse outcomes including HCC, need of liver transplantation and death was investigated.
There were 50 newly diagnosed HCC cases (4.9% of total PBC cases; 21 women and 29 men). A higher proportion of men developed HCC than women (13.6 vs. 2.6% P<0.001). The 5- and 10-year probabilities of HCC were 1.5% (95% CI 0.6–2.3%) and 2.8% (95% CI 1.2–4.4%), respectively. Overall, the median age at diagnosis was 73.6 years (IQR 62.1–81.5 years), without significant difference between women and men (73.5 years (IQR 61.2–81.2 years) and 74.7 years (IQR 62.8–81.6 years), respectively; P=0.783). There was one HCC case in the 20- to 39-year age group, seven cases in the 40- to 59-year age group, 25 cases in the 60- to 79-year age group, and 17 cases in those aged 80 years or above. Thirty-four patients with HCC died between 2000 and 2015. Eleven out of 21 women (52.4%) and 23 out of 29 men (79.3%) died. The median age at death was 78.6 years (IQR 66.9–83.3 years), without significant difference between women and men (80.8 years (IQR 75.6–87.2 years) and 76.3 years (IQR 65.2–81.8 years), respectively; P=0.146).
Liver transplantation was performed in 49 patients (4.8% of total PBC cases). Forty out of 802 were women (5.0%) and nine out of 214 were men (4.4%). The 5- and 10-year probabilities of transplantation were 4.6% (95% CI 3.0–6.2%) and 5.3% (95% CI 3.5–7.0%), respectively. The median age at liver transplantation was 54.0 years (IQR 48.4–60.7 years). Among those aged less than 40 years, none received transplantation. There were 34 liver transplantation cases in the 40- to 50-year age group, and 15 cases in the 60- to 79-year age group.
Four hundred and two PBC patients died between 2000 and 2015 (female cases 277, male cases 125). The median life expectancy was 72.4 years (IQR 61.4–81.2 years), without significant difference between women and men (72.5 years (IQR 61.7–82.0 years) and 71.6 years (IQR 59.1–79.5 years), respectively; P=0.217). The annual case fatality risk decreased from 10.8 to 6.4% between 2000 and 2015 (Poisson P=0.003; Table 3).
Table 3. Case fatality risk (%) of PBC cases in Hong Kong between 2000 and 2015.
| Year (no. of cases) | 2000 (n=158) | 2001 (n=178) | 2002 (n=209) | 2003 (n=226) | 2004 (n=252) | 2005 (n=269) | 2006 (n=288) | 2007 (n=317) |
|---|---|---|---|---|---|---|---|---|
| Annual number of deaths (case fatality risk, %) | ||||||||
| Female | 9 (7.3%) | 8 (5.4%) | 18 (10.4%) | 14 (7.4%) | 17 (8.3%) | 7 (3.3%) | 11 (4.6%) | 20 (7.7%) |
| Male | 8 (22.9%) | 5 (15.6%) | 3 (8.3%) | 2 (5.3%) | 7 (14.3%) | 9 (16.7%) | 7 (13.7%) | 6 (10.5%) |
| Total | 17 (10.8%) | 13 (7.3%) | 21 (10.0%) | 16 (7.1%) | 24 (9.5%) | 16 (5.9%) | 18 (6.3%) | 26 (8.2%) |
| Year (no. of cases) | 2008 (n=358) | 2009 (n=421) | 2010 (n=501) | 2011 (n=530) | 2012 (n=560) | 2013 (n=584) | 2014 (n=625) | 2015 (n=656) |
| Annual number of deaths (case fatality risk, %) | ||||||||
| Female | 11 (3.8%) | 19 (5.6%) | 22 (5.4%) | 19 (4.4%) | 24 (5.1%) | 22 (4.5%) | 26 (4.9%) | 30 (5.4%) |
| Male | 6 (9.2%) | 12 (15.2%) | 10 (10.9%) | 14 (14.7%) | 7 (7.9%) | 9 (9.8%) | 8 (8.3%) | 12 (12%) |
| Total | 17 (4.7%) | 31 (7.4%) | 32 (6.4%) | 33 (6.2%) | 31 (5.5%) | 31 (5.3%) | 34 (5.4%) | 42 (6.4%) |
PBC, primary biliary cholangitis.
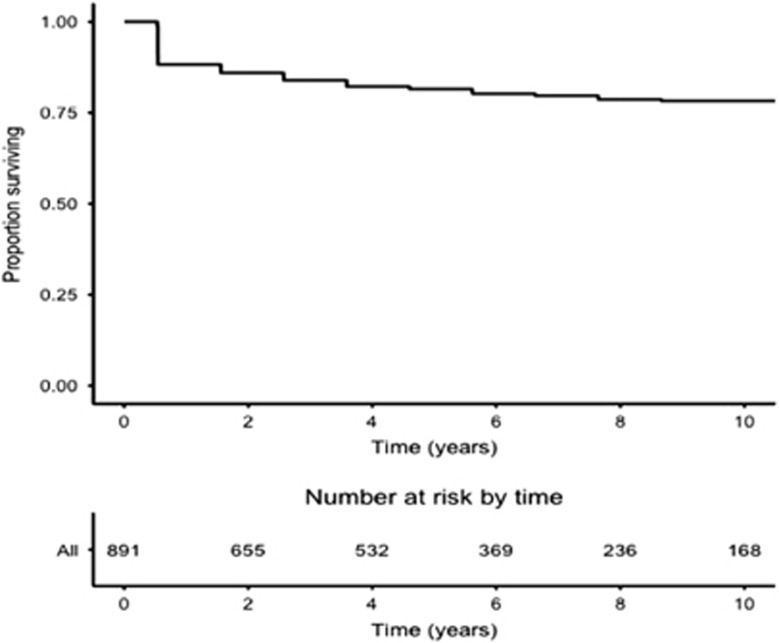
Overall, the 5- and 10-year survival rates were 81.5% (95% CI 78.8–84.3%) and 78.3% (95% CI 75.1–81.6%), respectively (Figure 2). By multivariate analysis, older age at diagnosis (hazards ratio (HR) 1.03; 95% CI 1.01–1.04) as well as presence of cirrhosis (HR 1.60; 95% CI 1.13–2.26) were significant risk factors, and UCDA prescription at diagnosis was a protective factor (HR 0.42; 95% CI 0.30–0.58), while gender did have statistical significance (HR for male gender: 1.24; 95% CI 0.87–1.76; Table 4).
Figure 2.
Kaplan–Meier survival plot for overall survival.
Table 4. HRs and 95% CIs for the association between different covariates and (A) overall survival, (B) transplant-free survival.
| Univariate analysis | 95% CI | Multivariate analysis | 95% CI | |
|---|---|---|---|---|
| A. Overall survival | ||||
| aAge | 1.03 | 1.02–1.05 | 1.03 | 1.01–1.04 |
| Male sex | 1.63 | 1.15–2.29 | 1.24 | 0.87–1.76 |
| Cirrhosis | 2.30 | 1.65–3.20 | 1.60 | 1.13–2.26 |
| UCDA | 0.35 | 0.26–0.48 | 0.42 | 0.30–0.58 |
| B. Transplant-free survival | ||||
| aAge | 1.02 | 1.01–1.03 | 1.01 | 1.0002–1.02 |
| Male sex | 1.58 | 1.16–2.16 | 1.23 | 0.89–1.70 |
| Cirrhosis | 2.56 | 1.91–3.43 | 2.05 | 1.51–2.80 |
| UCDA | 0.47 | 0.35–0.62 | 0.57 | 0.42–0.76 |
95% CI, 95% confidence interval; HR, hazards ratio; UCDA, ursodeoxycholic acid.
Age was treated as continuous variable in analysis.
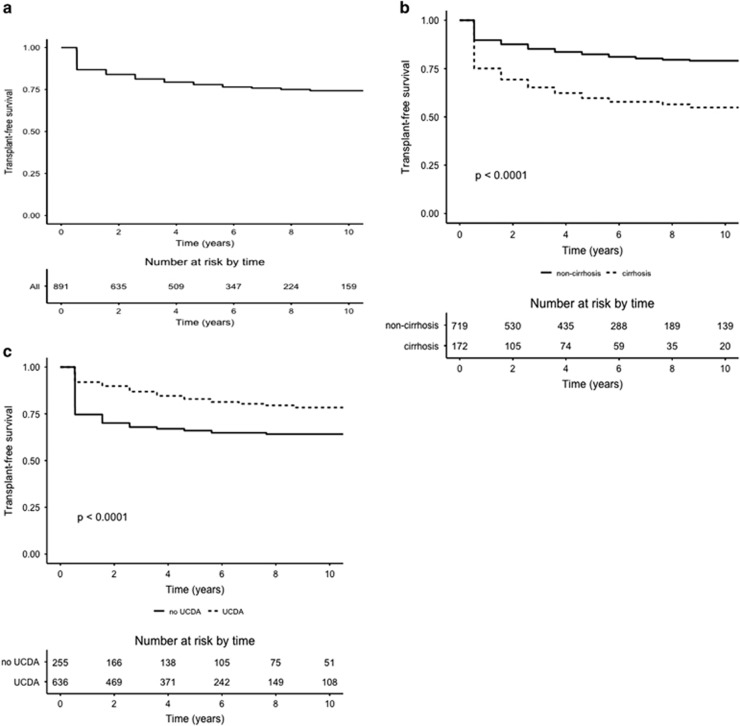
Overall, the 5- and 10-year transplant-free survival rates were 78.0% (95% CI 75.1–80.9%) and 74.3% (95% CI 71.1–77.7%), respectively (Figure 3a). By multivariate analysis, older age at diagnosis (HR 1.01; 95% CI 1.0002–1.02) as well as presence of cirrhosis (HR 2.05; 95% CI 1.51–2.80) were significant risk factors, and UCDA prescription at PBC diagnosis was a protective factor (HR 0.57; 95% CI 0.42–0.76), while gender did not have statistical significance (HR for male gender: 1.23; 95% CI 0.89–1.70) (Table 4). Figure 3b illustrate the comparison of transplant-free survival of patients according to their baseline cirrhosis and treatment status, respectively. Transplant-free survival rates were significantly higher in patients without cirrhosis (log-rank P<0.001) and in those who received UCDA (log-rank P<0.001).
Figure 3.
(a) Kaplan–Meier survival plot for overall transplant-free survival. (b) Kaplan–Meier survival plot for transplant-free survival stratified according to baseline cirrhosis status. (c) Kaplan–Meier survival plot for transplant-free survival stratified according to baseline treatment status. UCDA, ursodeoxycholic acid.
Discussion
The epidemiology of PBC has been well reported in the western countries, but not in the Asian population.3 Population-based studies investigating the epidemiology and natural history of PBC in the Asian population including the Chinese are lacking.
Previous studies usually recruited patients from a single hospital or tertiary referral centers,18, 19, 20, 21, 22 except for one performed in South Korea.6 The present territory-based study described the epidemiology and natural history of PBC in a well defined Chinese population.
The strength of this study is its population-based nature, in which the administrative electronic database system ensures complete capture of all PBC cases being followed up in the public healthcare setting. The inherent selection bias in studies conducted by a few hospitals or tertiary referral centers was minimized. Case ascertainment was based on ICD-9 coding, which has been shown to have high PPV and NPV of more than 90% from local studies.12, 15 These highly accurate rates were also confirmed in the present study on PBC that the PPV and NPV were 87% and 100%, respectively. Therefore, the numerators and denominators could be accurately defined in a Chinese population with a clearly defined geographic demarcation.
Studies from the West estimated an incidence rate of 3.3–58 per million person-years and prevalence of 19–402 per million.3 The estimates from our study were on the lower end of these ranges, with the average age-/sex-adjusted incidence rate being 8.4 per million person-years, and the age/sex-adjusted prevalence 56.4 per million. These data were consistent with the estimates reported in South Korea (8.6 per million person-years and 47.5 per million, respectively).6 More population-based studies are warranted to confirm whether PBC is less prevalent in the Asian population. Importantly, this could provide more insights into the genetic and environmental risk factors involved in the pathogenesis of PBC.
From the current study, the incidence rate and prevalence of PBC in Hong Kong had been increasing. The increase in the annual incidence rate could be related to increase in disease awareness, better diagnostic tests (e.g., more sensitive assays to detect AMA),23 and better diagnosis entry into the electronic database. In year 2009 and 2010, there was an upsurge in the incidence of PBC cases (Figure 1a), probably related to an intensive publicity of PBC in the media in Hong Kong in February 2009. As a result, there was an enhanced awareness of diagnosing and reporting this disease in 2009 and 2010. The overall increase in the prevalence could be attributed to an increase in the incidence and improved survival with UCDA (proportion of patients being prescribed with UCDA increased from 68.4 to 86.0% between 2000 and 2015).
The survival of patients with PBC was similar between the western and our Chinese cohorts. The 5- and 10-year survival rates were estimated to be 82 and 78%, respectively in our study, which were similar to the western population (survival rates were 88 and 77% at 5 years and 10 years, respectively).24 However the median life expectancy of patients with PBC in our cohort was only 71.5 years, which was much shorter than that of the general population in Hong Kong (81.2 years for men and 87.3 years for women in 2015).25
Our study also showed that PBC patients carried significant liver-related morbidity, with 4.8% developing HCC and 4.9% requiring liver transplantation. Notably, there were modifiable factors that could potentially influence transplant-free survival: older age at diagnosis (HR 1.01), presence of cirrhosis on diagnosis (HR 2.05) and UCDA prescription (HR 0.57). This underscores the importance of early diagnosis and prompt commencement of treatment. It has been recommended that all PBC patients with deranged liver function test should be treated with UCDA, regardless of disease stages.16
Classically, PBC is considered to affect patients aged between 30 and 65 years.26 A meta-analysis including 4,845 patients in the western population showed that the mean age at diagnosis was 54.5±12 years.24 However, several studies from other Asian countries outside of Hong Kong (including Taiwan, Singapore, and South Korea) showed that patients tended to be diagnosed with PBC at an older age, ranging from 55.6 to 57.4 years.6, 20, 21 Our study showed that the median age at diagnosis was 60.6 years. This estimate was very close to that reported from a retrospective study conducted by a local hospital from Hong Kong, which showed the median age at diagnosis was 59 years.18 In term of prevalence, we showed that PBC was commonest among patients aged 60–79 years, which was consistent with findings from other Asian studies.5, 6 Similar to other autoimmune diseases, we showed that PBC predominantly affected women (79%). Previous studies also revealed a female preponderance (77–87%) in Asian patients with PBC.6, 20, 22
There are a few limitations of our study. First, although the Hospital Authority is the only public healthcare provider, a small proportion of secondary and tertiary care was not covered (around 5–10%).7 Patients who were initially treated in private hospitals may be missed. As a result, it is possible that the incidence rate and prevalence of PBC were slightly underestimated. However, it is not uncommon that these patients will attend public hospitals or clinics later for follow-up because of the long-term disease monitoring or consultation for other medical problems, and therefore these PBC cases will still be captured in the public healthcare database system in the end. Second, the ICD-9 code of 571.6 encompasses both PBC and secondary biliary cirrhosis, and therefore the number of true PBC cases would be less than observed. To address this concern, we checked the coding validity by cross checking with patients from our hospital, and found that the PPV and NPV were 87 and 100% respectively. However, because of restricted access right, only the medical records in the CMS from our hospital cluster could be accessed for coding validation. Biases may arise from differences in coding practices across different clusters. Nevertheless, as the clinical data in CMS and CDARS are for clinical management purposes instead of reimbursement or insurance, there exist no financial rewards or initiatives. As such, the coding validity is likely to be consistent across different hospital clusters under the same management of structure of the Hospital Authority. Third, information regarding biochemical response to UCDA could not be retrieved, which may potentially underestimate the protective effect of UCDA in patients who are treatment-responsive. Fourth, data on concomitant viral hepatitis infection (e.g., chronic hepatitis B) were not available.
To address these limitations, future collaboration with all hepatology units from other hospitals (either public or private hospitals) in Hong Kong as well as prospective, multi-center studies with larger sample size can be carried out to ascertain the epidemiological link of risk factors for developing PBC in Chinese patients.
In summary, we described the epidemiology of PBC in a well defined Chinese population and identified factors associated with transplant-free survival. There was a considerable increase in both the incidence and prevalence of PBC in Hong Kong, which was associated with significant morbidity and mortality. Early diagnosis with prompt treatment improving disease outcome could potentially reduce the disease burden.
Study Highlights

Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Guarantor of the article: Ka-Shing Cheung, MBBS and Man-Fung Yuen, MD, PhD.
Authors contributions: Drs Ka-Shing Cheung and Wai-Kay Seto were involved with study concept and design; acquisition of data; analysis and interpretation of data; drafting of manuscript; Dr James Fung was involved with analysis and interpretation of data; and critical revision of the manuscript for important intellectual content. Professors Ching-Lung Lai and Man-Fung Yuen were involved with the study concept and design; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content; and study supervision. The corresponding authors had full access to all data, and were fully responsible for the data integrity and statistical analysis. All authors revised the manuscript and approved the final version of this article.
Conflict of Interest: WK Seto is an advisory board member of Bristol-Myers Squibb and Gilead Sciences, and received speaker fees from Bristol-Myers Squibb, Gilead Sciences and Novartis. J Fung received research funding from Novartis. CL Lai received speaker fees and is an advisory board member of Bristol-Myers and Gilead Sciences. MF Yuen received speaker fees and research funding and is an advisory board member of Bristol-Myers Squibb, Novartis, Gilead Sciences and Roche Diagnostics. The remaining authors declare no conflict of interest.
Funding source: None.
Supplementary Material
References
- Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet 2015; 386: 1565–1575. [DOI] [PubMed] [Google Scholar]
- Beuers U, Gershwin ME, Gish RG et al. Changing nomenclature for PBC: from 'cirrhosis' to 'cholangitis'. Hepatology 2015; 62: 1620–1622. [DOI] [PubMed] [Google Scholar]
- Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol 2012; 56: 1181–1188. [DOI] [PubMed] [Google Scholar]
- Selmi C, Bowlus CL, Gershwin ME et al. Primary biliary cirrhosis. Lancet 2011; 377: 1600–1609. [DOI] [PubMed] [Google Scholar]
- Myers RP, Shaheen AA, Fong A et al. Epidemiology and natural history of primary biliary cirrhosis in a Canadian health region: a population-based study. Hepatology 2009; 50: 1884–1892. [DOI] [PubMed] [Google Scholar]
- Kim KA, Ki M, Choi HY et al. Population-based epidemiology of primary biliary cirrhosis in South Korea. Aliment Pharmacol Ther 2016; 43: 154–162. [DOI] [PubMed] [Google Scholar]
- The Hospital AuthorityHospital Authority statistical report 2012–2013. Available at http://www.ha.org.hk/haho/ho/stat/HASR1415_2.pdf Accessed 12 January 2017.
- The Hospital AuthorityIntroduction. Available at http://www.qeh.org.hk/visitor/ha_visitor_index.asp?Content_ID=10008&Lang=EN&Dimension=100Accessed 12 January 2017.
- Wong CPHealth informatics development in the hospital authority. Available at http://www.ha.org.hk/haconvention/Accessed Accessed 12 January 2017.
- Man KK, Ip P, Hsia Y et al. ADHD drug prescribing trend is increasing among children and adolescents in Hong Kong. J Atten Disord 2014. (e-pub ahead of print). Available at http://dx.doi.org/10.1177/1087054714536047. [DOI] [PubMed]
- Chiu SS, Lau YL, Chan KH et al. Influenza-related hospitalizations among children in Hong Kong. N Engl J Med 2002; 347: 2097–2103. [DOI] [PubMed] [Google Scholar]
- Chan EW, Lau WC, Leung WK et al. Prevention of Dabigatran-Related Gastrointestinal Bleeding With Gastroprotective Agents: A Population-Based Study. Gastroenterology 2015; 149: 586–595.e583. [DOI] [PubMed] [Google Scholar]
- He Y, Chan EW, Man KK et al. Dosage effects of histamine-2 receptor antagonist on the primary prophylaxis of non-steroidal anti-inflammatory drug (NSAID)-associated peptic ulcers: a retrospective cohort study. Drug Saf 2014; 37: 711–721. [DOI] [PubMed] [Google Scholar]
- Man KK, Chan EW, Coghill D et al. Methylphenidate and the risk of trauma. Pediatrics 2015; 135: 40–48. [DOI] [PubMed] [Google Scholar]
- Wong OF, Ho PL, Lam SK. Retrospective review of clinical presentations, microbiology, and outcomes of patients with psoas abscess. Hong Kong Med J 2013; 19: 416–423. [DOI] [PubMed] [Google Scholar]
- Lindor KD, Gershwin ME, Poupon R et al. Primary biliary cirrhosis. Hepatology 2009; 50: 291–308. [DOI] [PubMed] [Google Scholar]
- Census and Statistics DepartmentHong Kong Statistics. Available at http://www.censtatd.gov.hk/hkstat/sub/sp150.jsp?tableID=002&ID=0&productType=8. Accessed 12 January 2017.
- Wong GL, Hui AY, Wong VW et al. A retrospective study on clinical features and prognostic factors of biopsy-proven primary biliary cirrhosis in Chinese patients. Am J Gastroenterol 2005; 100: 2205–2211. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Y, Wang L et al. Prevalence of primary biliary cirrhosis in adults referring hospital for annual health check-up in Southern China. BMC Gastroenterol 2010; 10: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CW, Hung HH, Huo TI et al. Natural history and prognostic factors of primary biliary cirrhosis in Taiwan: a follow-up study up to 18 years. Liver Int 2008; 28: 1305–1313. [DOI] [PubMed] [Google Scholar]
- Wong RK, Lim SG, Wee A et al. Primary biliary cirrhosis in Singapore: evaluation of demography, prognostic factors and natural course in a multi-ethnic population. J Gastroenterol Hepatol 2008; 23: 599–605. [DOI] [PubMed] [Google Scholar]
- Gu EL, Yao GB. The clinical characteristics of primary biliary cirrhosis in China: a systematic review. Zhonghua Gan Zang Bing Za Zhi 2009; 17: 861–866. [PubMed] [Google Scholar]
- Miyakawa H, Tanaka A, Kikuchi K et al. Detection of antimitochondrial autoantibodies in immunofluorescent AMA-negative patients with primary biliary cirrhosis using recombinant autoantigens. Hepatology 2001; 34: 243–248. [DOI] [PubMed] [Google Scholar]
- Lammers WJ, van Buuren HR, Hirschfield GM et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology 2014; 147: 1338–1349.e1335; quiz e1315. [DOI] [PubMed] [Google Scholar]
- Census and Statistics DepartmentHong Kong Statistics. Available at http://www.fhb.gov.hk/statistics/en/statistics/life_expectancy.htm. Accessed 12 January 2017.
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005; 353: 1261–1273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.