Abstract
Receptor tyrosine kinases (RTKs) are membrane receptors that play a vital role in various biological processes, in particular, cell survival, cell proliferation, and cell differentiation. These cellular processes are composed of multitiered signaling cascades of kinases starting from ligand binding to extracellular domains of RTKs that activate the entire pathways through tyrosine phosphorylation of the receptors and downstream effectors. A previous study reported that, based on proteomics data, RTKs were a major candidate target for osteosarcoma. In this study, activation profiles of six candidate RTKs, including c-Met, c-Kit, VEGFR2, HER2, FGFR1, and PDGFRα, were directly examined from chemonaive fresh frozen tissues of 32 osteosarcoma patients using a multiplex immunoassay. That examination revealed distinct patterns of tyrosine phosphorylation of RTKs in osteosarcoma cases. Unsupervised hierarchical clustering was calculated using Pearson uncentered correlation coefficient to classify RTKs into two groups—Group A (c-Met, c-Kit, VEGFR2, and HER2) and Group B (FGFR1 and PDGFRα)—based on tyrosine phosphorylation patterns. Nonactivation of all Group A RTKs was associated with shorter overall survival in stage IIB osteosarcoma patients. Percentages of tumor necrosis in patients with inactive Group A RTKs were significantly lower than those in patients with at least one active Group A RTK. Paired primary osteosarcoma cells with fresh osteosarcoma tissue were extracted and cultured for cytotoxicity testing. Primary cells with active Group A RTKs tended to be sensitive to doxorubicin and cisplatin. We also found that osteosarcoma cells with active Group A RTKs were more proliferative than cells with inactive Group A RTKs. These findings indicate that the activation pattern of Group A RTKs is a potential risk stratification and chemoresponse predictor and might be used to guide the optimum chemotherapy regimen for osteosarcoma patients.
Introduction
Osteosarcoma is an aggressive primary bone sarcoma that occurs predominantly in children and teenagers [1]. Current treatment strategies for osteosarcoma include surgery to remove the tumor and chemotherapy [2]. The most effective regimen is based on a combination of methotrexate, cisplatin, and doxorubicin (MAP). Unfortunately, not all patients have a good response to the chemotherapeutic treatment; many patients with high-grade osteosarcoma develop chemoresistance to the MAP regimen, leading to poor clinical outcomes [3], [4]. Additionally, conventional chemotherapy can cause various side effects that worsen patient outcomes. The ability to identify patients who will respond poorly to chemotherapy is a promising approach for treating patients more effectively and with less toxic effects.
In our previous work, we established a list of target proteins of FDA-approved drugs based on results reported in several proteomics studies of osteosarcoma [5]. Interestingly, it was found that receptor tyrosine kinases (RTKs) including fibroblast growth factor receptor 1 (FGFR1), platelet-derived growth factor receptor alpha (PDGFRα), mast/stem cell growth factor receptor Kit (c-Kit), vascular endothelial growth factor receptor 2 (VEGFR2), hepatocyte growth factor receptor (c-Met), and receptor tyrosine-protein kinase erbB-2 (HER2) were a major target group. Expression levels of these RTKs were higher in osteosarcoma cells compared to osteoblastic cells.
Since the discovery of the first RTK in 1978, RTKs have been shown to be important growth factor receptors that regulate critical cellular processes including cell survival, proliferation, differentiation, metabolism, cell-cell communication, cell migration, and cell-cycle control [6], [7]. The human RTK family includes 58 members which fall into 20 subfamilies [8]. All known RTKs share a conserved molecular architecture with extracellular ligand-binding domains, a single transmembrane region, and a cytoplasmic kinase domain that is activated by tyrosine phosphorylation upon dimerization or oligomerization [8].
The FGFR family is comprised of four main members including FGFR1, FGFR2, FGFR3, and FGFR4 [9]. The extracellular region of all FGFRs contains three Ig-like domains that bind to FGFs in the presence of the accessory molecule heparin [10]. The main result of activation of FGF signals is to trigger the RAS–mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)–AKT signaling pathways which subsequently induce cell proliferation and cellular survival, respectively [11].
The PDGFR family includes PDGFR, Kit, CSF1R, and Flt3 which contain different numbers of extracellular Ig-like domains [10]. Binding of homodimeric or heterodimeric PDGFR (PDGFR-α an PDGFR-β) to different PDGFs (PDGFA, PDGFB, PDGFC, and PDGFD) transduces various signals and generates broad biological functions under diverse physiological and pathological conditions [12], [13]. A system of the PDGFR/PDGF complex induces cancer cell proliferation, angiogenesis, metastasis, and the development of tumor-associated fibroblasts [12], [14]. c-KIT receptor is also a member of the PDGFR family. That receptor binds to stem cell factor (SCF) molecules at the Ig-like domains of c-KIT [8]. Upon activation, the c-KIT/SCF system triggers various downstream pathways, mainly MAP kinase, that regulate cell survival and proliferation [15].
The VEGFR family (VEGFR1, VEGFR2, and VEGFR3) expresses Ig-like extracellular domains which are structurally related to the PDGFR family [16]. The VEGFR receptor and their co-receptors bind to distinct VEGF ligands including VEGFA, VEGFB, VEGFC, VEGFD, and placenta growth factor [17]. This different binding of the VEGFR/VEGFs regulates various biological mechanisms, mainly angiogenesis, vasculogenesis, lymphangiogenesis, permeability, inflammatory cell recruitment, and fatty acid uptake.
The c-MET receptor, also known as the hepatocyte growth factor receptor (HGF receptor), contains three extracellular domains including the Sema, the PSI, and the IPT domains [18]. Upon binding of the c-MET receptor and HGF, the complex triggers diverse signaling cascades, mainly the MAP kinase, the PI3K-Akt axis, the signal transducers and activators of transcription (STAT) pathway, and the IκBα–NF-κB complex [19], [20], [21], [22]. The activation of c-MET induces tissue remodeling as well as promoting cell survival, proliferation, and migration that facilitate invasive growth and metastasis of cancer cells.
HER2 receptor (erbB2, HER2/neu) is a member of the human epidermal growth factor receptor family that also includes EGFR (HER1, erbB1), HER3 (erbB3), and HER4 (erbB4) [23]. The extracellular region of HER2 consists of four domains: domains I and III which comprise the β helix LRR-like “solenoid” domains as well as the cysteine-rich domains II and IV [8]. HER2 does not contain a ligand-binding domain, which makes this receptor highly active [24]. Activation of HER2 induces important biological mechanisms involved in cell survival, proliferation, and cell-cycle progression through the PI3K/AKT and RAF/MEK/MAPK pathways [24].
Phosphorylated RTKs subsequently recruit adaptor proteins to trigger various downstream cascade signaling. Much evidence indicates that aberrant activation of RTKs, including gene amplification, receptor overexpression, autocrine activation, and gain-of-function mutations, has been causally linked to cancer [25]. For example, in gastric, lung, and esophageal tumors, it was found that the cancers with MET gene amplification were more sensitive to MET kinase inhibitors than those not carrying this aberrancy [26], [27]. Other examples include breast cancer with ERBB2 gene amplification, GIST with PDGFRα gene mutation, GIST with c-KIT gene mutation, and CML with FGFR1 gene translocation. The subset of patients with these aberrancies is highly sensitive to particular RTK inhibitors [28].
In this study, we examined further the activation state of those candidate RTKs directly in clinical samples (frozen osteosarcoma tissues) using the multiplex immunoassay, a rapid and clinically applicable method. The results of this experiment provide a better understanding of the relationship between activated RTKs and clinical outcomes in addition to identifying distinct groups among osteosarcoma patients. Moreover, the activated RTK patterns provide potentially important clues for further development of tailored therapy for the treatment of osteosarcoma.
Materials and Methods
Patients and Tissue Samples
A total of 32 patients diagnosed with stage IIB osteosarcoma and treated at Maharaj Nakorn Chiang Mai Hospital, Thailand, between 2010 and 2016 were included in this study. Patients were followed up for survival analysis for at least 24 months (until 30 June 2016). All biopsy samples were obtained prior to neoadjuvant treatments and were separated into three parts: one was kept as formalin-fixed, paraffin-embedded tissues for clinical diagnosis; the second was stored as fresh frozen tissues and used for determination of tyrosine phosphorylation of RTKs; and the third was processed further and cultured as primary cells. Tissue specimens were freshly frozen at −80°C within 30 minutes of surgery and stored until use.
To evaluate the percentage of tumor necrosis, tissue samples were obtained after the patients had been treated with chemotherapy. The patients' clinical information is shown in Table 1. This research protocol has been approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University.
Table 1.
Characteristics of Osteosarcoma Patients (n = 32)
| Factor | Patients (n, %) |
|---|---|
| Age (median = 15.5, years) | |
| ≤15 | 16, 50.0% |
| >15 | 16, 50.0% |
| Gender | |
| Male | 13, 40.6% |
| Female | 19, 59.4% |
| Site | |
| Extremities | 32, 100.0% |
| Axial | - |
| Tumor size (cm) | |
| ≤ 8 | 18, 56.2% |
| > 8 | 14, 43.8% |
| Metastasis | |
| Yes | 10, 31.2% |
| No | 22, 68.8% |
| Chemoresistance (n = 18) | |
| Good responder (tumor necrosis ≥90%) | 5, 27.8% |
| Poor responder (tumor necrosis <90%) | 13, 72.2% |
| Treatment acceptance | |
| Complied | 22, 68.8% |
| Against | 8, 25.0% |
| Lost to follow-up | 2, 6.2% |
| Status | |
| Alive | 17, 53.1% |
| Dead | 13, 40.6% |
| Lost to follow-up | 2, 6.3% |
Tissue Extraction and Multiplex Immunoassay
Fresh frozen tissues (100 mg) were cut into small pieces and crushed in liquid nitrogen using a chilled mortar and pestle. The tissue powder was then incubated in 1 ml of Milliplex MAP lysis buffer (Merck, Darmstadt, Germany) containing phosphatase inhibitors including 1 mM sodium orthovanadate and freshly added protease inhibitor cocktail (Merck, Darmstadt, Germany) with agitation on a rocking mixer at 4°C for 15 minutes. The lysate was centrifuged at 10,000×g for 10 minutes at 4°C. Supernatant was collected to determine protein concentration with BCA assay (Thermo Fisher Scientific, Waltham, MA), and lysate samples containing 25 μg protein were used and analyzed using multiplex immunoassay.
An RTK phosphoprotein magnetic bead panel kit was used, composed of six types of RTKs, including c-Kit, VEGFR2, c-Met, HER2, FGFR1, and PDGFRα (Merck, Darmstadt, Germany). The semiquantification of tyrosine phosphorylation levels of RTKs of all samples and controls (serum stimulated and unstimulated) provided in the kit was determined using manufacturer's instructions and detected by a Bio-Plex MAGPIX Multiplex Reader (Bio-Rad, Hercules, CA). The results were recorded as median fluorescence intensity (MFI) of RTKs of osteosarcoma cases normalized by blank controls. The MFI which was higher than the blank controls was considered as a positive result.
Data Analysis
Hierarchical cluster analysis was performed using GENESIS software version 1.7.7 (Bioinformatics Group, Institute of Knowledge Discovery, Graz University of Technology, Graz, Austria).
Patient-Derived Osteosarcoma Cells
Patient-derived osteosarcoma cells were extracted, cultured, and characterized following the previously described procedures [29]. This involved mincing chemonaive biopsy tissues and incubating in 5 mg/ml collagenase type I solution (Gibco, Waltham, MA) at 37°C for 18 hours. Cells were pelleted by centrifugation and then cultured in freshly prepared Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (Gibco, Waltham, MA) at 37°C in a humidified 5% CO2 incubator. All osteosarcoma primary cells were characterized for osteogenicity; cancer markers including MMP-9 and collagen type X were determined by real-time reverse transcriptase polymerase chain reaction according to previously described protocol [29].
Cell Viability Assay
Cell viability was determined using MTT assay according to previously published protocols with some modifications [30]. Primary osteosarcoma cells were seeded in 96-well tissue culture plates (5 × 103 cells/100 μl freshly prepared DMEM/well) and incubated at 37°C, 5% CO2 overnight. The cells were treated with doxorubicin or cisplatin at concentrations of 0, 0.01, 0.1, 1, 10, and 100 μM for 72 hours. After 72-hour incubation, the culture medium was removed, and 100 μl of fresh medium containing 5 mg/ml of MTT solution was added to each well and then incubated for 2 hours at 37°C. The MTT-formazan crystal was dissolved in 100 μl of dimethyl sulfoxide with vigorous mixing. Finally, measurement was performed using a spectrophotometer with absorbance at 550 nm.
Statistical Analysis
Survival analyses were performed using STATA version 11 (StataCorp LLC). Association between activation profiles of RTKs and overall survival of osteosarcoma patients in this study was analyzed by univariate survival analysis using the Kaplan-Meier method and the log-rank test. P values < .05 were considered to be statistically significant. Significant correlation between activation patterns of RTKs and percentage of tumor necrosis was tested using the Mann-Whitney U test by Prism 7 version 7.0c (GraphPad Software, Inc.).
Results
Distinct Levels of Tyrosine Phosphorylation of RTKs in Osteosarcoma
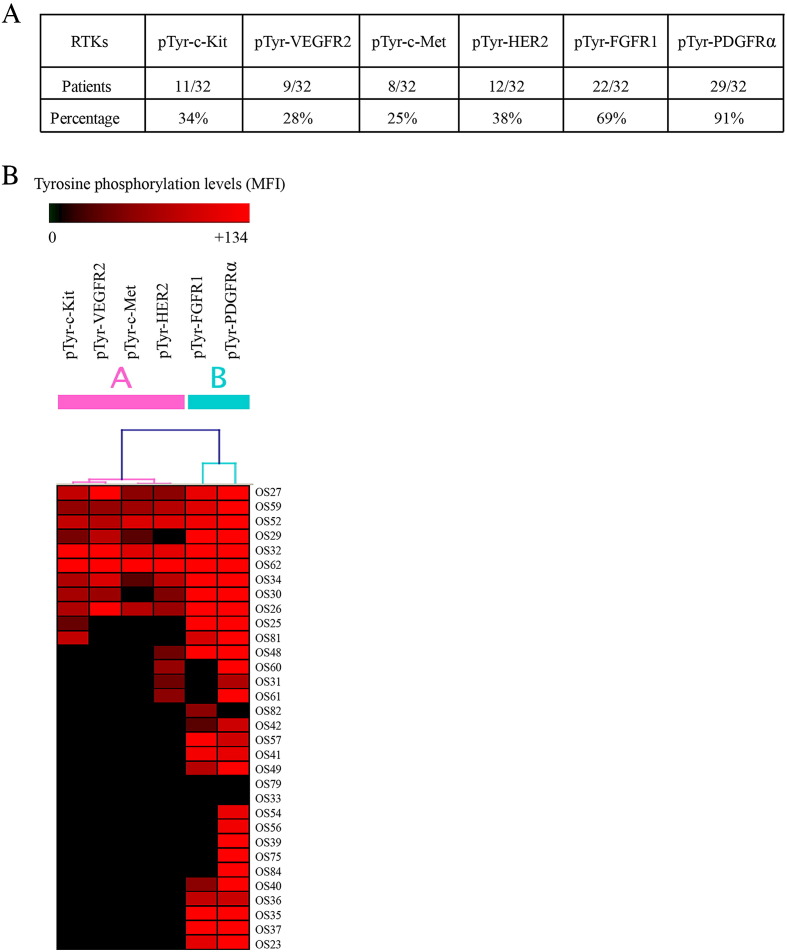
A multiplex immunoassay was used to detect tyrosine phosphorylation levels of six RTKs in fresh frozen osteosarcoma tissues simultaneously. Levels of tyrosine phosphorylation indicating the activation state of RTKs were determined by MFI. The majority of the osteosarcoma tissue samples showed activation of PDGFRα (90.63%) and FGFR1 (68.75%). Unlike these two RTKs, tyrosine phosphorylation of the others, c-Kit, VEGFR2, c-Met, and HER2, ranged from 25.00% to 37.50% (Figure 1A).
Figure 1.
Tyrosine phosphorylation of tested RTKs in osteosarcoma. (A) Number and percentage of osteosarcoma cases in which RTKs were phosphorylated at tyrosine residues. (B) Unsupervised hierarchical clustering of six RTKs based on levels of tyrosine phosphorylation (MFI) of the 32 osteosarcoma cases (GENESIS software). Osteosarcoma case numbers are shown on the right.
Hierarchical Clustering Analysis Classification of Tested RTKs Based on Activation Profiles in Osteosarcoma
The MFI of each of the RTKs is shown as a heat map to illustrate the activation patterns of the individual tissue samples (Figure 1B). Based on levels of tyrosine phosphorylation of tested RTKs, RTKs were classified into two subgroups based on unsupervised hierarchical clustering using Pearson uncentered correlation coefficient (GENESIS software) [31]. Group A included pTyr-c-Kit, pTyr-VEGFR2, pTyr-c-Met, and pTyr-HER2; Group B included pTyr-FGFR1 and pTyr-PDGFRα.
Hierarchical clustering was also performed to classify the osteosarcoma patients based on the tyrosine phosphorylation profiles of all tested RTKs in individual cases. Although various metrics were applied in an attempt to identify subsets of osteosarcoma, active RTK patterns of the osteosarcoma cases were too heterogeneous to be classified appropriately (data not shown).
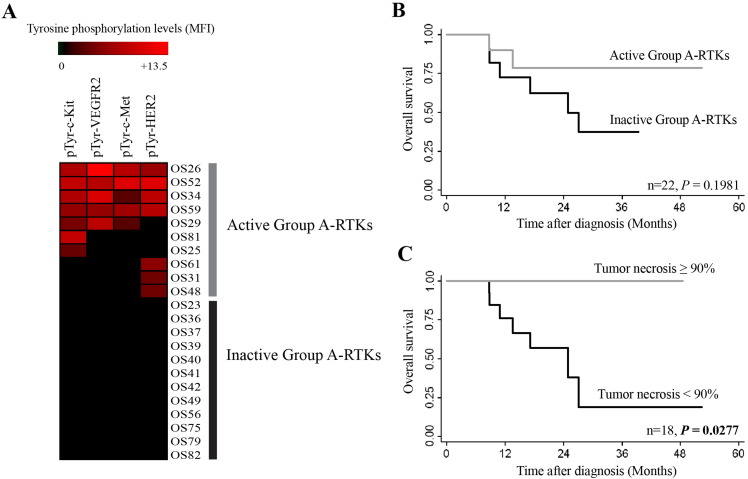
Association of Inactive Group A-RTKs with Worst Overall Survival of Stage IIB Osteosarcoma Patients
Hierarchical clustering of osteosarcoma cases uncovered differences between Group A and Group B RTKs with similar tyrosine phosphorylation profiles. Each set of RTKs was analyzed separately to determine the association with overall survival of the patients. Retrospective data of 22 osteosarcoma patients who had been treated with neoadjuvant chemotherapy and/or undergone surgery at Maharaj Nakorn Chiang Mai Hospital and who accepted and complied with treatments were used in the survival analysis. Two patients were lost to follow-up and were excluded from the study (Table 1). Group A-RTKs, which included activated c-Kit, VEGFR2, c-Met, and HER2, patients were further subdivided into two groups based on the activation status of these RTKs (Figure 2A).
Figure 2.
Analysis of median survival of stage IIB osteosarcoma by activation states of Group A-RTKs (c-Kit, VEGFR2, c-Met, and HER2). (A) Active (osteosarcoma tissues with at least one RTK tyrosine phosphorylated) and inactive (none of the RTK tyrosine phosphorylated). (B) Kaplan-Meier curves of overall survival based on activation status of RTKs. (C) Kaplan-Meier curves based on percentage of tumor necrosis. (P values were calculated with the log-rank test).
Analysis found that patients carrying inactive Group A-RTKs tended to have shorter overall survival compared to patients who had at least one active Group A-RTK, although the differences were not statistically significant (Figure 2B). The significant negative prognosticator observed in this study was the percentage of tumor necrosis (P = .0227) (Figure 2C). Among Group B-RTKs, no correlation between active status of RTKs and overall survival was observed among the osteosarcoma patients (data not shown).
Differential Chemosensitivity of Osteosarcoma Patients Associating with Group A-RTK Activation Profiles
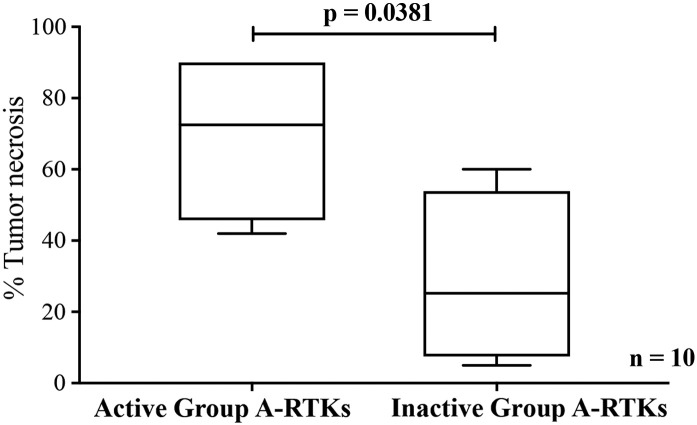
Because the survival analysis results showed osteosarcoma patients with inactive Group A-RTKs tended to have a worse prognosis, further analysis was conducted into the association between activation status of RTKs and both chemoresistance and metastasis. Patients in the cohort that had been treated with the same chemotherapy regimen, including doxorubicin and cisplatin, were identified (n = 10), and their percentages of tumor necrosis after treatment were evaluated by an experienced pathologist. It was found that osteosarcoma patients with active Group A-RTKs were more sensitive to chemotherapy than patients with inactive Group A-RTKs and that the percentage of tumor necrosis was statistically significantly lower in the inactive Group A-RTK patients (P = .0381) (Figure 3). No correlation between the activation status of Group A-RTKs and metastasis was observed (data not shown).
Figure 3.
Boxplot of percentages of tumor necrosis of osteosarcoma tissue samples obtained after neoadjuvant treatments (doxorubicin and cisplatin) in patients with at least one active Group A-RTK and with no active Group A-RTKs. (P values were calculated with the Mann-Whitney U test).
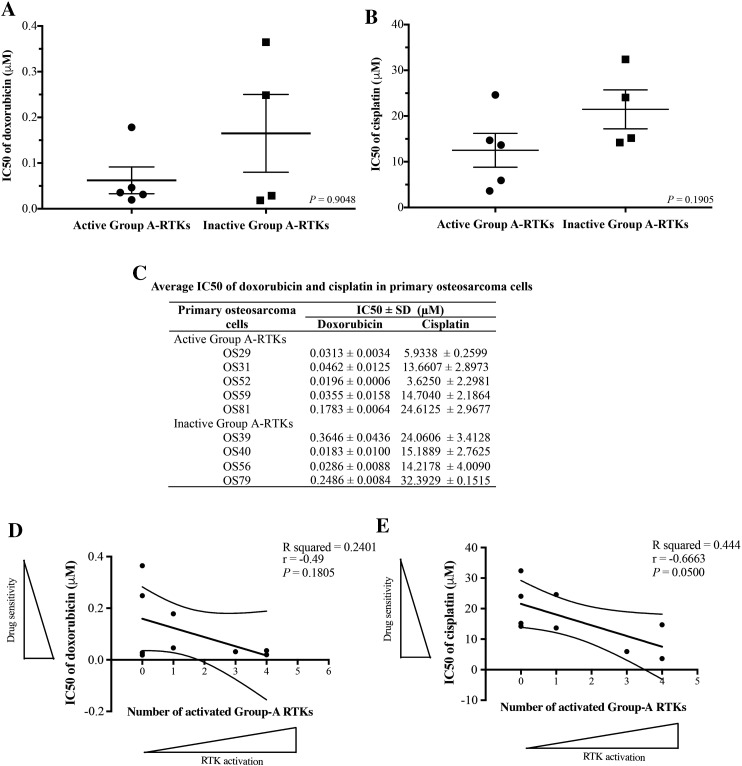
Association of Differential Drug Sensitivity of Primary Osteosarcoma Cells with Activation Patterns of Group A-RTKs
Paired primary osteosarcoma cells with fresh frozen tissue specimens from nine patients who had been treated with either doxorubicin or cisplatin were used for in vitro cell viability assay. One sample was excluded from the experiment because it did not grow in the culture. All primary cells were treated with either doxorubicin or cisplatin at various concentrations and examined for cell viability using MTT assay. The majority of the primary osteosarcoma cells with active Group A-RTKs were found to be more sensitive to both doxorubicin and cisplatin treatments compared to those cells with inactive Group A-RTKs (Figure 4, A and B). Summary of the averages of the half maximal inhibitory concentration (IC50) of doxorubicin and cisplatin from three independent experiments is presented in Figure 4C. In addition, it was found that sensitivity to doxorubicin and cisplatin had a tendency to increase in primary osteosarcoma cells with a higher number of activated Group A-RTKs (Figure 4, D and E).
Figure 4.
In vitro cell viability of osteosarcoma primary cells using MTT assay after treatment with (A) doxorubicin and (B) cisplatin at indicated drug concentrations. (C) IC50 averages of doxorubicin and cisplatin from three independent experiments. Graph shows an association between the number of activated Group A-RTKs and sensitivity to (D) doxorubicin and (E) cisplatin. Active Group A-RTKs: at least one positive tyrosine phosphorylation of c-Kit, VEGFR2, c-Met or HER2. Inactive Group A-RTKs: unphosphorylation of c-Kit, VEGFR2, c-Met, and HER2 (results of matched frozen tissues from multiplex immunoassay).
Increasing Proliferation of Osteosarcoma Cells Carrying Active Group A-RTKs
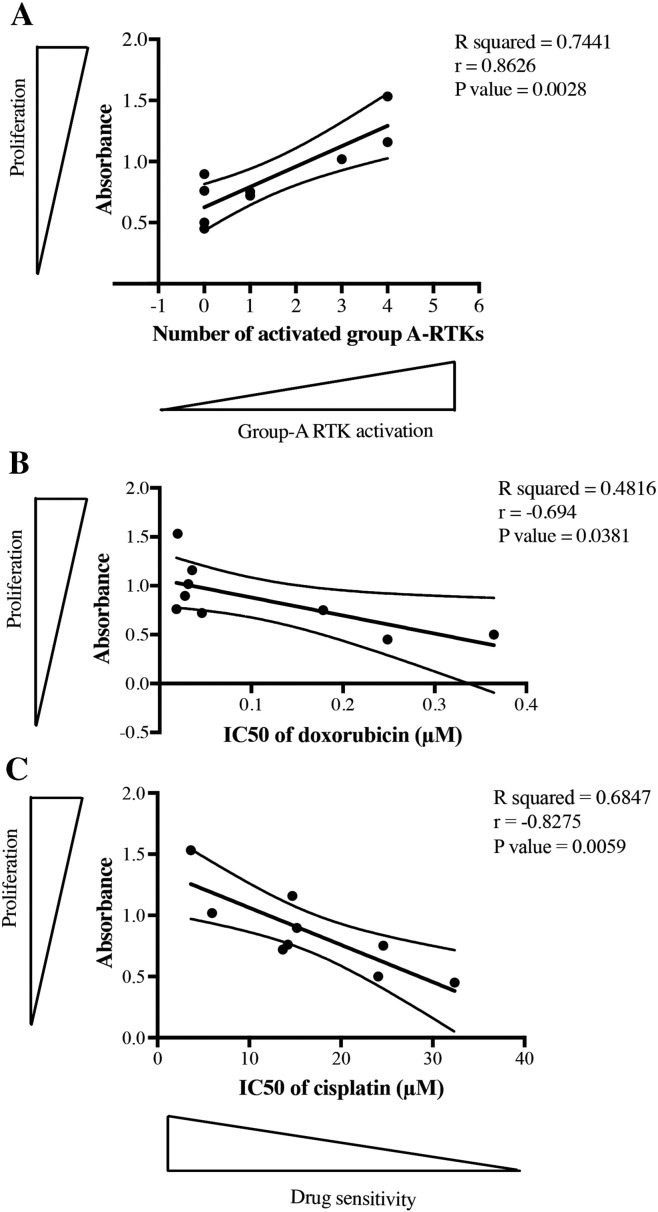
Results of the percentages of tumor necrosis and in vitro cytotoxicity testing indicate that active Group A RTKs appeared to induce higher chemoresponse than inactive ones. In this study, proliferation of osteosarcoma cells was also measured using MTT assay. The results showed that proliferation rates of primary osteosarcoma cells were significantly increased in cells carrying higher numbers of active Group A RTKs (P = .0028) (Figure 5A). In addition, we found that more proliferative osteosarcoma cells were more sensitive to both doxorubicin and cisplatin treatment (Figure 5, B and C).
Figure 5.
Association of proliferation of primary osteosarcoma cells with (A) the activation of Group A RTKs, (B) sensitivity to doxorubicin, and (C) sensitivity to cisplatin. P values of the linear relationship between the two data sets were calculated using the Pearson correlation coefficient.
Discussion
Aberrant activation of RTKs has emerged as a key factor in cancer progression and development [32]. However, there have only been limited findings on the association between the activation state of RTKs and clinical outcomes in osteosarcoma that have been reported. A previous study which was based on combining data from previously published research on proteomics found that some RTKs are overexpressed in osteosarcoma cells [5]. Following up on those results, we decided to continue working on these candidate RTKs, examining their activation profiles in individual patients, as well as determining correlations between patterns of activation status of RTKs and clinical outcomes. In this study, multiplex immunoassay was used to measure semiquantitative levels of tyrosine phosphorylation of six RTKs in fresh frozen tissues obtained from osteosarcoma patients. The results revealed frequent activation of FGFR1 (69%) and PDGFRα (91%), whereas lower numbers of osteosarcoma specimens showed activation of c-Kit (34%), VEGFR2 (28%), c-Met (25%), and HER2 (38%). Interestingly, we found that nonactivation of Group A-RTKs, including c-Kit, VEGFR2, c-Met, and HER2, was potentially associated with shorter overall survival and lower sensitivity to chemotherapy in stage IIB osteosarcoma patients, whereas patients with activation in one of these RTKs had a better prognosis. The current study clearly demonstrated that the percentage of tumor necrosis was higher in patients with active Group A-RTKs. Sensitivity to doxorubicin and cisplatin was further examined in patient-derived osteosarcoma cells. Even though the differences between groups in the in vitro cytotoxicity testing were not statistically significant, the results did show a consistent trend in the percentage of tumor necrosis in the tissue samples (Figures 3 and 4). The majority of primary osteosarcoma cells with distinct RTK activation profiles responded to doxorubicin and cisplatin at differential levels compared to those with non-RTK activation. The more active the Group A-RTKs were, the higher was the observed chemotherapeutic sensitivity (Figure 4, D and E).
Most studies of the prognostic significance of RTKs in osteosarcoma have focused on an expression of the receptors. Unfortunately, the correlation between levels of RTK expression and clinical outcome has remained inconclusive. For example, one study showed that an overexpression of c-Kit had a nonstatistically significant association with the worst outcomes in pediatric osteosarcomas (n = 56), whereas another study demonstrated that protein levels of c-Kit did not predict the prognosis (n = 100) [33], [34]. A meta-analysis of five studies evaluating the correlation of HER2 overexpression with 2-year survival found a tendency toward the worst clinical outcomes, but the results of the overall analysis were not statistically significant [35].
Recent data from a large multicenter randomized study, EURAMOS-1, suggested that the MAP regimen is the gold standard for treatment of osteosarcoma [36]. Moreover, poor response to chemotherapy has been confirmed as a strong negative prognostic indicator for survival. However, a major obstacle in improving patient survival is that no prognostic factors have been confirmed to be predictive markers for risk stratification or chemotherapy sensitivity in osteosarcoma. In addition, many attempts to discover the relevant targeted therapy in osteosarcoma have met with difficulties, mainly due to the inter- and intraheterogeneity of osteosarcoma. The ability to predict which patients are more likely to benefit from currently available chemotherapy would help ensure the use of the right regimen for the right patients as well as minimize toxicity. The findings from the present study suggest the potential for using active RTKs patterns in predicting chemotherapy sensitivity and in identifying good responders who will gain a true benefit from the two-drug regimen of doxorubicin and cisplatin. Importantly, it has been reported that this regimen can cure 50% of patients with localized osteosarcoma [37]. This study suggests that alternative treatment regimens might be more effective in the poor responders carrying inactive Group A-RTKs and might improve their survival rate.
These findings suggest that active RTKs play a role in the alteration of biological behavior of osteosarcoma cells, leading to increased drug sensitivity. Considering the mechanism of action of the drugs tested and the major cellular processes regulated by RTKs, we hypothesized that chemosensitive cells with active Group A-RTKs might have a higher proliferation rate than the other group and thus an increased responsiveness to chemotherapy. This idea was supported by two factors. First, doxorubicin and cisplatin are nonselective chemotherapies which target highly proliferative cells. Thus, they also kill active dividing normal cells, leading to toxic side effects in various tissues [38], [39]. Second, tyrosine-phosphorylated RTKs can provoke downstream signaling cascades that eventually induce activation of various pathways, mainly the MAPK family, PI3K, and Janus kinase/STAT proteins that govern cellular processes including cell proliferation, cell survival, and differentiation [40], [41]. The coactivation of Group A-RTKs might increase osteosarcoma cell proliferation and, in turn, enhance sensitivity to chemotherapy. In this study, proliferation of primary osteosarcoma cells was examined using MTT assay. The results support our hypothesis that more proliferative osteosarcoma cells carry more active Group A RTKs and are also more sensitive to doxorubicin and cisplatin treatment (Figure 5). This finding is consistent with results of a study of HER2-positive breast cancer which found that HER2-positive patients were significantly more sensitive to doxorubicin compared to HER2-negative patients and that most of the HER2-overexpression cell lines were highly proliferative as well as being sensitive to doxorubicin treatment [42]. Another study of primary uterine serous papillary carcinoma cell lines (USPC) overexpressing HER2 described it in the same manner: USPC overexpressing HER2 in vitro were more responsive to platinum compounds compared to HER2 low-expressing cells [43].
In conclusion, this study revealed a relationship between activation profiles of the RTKs c-Met, c-Kit, VEGFR2, and HER2, and the overall survival of nonmetastatic-stage (IIB) osteosarcoma patients. In addition, coactivation of RTKs augmented the response to a standard osteosarcoma chemotherapy regimen, particularly one based on doxorubicin and cisplatin. Together, these findings suggest an important correlation between activation patterns of RTKs and clinical outcomes in osteosarcoma that warrants further study, particularly of potential mechanisms of RTKs in chemoresponsive phenotypes. In addition, RTKs potentially have the capacity to identify chemoresistant patients early in treatments, thus allowing for the optimization of the use of new therapeutic options in combination with presently available drugs.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Acknowledgments
Acknowledgements
This work was supported by grant no. P-15-50265 from the National Science and Technology Development Agency; Faculty of Medicine, Chiang Mai University; National Research University fund; and the Excellence Center in Osteology Research and Training Center. The authors would also like to express their sincere thanks to Dr. G. Lamar Robert, PhD, and Assoc. Prof. Dr. Chongchit Sripun Robert, PhD, for editing the English manuscript.
References
- 1.Settakorn J, Lekawanvijit S, Arpornchayanon O, Rangdaeng S, Vanitanakom P, Kongkarnka S, Cheepsattayakorn R, Ya-In C, Thorner PS. Spectrum of bone tumors in Chiang Mai University Hospital, Thailand according to WHO classification 2002: A study of 1,001 cases. J Med Assoc Thail. 2006;89:780–787. [PubMed] [Google Scholar]
- 2.Ferrari S, Serra M. An update on chemotherapy for osteosarcoma. Expert Opin Pharmacother. 2015;16(18):2727–2736. doi: 10.1517/14656566.2015.1102226. [DOI] [PubMed] [Google Scholar]
- 3.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J Clin Oncol. 2015;33:3029–3035. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botter SM, Neri D, Fuchs B. Recent advances in osteosarcoma. Curr Opin Pharmacol. 2014;16:15–23. doi: 10.1016/j.coph.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Chaiyawat P, Settakorn J, Sangsin A, Teeyakasem P, Klangjorhor J, Soongkhaw A, Pruksakorn D. Exploring targeted therapy of osteosarcoma using proteomics data. Onco Targets Ther. 2017;10:565–577. doi: 10.2147/OTT.S119993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4(5):361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 7.Sun C, Bernards R. Feedback and redundancy in receptor tyrosine kinase signaling: relevance to cancer therapies. Trends Biochem Sci. 2014;39(10):465–474. doi: 10.1016/j.tibs.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16(2):139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Choura M, Rebai A. Receptor tyrosine kinases: from biology to pathology. J Recept Signal Transduct Res. 2011;31(6):387–394. doi: 10.3109/10799893.2011.625425. [DOI] [PubMed] [Google Scholar]
- 11.Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev. 2014;34(2):280–300. doi: 10.1002/med.21288. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y. Multifarious functions of PDGFs and PDGFRs in tumor growth and metastasis. Trends Mol Med. 2013;19(8):460–473. doi: 10.1016/j.molmed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Appiah-Kubi K, Wang Y, Qian H, Wu M, Yao X, Wu Y, Chen Y. Platelet-derived growth factor receptor/platelet-derived growth factor (PDGFR/PDGF) system is a prognostic and treatment response biomarker with multifarious therapeutic targets in cancers. Tumour Biol. 2016;37:10053–10066. doi: 10.1007/s13277-016-5069-z. [DOI] [PubMed] [Google Scholar]
- 14.Demoulin JB, Essaghir A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev. 2014;25(3):273–283. doi: 10.1016/j.cytogfr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Ashman LK, Griffith R. Therapeutic targeting of c-KIT in cancer. Expert Opin Investig Drugs. 2013;22(1):103–115. doi: 10.1517/13543784.2013.740010. [DOI] [PubMed] [Google Scholar]
- 16.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153(1):13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapisarda A, Melillo G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res. 2012;114:237–267. doi: 10.1016/B978-0-12-386503-8.00006-5. [DOI] [PubMed] [Google Scholar]
- 18.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11(12):834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 19.Pelicci G, Giordano S, Zhen Z, Salcini AE, Lanfrancone L, Bardelli A, Panayotou G, Waterfield MD, Ponzetto C, Pelicci PG. The motogenic and mitogenic responses to HGF are amplified by the Shc adaptor protein. Oncogene. 1995;10:1631–1638. [PubMed] [Google Scholar]
- 20.Boccaccio C, Ando M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio PM. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391:285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YW, Wang LM, Jove R, Vande Woude GF. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene. 2002;21:217–226. doi: 10.1038/sj.onc.1205004. [DOI] [PubMed] [Google Scholar]
- 22.Fan S, Gao M, Meng Q, Laterra JJ, Symons MH, Coniglio S, Pestell RG, Goldberg ID, Rosen EM. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene. 2005;24:1749–1766. doi: 10.1038/sj.onc.1208327. [DOI] [PubMed] [Google Scholar]
- 23.Moasser MM. Targeting the function of the HER2 oncogene in human cancer therapeutics. Oncogene. 2007;26(46):6577–6592. doi: 10.1038/sj.onc.1210478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollock NI, Grandis JR. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21(3):526–533. doi: 10.1158/1078-0432.CCR-14-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casaletto JB, McClatchey AI. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat Rev Cancer. 2012;12(6):387–400. doi: 10.1038/nrc3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smolen GA, Sordella R, Muir B, Mohapatra G, Barmettler A, Archibald H, Kim WJ, Okimoto RA, Bell DW, Sgroi DC. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A. 2006;103:2316–2321. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutterbach B, Zeng Q, Davis LJ, Hatch H, Hang G, Kohl NE, Gibbs JB, Pan BS. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res. 2007;67:2081–2088. doi: 10.1158/0008-5472.CAN-06-3495. [DOI] [PubMed] [Google Scholar]
- 28.Janne PA, Gray N, Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat Rev Drug Discov. 2009;8(9):709–723. doi: 10.1038/nrd2871. [DOI] [PubMed] [Google Scholar]
- 29.Pruksakorn D, Teeyakasem P, Klangjorhor J, Chaiyawat P, Settakorn J, Diskul-Na-Ayudthaya P, Chokchaichamnankit D, Pothacharoen P, Srisomsap C. Overexpression of KH-type splicing regulatory protein regulates proliferation, migration, and implantation ability of osteosarcoma. Int J Oncol. 2016;49:903–912. doi: 10.3892/ijo.2016.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 31.Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18(1):207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 33.Entz-Werle N, Marcellin L, Gaub MP, Guerin E, Schneider A, Berard-Marec P, Kalifa C, Brugiere L, Pacquement H, Schmitt C. Prognostic significance of allelic imbalance at the c-kit gene locus and c-kit overexpression by immunohistochemistry in pediatric osteosarcomas. J Clin Oncol. 2005;23:2248–2255. doi: 10.1200/JCO.2005.03.119. [DOI] [PubMed] [Google Scholar]
- 34.Sulzbacher I, Birner P, Toma C, Wick N, Mazal PR. Expression of c-kit in human osteosarcoma and its relevance as a prognostic marker. J Clin Pathol. 2007;60:804–807. doi: 10.1136/jcp.2005.032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li YG, Geng X. A meta-analysis on the association of HER-2 overexpression with prognosis in human osteosarcoma. Eur J Cancer Care (Engl) 2010;19(3):313–316. doi: 10.1111/j.1365-2354.2008.00970.x. [DOI] [PubMed] [Google Scholar]
- 36.Whelan JS, Bielack SS, Marina N, Smeland S, Jovic G, Hook JM, Krailo M, Anninga J, Butterfass-Bahloul T, Bohling T. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Ann Oncol. 2015;26:407–414. doi: 10.1093/annonc/mdu526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whelan JS, Jinks RC, McTiernan A, Sydes MR, Hook JM, Trani L, Uscinska B, Bramwell V, Lewis IJ, Nooij MA. Survival from high-grade localised extremity osteosarcoma: combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann Oncol. 2012;23:1607–1616. doi: 10.1093/annonc/mdr491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 39.Florea AM, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3(1):1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 41.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117(Pt 8):1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 42.Campiglio M, Somenzi G, Olgiati C, Beretta G, Balsari A, Zaffaroni N, Valagussa P, Menard S. Role of proliferation in HER2 status predicted response to doxorubicin. Int J Cancer. 2003;105:568–573. doi: 10.1002/ijc.11113. [DOI] [PubMed] [Google Scholar]
- 43.Cross SN, Cocco E, Bellone S, Anagnostou VK, Brower SL, Richter CE, Siegel ER, Schwartz PE, Rutherford TJ, Santin AD. Differential sensitivity to platinum-based chemotherapy in primary uterine serous papillary carcinoma cell lines with high vs low HER-2/neu expression in vitro. Am J Obstet Gynecol. 2010;203:162 e161–168. doi: 10.1016/j.ajog.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]