Abstract
Pulmonary hamartomas are rare benign tumors consisting of multiple mesenchymal cell lines like cartilage, bone and fat. We discuss an interesting case of a 53-year-old male patient, who was referred to our clinic for persistent cough. Chest X-ray revealed a left suprahilar density associated with plate like atelectasis, which on chest CT was found to be a densely calcified nodule, causing narrowing of the left upper lobe (LUL) bronchus with calcified bilateral hilar lymph nodes. A bronchoscopy revealed a smooth endobronchial mass with calcification, which was removed. Histopathology revealed pulmonary hamartoma.
1. Introduction
Pulmonary hamartomas are rare tumors with a population incidence of 0.25%, constituting about 8% of all benign lung neoplasms [1], [2], [3]. These benign neoplasms are composed of varying proportions of cartilage, fat, fibrous tissue and entrapped respiratory epithelium [1], [2], [3]. Only 10% of pulmonary hamartomas occur endobronchially, while the rest are peripheral [2]. They occur more commonly in males and the peak incidence is in the six to seventh decade of life [4], [5]. They are usually small and well demarcated. Average size is 1.5 cm, but some can grow as big as 6 cm, causing irreversible destruction of lung tissue [4], [5], [6].
Hereby, we discuss an interesting case of endobronchial hamartoma with chronic cough as the presenting symptom.
2. Case report
A 53 year old male, who was following with Allergy and Immunology for asthma was referred to our pulmonary clinic for persistent cough for the last 10 months that could not be controlled despite aggressive treatment of his asthma, allergic rhinitis and gastroesophageal reflux disease (GERD). His cough was triggered by a ticklish sensation in his central and left upper chest area and was associated with post tussive shortness of breath and dizziness. He denied any other associated symptoms like wheezing, chest pain, fever, chills, sputa and weight loss. Past medical history included Asthma, Allergic rhinitis, Gastroesophageal Reflux, Hypertension, Diabetes Mellitus, Liver Cancer (cancer free after resection) and Post Traumatic Stress Disorder. He was a former smoker with <5 pack year history of smoking and worked as a technician in the Navy. He denied any asbestos exposure while working in Navy. There was no recent travelling history. He had a dog at home. His father had lung cancer but details were unknown. He was using symbicort twice a day and albuterol four times a day for asthma at the time of presentation. Before he was referred to the pulmonary clinic, he got treated with oral bursts of prednisone, for his cough as it was thought to be from upper respiratory tract infection (URI) related asthma exacerbation. His ACE inhibitor was stopped. With his known history of allergic rhinitis, the treatment was optimized by giving oral Cetirizine in addition to nasal saline and steroid sprays, and increasing the control of environmental factors to avoid common allergen exposures. GERD treatment was also optimized by increasing the dose of PPI. The cough persisted after optimizing treatment for the common causative factors, therefore further work up including IgE, RAST panel (both found to be in normal limits) and Chest X-ray was ordered by the allergist. Pulmonary function tests at the time, did not show any obstruction but were significant for mild restrictive disease. The results of the chest X-ray showed previously known healed calcified lymph nodes most prominent in the left hilar region and development of a new long linear density which was about 5mm thick arising from left hilar region superiorly and extending laterally, when compared with the old roentgenograms. (Fig. 1A–B). Atelectasis was suspected but no other infiltrate was noted at that time. Patient was then referred to pulmonary clinic due to the above mentioned abnormal findings on Chest X-ray. On presentation, he was hypertensive to 174/96 mmHg, tachypneic with respiratory rate of 24/min, tachycardic with heart rate of 118/min and oxygen saturation on room air was normal at 97%. He was afebrile. There were no stigmata of active sinus inflammation and no wheezing was heard on auscultation. His lab findings were significant for a normal white cell count of 7.8 with eosinophilia of 4.2%. Chest CT done subsequent to the chest X-ray showed a densely calcified nodule in the left perihilar region, narrowing of the left upper lobe (LUL) bronchus with plate like atelectasis of the LUL (Fig. 1C–H). A bronchoscopy was performed which revealed, a smooth mass occluding the LUL bronchus, without gross evidence of hemorrhage, necrosis or dilated blood vessel and with minimal reaction in the surrounding airways. There was evidence of calcification in the inferior and lateral aspect of the mass (Fig. 2A–B). Histopathology showed a hypercellular submucosal cartilaginous nodule with islands of cartilage close to the bronchial epithelium. The patient was then treated with laser resection of the tumor and balloon dilation of the LUL bronchus which resulted in complete resolution of his symptoms. He was followed up with CT scans for 2 years. His cough returned and a repeat CT scan showed LUL bronchus obstruction and atelectasis. He underwent a repeat bronchoscopy at that time that showed LUL scarring and obstruction for which balloon dilation was attempted without success. At that time patient was referred to thoracic surgery and underwent LUL resection. On histopathology exam, there was no malignancy seen in the resected specimen. There was a partially occlusive endobronchial inflammatory granulation tissue polyp. An ill-defined mass was seen close to the hilum, consisting of, multiple islands of benign cartilage, slit like bronchial epithelium and adipose tissue in a disorganized manner suggestive of pulmonary hamartoma. (Fig. 3A–D). Left hilar lymph nodes were calcified and necrotic with non-infective granulomata.
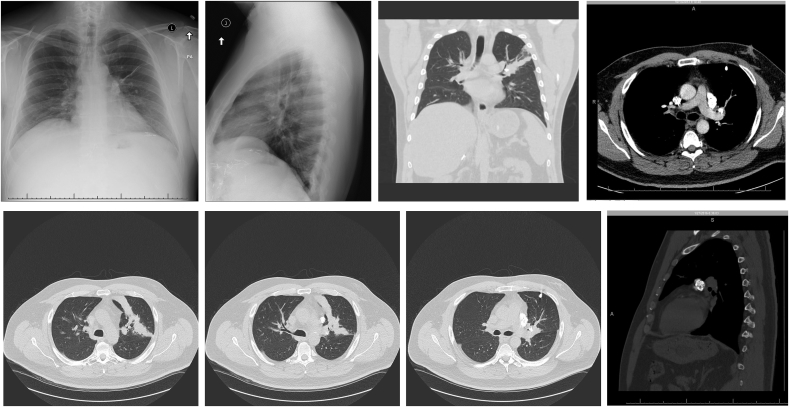
Fig. 1.
A: Dense calcified mass (arrow) overlying the left hilum associated with linear atelectasis extending laterally from the hilum and hyperinflation of the left upper lobe consistent with partial airway obstruction and peripheral air trapping. B: Chest X-ray Lateral View: Partial left upper lobe collapse and hilar calcifications. C: CT Chest Coronal View Without Contrast: Densely Calcified rounded mass causing LUL Bronchus narrowing and Calcified left hilar lymph nodes. D: CT Chest Axial View with contrast: Irregular Lobulated Calcified mass in LUL Bronchus causing plate like atelectasis with bilateral calcified hilar lymph nodes. E: CT Chest without contrast Axial View: Plate like LUL Partial Atelectasis due to LUL Bronchus narrowing. F: Caclified Lymphnodes in the Left Supra-hilar region. G: LUL Bronchus narrowing from a calcified endobronchial mass with surrounding non-calcified tissue. H: CT Chest Coronal View: Classic appearance of “popcorn calcification” in the left supra hilar region.
Fig. 2.
A: LUL Endobronchial Mass with smooth surface and inferolateral calcification. B: The anterior segment of the left upper lobe completely obstructed with scaring tissue and protrusion of the mass.
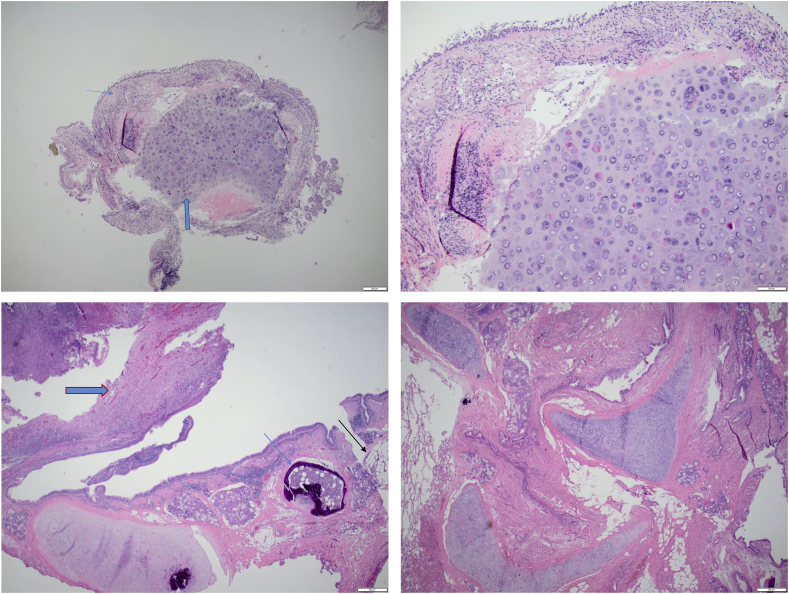
Fig. 3.
A: Benign lobulated mature cartilage ( ) covered by respiratory epithelium (
) covered by respiratory epithelium ( ) (H&E biopsy specimen ×2). B: The benign Hyaline cartilage is hypercellular with slightly irregular surface (×10). C: Inflammatory polyp (
) (H&E biopsy specimen ×2). B: The benign Hyaline cartilage is hypercellular with slightly irregular surface (×10). C: Inflammatory polyp ( ) and osseous metaplasia of peribronchial cartilage (
) and osseous metaplasia of peribronchial cartilage ( ) and adjacent fat (
) and adjacent fat ( ) (H&E resection specimen ×2). D: Disordered mass forming proliferation of mature cartilage, fibrovascular connective tissue, benign adipose tissue and slit like entrapped respiratory epithelium, consistent with Hamartoma. (H&E resection specimen ×2).
) (H&E resection specimen ×2). D: Disordered mass forming proliferation of mature cartilage, fibrovascular connective tissue, benign adipose tissue and slit like entrapped respiratory epithelium, consistent with Hamartoma. (H&E resection specimen ×2).
3. Discussion
Pulmonary Hamartoma is a benign tumor that contains mesenchymal and epithelial elements. It results from neoplastic transformation of a primitive mesenchymal cell that differentiates into chondroid, adipose and smooth muscle cells. As a result they can have multiple diverse mesenchymal populations including fat, cartilage and smooth muscle. A hamartoma is distinguished from other benign endobronchial tumors like Chondromas or Lipomas by the presence of at least two mesenchymal elements as compared to the latter, which have only one mesenchymal element ie either cartilage or fat respectively. Genetic rearrangement of chromosome band 12q15 is frequently found in pulmonary hamartoma [7]. Less commonly seen is a translocation t(12; 14) (q15; q24) which typically occurs in leiomyomas [7].
Our patient was evaluated for various etiologies of chronic cough in order to obtain the correct diagnosis. Common causes of chronic cough include environmental or occupational irritants, primary or secondary smoking, use of angiotensin-converting enzyme (ACE) inhibitors, abnormal chest radiographic findings, asthma, upper airway cough syndrome due to a variety of rhinosinusitis conditions, non-asthmatic eosinophilic bronchitis, and suppurative lung disease [8]. The patient was not on ACE inhibitors and he was on maximum therapy for asthma and allergic rhinitis with no improvement in his cough. An abnormal chest X-ray caused us to obtain a chest CT, which ultimately led us to the diagnosis.
Symptoms of hamartoma depend on the location of the tumor. Patients can be asymptomatic with peripheral parenchymal hamartomas, with incidental discovery on chest imaging obtained for another reason. If located endobronchially, they can cause irritation of bronchial mucosa, causing persistent cough as a presenting symptom like in our patient. Endobronchial hamartomas can be associated with obstruction of the bronchus resulting in symptoms of fever, cough, expectoration, wheezing, and dyspnea. Complications like post obstructive pneumonia and lobar atelectasis occur, as was seen in our patient [9]. Hemoptysis can occur if it impinges on vascular structures and causes invasion or perforation. Massive hemoptysis has been reported in the literature [10], [11], [12].
Conventional radiography can be nonspecific and related to post-obstructive changes, such as atelectasis, pneumonia, and bronchiectasis [13]. Our patient initially showed findings of plate like atelectasis. In parenchymal hamartoma, the typical radiographic appearance is in the form of a solitary pulmonary nodule that may contain popcorn calcification, which was evident in our patient as well [13]. However, in some cases, endobronchial hamartomas may be poorly demonstrated or may not be seen at all on chest radiograph.
Chest CT shows collections of fat alternating with foci of calcification. It typically contains more fat tissue than parenchymal elements. In our case the tumor was seen as an irregular densely calcified left suprahilar mass associated with plate like atelectasis of the anterior LUL. Presence of calcified hilar masses increased our suspicion for infectious causes like Histoplasmosis, and Tuberculosis. Infectious causes, primary calcified tumors other than hamartomas, and foreign bodies, were all excluded in our patient after the appropriate work up and pathologic exam of the resected specimen.
Grossly, endobronchial hamartomas usually project as broad-based, polypoid nodules within the lumen of a large bronchus as was seen in our case. They tend to have a bulging cut surface and are white or grey with a firm consistency [13]. Histologically, most central and peripheral pulmonary hamartomas contain a predominance of cartilage (chondromatous hamartoma), as well as variable amounts of fibromyxoid connective tissue, fat, bone, and smooth muscle in decreasing order of frequency [13]. Fat may be more abundant in endobronchial lesions, and there is a constant association with the bronchial wall [13]. Slit-like spaces lined by entrapped, benign, pulmonary epithelium is a constant feature of both endobronchial and parenchymal hamartomas, and the surface of endobronchial hamartomas is lined by respiratory epithelium or metaplastic squamous epithelium [13].
In our case, endobronchial biopsy and surgical resection specimens showed bronchial epithelium with metaplastic changes and islands of cartilage arranged in a disorganized manner suggestive of hamartoma. There were also unrelated scattered partially calcified granulomata that were negative for mycobacteria by Acid-Fast Bacteria stain and fungal organisms by Gomori Methenamine Silver stain. There was no evidence of malignancy.
4. Conclusion
The treatment of endobronchial hamartomas is conservative in the absence of chronic post-obstructive lung injury and includes endoscopic excision, wedge resection, or sleeve excision, whereas lobectomy may be necessary in cases with recurrent pneumonia. In our case, after initial biopsy, chondroma vs hamartoma was suspected. The patient was offered resection of the mass endoscopically versus surgical options and he chose the former. He underwent laser resection of the mass with balloon dilation of LUL Bronchus with complete symptomatic relief. Unfortunately, after 2 years, he had recurrence of tumor growth with narrowing of the LUL bronchus causing LUL atelectasis and recurrence of unrelenting cough. Patient then elected to undergo LUL lobectomy with complete resolution of his symptoms. He remains well and symptom free after 1 year of follow up post resection.
References
- 1.Syahminan S., Zeid A., Carolyn A. Pictorial essay of radiological features of benign intrathoracic masses. Ann. Thorac. Med. 2015 Oct-Dec;10(4):231–242. doi: 10.4103/1817-1737.160365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David O., Beasley M.B., Minardi A.J., Jr., Malek F., Kovitz K.L. Management of endobronchial hamartoma. J. La State Med. Soc. 2003 Mar-Apr;155(2):110–112. [PubMed] [Google Scholar]
- 3.Agnieszka C., Macura Katarzyna J. Evaluation of solitary pulmonary nodule detected during computed tomography examination. Pol. J. Radiol. 2012 Apr-Jun;77(2):22–34. doi: 10.12659/pjr.882967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarioglu N., Susur A., Goksel T., Paksoy S., Erel F. An unexpected cause of hemoptysis: endobronchial lipomatous hamartoma. Med. Arch. 2014;68(1):65–66. doi: 10.5455/medarh.2014.68.65-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gjevre J.A., Myers J.L., Prakash U.B. Pulmonary hamartomas. Mayo Clin. Proc. 1996;71:14–20. doi: 10.4065/71.1.14. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Zehani-Kassar A., Ayadi-Kaddour A., Marghli A., Ridene I., Kilani T., El Mezni F. Clinical characteristics of resected bronchial hamartoma. Study of seven cases. Rev. Mal. Respir. 2011 May;28(5):647–653. doi: 10.1016/j.rmr.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher J.A., Longtine J., Wallace K., Mentzer S.J., Sugarbaker D.J. Cytogenetic and histologic findings in 17 pulmonary chondroid hamartomas: evidence for a pathogenetic relationship with lipomas and leiomyomas. Genes Chromosom. Cancer. 1995;12:220–223. doi: 10.1002/gcc.2870120310. [DOI] [PubMed] [Google Scholar]
- 8.Achilleos A. Evidence-based evaluation and management of chronic cough. Med. Clin. North Am. 2016 Sep;100(5):1033–1045. doi: 10.1016/j.mcna.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Ishibashi H., Akamatsu H., Kikuchi M., Sunamori M. Resection of endobronchial hamartoma by bronchoplasty and transbronchial endoscopic surgery. Ann. Thorac. Surg. 2003 Apr;75(4):1300–1302. doi: 10.1016/s0003-4975(02)04624-6. [DOI] [PubMed] [Google Scholar]
- 10.Garcha P., Machuzak M., Arrossi A., Stoller J.K. Endobronchial hamartoma causing massive hemoptysis. J. Bronchology Interv. Pulmonol. 2009;16(4):298–300. doi: 10.1097/LBR.0b013e3181bf0edc. [DOI] [PubMed] [Google Scholar]
- 11.Sharkey R.A., Mulloy E.M., O'Neill S. Endobronchial hamartoma presenting as massive hemoptysis. Eur. Respir. J. 1996;9(10):2179–2180. doi: 10.1183/09031936.96.09102179. [DOI] [PubMed] [Google Scholar]
- 12.Kleinman J., Zirkin H., Feuchtwanger M.M., Hertzanu Y., Walfisch S. Benign hamartoma of the lung presenting as massive hemoptysis. J. Surg. Oncol. 1986;33(1):38–40. doi: 10.1002/jso.2930330111. [DOI] [PubMed] [Google Scholar]
- 13.Wilson R.W., Kirejczyk W. Pathological and radiological correlation of endobronchial neoplasms: Part I, Benign tumors. Ann. Diagn. Pathol. 10/1997;1(1) doi: 10.1016/s1092-9134(97)80007-x. [DOI] [PubMed] [Google Scholar]