Abstract
Introduction
The reasons for the long term complete or partial loss of islet graft function are unknown, but there are obviously others than just pure allogeneic graft rejection. Earlier studies have shown that deposition of islet amyloid polypeptide (IAPP) amyloid in transplanted islets may indicate a mechanism for loss of β-cells.
Materials and Methods
Sections from liver material from four deceased islet-bearing recipients have been scrutinized for the presence of amyloid. Clinical data and certain aspects of the islet graft pathology of these patients have been published previously.
Result
With this extended histological analysis we demonstrate the occurrence of amyloid deposits in islets transplanted into the liver in three out of four type 1 diabetic patients.
Conclusion
The finding adds evidence to the assumption that aggregation of islet amyloid polypeptide might be an important cause of progressing β-cell dysfunction in clinically transplanted islets.
Keywords: Islet amyloid, transplant pathology, type 1 diabetes, beta cell, islet graft
Introduction
With the introduction of the glucocorticoid-free immunosuppressive regimen there was a considerable increase of the success rate of clinical islet transplantation (1). However, within some 5 years most patients had to resume exogenous insulin therapy, demonstrating a progressive deterioration of islet graft function over time (2). The reasons for this failure are not known but there are obviously others than just pure allogeneic graft rejection. In order to obtain a more complete understanding of this process a detailed morphological characterization of intraportally grafted islets in autopsy material would be of great interest. In a recent report we described the presence of wide spread amyloid depositions, consisting of the β-cell IAPP, in human pancreatic islets intraportally grafted into a diabetic patient 60 and 6 months prior to his death (3). In previous studies we and others had described amyloid deposits in human islets or mouse islets, transgenic for human IAPP, transplanted into nude mice (4, 5). This may be of great interest since aggregated IAPP, either as amyloid fibrils or as oligomeric assemblies, is believed to be of importance for the loss of β-cells in type 2 diabetes (6, 7). Consequently, deposition of IAPP amyloid in transplanted islets may indicate a mechanism for loss of β-cells. Our first report was based on the findings in one individual only. The present study was therefore carried out in order to extend our knowledge as regards the formation of amyloid in clinically transplanted human islets. Since there is evidence to suggest that there is a prohormone convertase 2 (PC2) deficiency in experimentally grafted human islets (8) we also examined the presence of prohormone convertases in these clinically grafted islets.
Results
Liver material from 4 deceased islet-bearing recipients has been made available for the demonstration of amyloid depositions in grafted pancreatic islets. Neither of the recipients was obese and they had all therapy-controlled hypertension. Excised pancreas was preserved in the two-layer cold storage solution or in University of Wisconsin solution and the ischemic period lasted 2 to14 hours. Two of the recipients (identity acronyms 001, 002) were described by the Edmonton team (9), one (003) by the Milan group (10) and the final one (004) by the Nordic Network for Clinical Islet Transplantation (3).
Patient 001
This patient (9) died almost two years after transplant from a methadone overdose. He was using a small dose of insulin at death and had slightly elevated serum HbA1C levels (table 1). Fasting serum C-peptide concentrations were fairly low. The implant was a mixture from three donors, two with BMI above 30 and age varying from 32 to 52. A total of 28 islets were recovered in the liver blocks available. These islets were possible to follow by means of serial sectioning. In seven of them amyloid deposits were found in grades 1+ to 3+ (table 2). The amyloid was widely spread in the affected islets and appeared around capillaries and at the outer border of the islets (figures 1 A and B). More nodular deposits were sometimes seen. Intracellular amyloid was not possible to identify light microscopically. As expected, the material was labeled with antibodies against IAPP (not shown).
Table 1. Glucose homeostasis at death in islets-grafted patients.
| Recipient | 001 | 002 | 003 | 004 |
|---|---|---|---|---|
|
| ||||
| Sex | Man | Man | Woman | Man |
|
| ||||
| Age at death | 43 | 46 | 43 | 55 |
|
| ||||
| Time postx (mo) | 22 | 17 | 50 | 60 |
|
| ||||
| Insulin requirement/day (U/Kg/day) | 0.54 | 0 | 0 | 0.7 |
|
| ||||
| HbA1c | 6.9 | 6.1 | 6.3 | 6.7 |
|
| ||||
| Fasting C-peptide (nmol/l) | 0.38 | 0.67 | 0.80 | 0.34 |
|
| ||||
| BMI | 26.4 | 25.2 | 20.7 | 24 |
|
| ||||
| Daclizumab | Daclizumab | Azathioprine | Daclizumab | |
| Immunosupression | Tacrolimus | Tacrolimus | Cyclosporin A | Tacrolimus |
| Sirolimus | Sirolimus | Prednisolone | Sirolimus | |
|
| ||||
| Smoking | No | No | No | No |
|
| ||||
| Hypertension | Controlled by therapy | Controlled by therapy | Controlled by therapy | Controlled by therapy |
|
| ||||
| Cause of death | Accident | Ml | Ml | Ml |
|
| ||||
| No. donor | 3 | 2 | 2 | 4* |
|
| ||||
| Sex | F+M+M | M+M | M+M | F+F/M/M |
|
| ||||
| Age | 32+41+52 | 50+53 | 27+44 | 64+60/58/54 |
|
| ||||
| BMI | 31.5+23.9+33.9 | 31.8+25.6 | 24.1+27.5 | 23+21.9/36.9/23.7 |
|
| ||||
| Cold ischemia time (hours) | 8+2.5+14 | 7.5+2 | <8+<8 | 6.2+7/10/8 |
|
| ||||
| Preservation solution | TLM+UW+TLM | TLM+UW | UW+UW | UW/UW/UW |
Patient 004 received islets from 4 different donors at the three time points 60, 56 and 6 months before death, Ml = Myocardial infarction, TLM = Two layer method, UW = University of Wisconsin solution, F=female, M=male
Table 2. Histological assessment of islet amyloid contents in livers from four autopsied pancreatic islet recipients.
| 001 | 002 | 003 | 004 | |
|---|---|---|---|---|
| Number of islets assessed | 28 | 14 | 9 | 89 |
| Amyloid depositions | ||||
| 0 | 21 | 14 | 5 | 51 |
| 1+ | 1 | 0 | 0 | 0 |
| 2+ | 2 | 0 | 2 | 5 |
| 3+ | 4 | 0 | 0 | 19 |
| 4+ | 0 | 0 | 2 | 14 |
Patient 004, received islets from 3 different donors at time points 60, 56 and 6 months before death.
Figure 1.
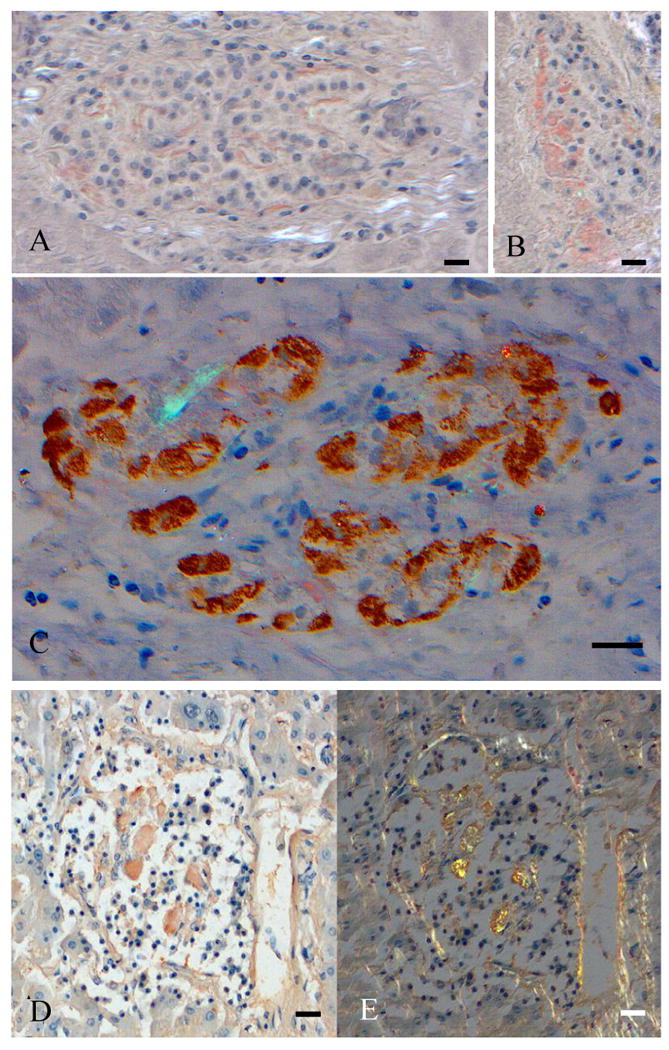
Intraportal islet grafts stained with Congo red, all of them showing wide-spread amyloid deposits. A and B show two islets from patient 001, and the wide-spread character of the amyloid material (stained red) is obvious. In C is an islet from 004 with α-cells brown after immunolabeling with antiglucagon antibodies and amyloid stained red. Bar 40 μM. In D and E show an islet from 003, visualized in ordinary light (D) and in polarized light (E). There is a bright yellow-green birefringence in B. Bar 40 μM.
Patient 002
This patient (9) was off insulin 17 months after transplant when he died of a myocardial infarct. He had the lowest HbA1C value of the 4 patients examined and the second highest fasting C-peptide value (table 1). Islets were from two male donors aged 50 and 53 and one with a BMI above 30. Only 14 islets were available for assessment and none of them were found to contain amyloid deposits, neither after Congo red staining viewed in cross polarized light or with fluorescence microscope (table 2).
Patient 003
This patient (10) died of a myocardial infarct more than four years after transplant. She was off insulin, had slightly elevated serum HbA1C levels and the highest serum C-peptide concentration of the 4 investigated patients (table 1). This patient was the only one on glucocorticoid immunosuppressive regime. Implanted islets were isolated from 2 male donors both with BMI below 28. In the sections still available for amyloid diagnosis nine islets were identified. In 4 of them amyloid deposits were recognized, two of them with pronounced deposits (table 2; figures 1 D and E). The amyloid had typical affinity for Congo red and showed bright green birefringence.
Patient 004
The finding of amyloid in this patient was reported earlier (3). This patient also died from a myocardial infarct 60 months after the first transplant. He had slightly elevated HbA1C levels, fairly low serum C-peptide concentrations and required a low dose of insulin. The first transplant was isolated from two female donors while the second and third implants were from single male donors, given 4 and 54 months after the first implant, respectively. These four donors had the highest age (54-64) and one of them had a BMI above 30. High numbers of islets had been recovered since the availability of liver tissue was essentially unlimited. More than 40 % of the examined islets contained amyloid deposits in greater or smaller amounts as assessed by means of Congo red staining (ordinary and polarized light) (figure 1 C) and immunolabeling at both the light and electron microscopical level.
Immunolabeling with antibodies against PC1/3 and PC2
In double immunolabeling experiments, monoclonal antibodies against PC1/3 and PC2 antibodies were used together with antiinsulin antibodies. PC2 labeling was detected in insulin containing cells and also in non-insulin reactive cells in all three investigated grafts, (figures 2 A and B). PC1/3 reactivity was restricted to insulin reactive cells (not shown). The fairly strong and even labeling with antibodies against the processing enzymes did not differ from that seen in islets in normal human pancreas.
Figure 2.
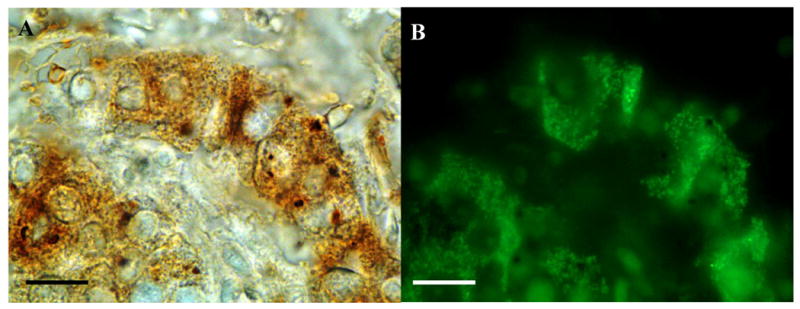
Intraportal islet graft (004) with PC2 reactivity visualized with DAB (A) and green insulin reactivity visualized by Alexa 488 (B). All insulin positive cells express PC2. Bar 20 μM.
Discussion
We reported earlier the wide-spread occurrence of amyloid in islets transplanted into the liver of a type 1 diabetic individual and suggested that this may be a common event of importance for the long term loss of β-cell function in such cases. Indeed, when human islets or islets from transgenic mice expressing human IAPP are transplanted into nude mice, amyloid develops rapidly in the transplanted islets (4, 5, 11, 12). The present study extends and strengthens our previous observation that development of IAPP amyloid deposits in transplanted islets is common also in the clinical situation when islets are infused into the portal system of the liver.
In their previous histological assessment of clinical islet grafts the Edmonton group reported that the two autopsy specimens available were both negative for amyloid. We can now demonstrate that at least in one of the two patients (001) there were amyloid depositions as demonstrated by Congo red staining with polarization microscopy (figures 1 A and B). The reasons for this discrepant reporting can simply be that the islets investigated in the first report were, indeed, islets lacking amyloid. Also in the present study as many as three out of four islets were amyloid negative and in the Nordic network patient (3) almost six out of ten. It should, however, be pointed out that the diagnosis of amyloid in pancreatic islets may be difficult and requires considerable experience. By the same token it might be argued that the fact that we were unable to demonstrate islet amyloid in the second Edmonton patient (002) was due to the fact that just 14 islets have been scrutinized. It might well be that with a greater material some amyloid positive islets might have shown up here as well. It should be pointed out that in our first published patient (3) sufficient amounts of fixed tissue were present to make electron microscopical examinations possible. In the three newly added patients this was not the case.
Although our clinical material is still very small it is nevertheless intriguing to notice that the patient in whom we found no amyloid depositions in the grafted islets (002) had the shortest observation time. The two patients with most depositions (004 and 003) had survived for more than 5 and 4 years, respectively, after the first islet transplantation. Amyloid formation in the clinical setting may be a slow process and its importance for the functional impairment of grafted islets is still not known. There is, however, strong support for the effect of islet amyloid on islet function in a primate diabetes model (13).
The mechanisms behind the formation of amyloid in the grafted islets are not fully understood. Insufficiencies in the revascularization of the grafted islets have been one intriguing reason for the long-term failure of grafted (14). It is therefore of great interest that it was recently shown that intramuscular islet transplantation might offer advantages, since it induces an almost normal blood supply to the grafted islets (15). Against that background it will be important to analyze such islets for amyloid deposition. It should be noted though that there was no difference in amyloid development in human islets transplanted into the liver, subcapsularly in the kidney or in the spleen of nude mice (5). Another important issue is the species-dependent difference in amyloid formation. The fibril formation propensity varies strongly between species depending on the primary structure of IAPP (16). Xenotransplantation with porcine islets – although it involves considerable problems – may therefore offer an interesting alternative since porcine IAPP does not aggregate into amyloid fibrils (17).
There is evidence that prolAPP may be even more amyloidogenic than mature IAPP and that the very first, intracellular amyloid is formed from the propeptide. The report of reduced β-cell PC2 immunolabeling and hyperpro-insulinemia after transplantation of human islets to nude mice was therefore interesting (8). However, in this study we detect PC2 labeling in β-cells from all three patients examined. Therefore, it is possible that the disappearance of PC2 is a transient phenomenon, which still may be of importance in the initiation of islet amyloid formation. This issue deserves more studies.
Our finding that amyloid often develops in transplanted islets should increase our efforts to unravel the effect of aggregation of IAPP on the function of transplanted human islets. Experimentally, it has been shown that aggregated human IAPP added to isolated islets in vitro exerts a toxic effect to the β-cell. Several different mechanisms have been suggested, including formation of pathological cell membrane pores. It should be mentioned however, that early islet amyloid formation takes place intracellularly (4, 18, 19), indicating that interference with intracellular events is of relevance. The site of the early aggregation events may be important for the development of future therapeutical alternatives.
Materials and Methods
Patient characteristics
Relevant information on glucose homeostasis is given in table 1 and on transplantation outcome (donor numbers, time posttransplantation) is given in table 2. The information has been extracted from earlier publications (3, 9, 10).
Staining methods
From three of the patients (001, 002 and 004) several blocks of formalin-fixed and paraffin-embedded liver autopsies were available. Material from the Italian case (003) was in the form of unstained sections from 7 blocks, containing 1-2 formalin-fixed and paraffin embedded specimens. Islets were localized in the sections by means of immunostaining for insulin and/or glucagon. All specimens were stained for amyloid with Congo red and examined under ordinary and polarized light (20). Other specimens were immunostained with rabbit antibodies against IAPP (A110;(21)) and visualized by goat anti rabbit antibodies labeled with Alexa-488 (Invitrogen, Stockholm, Sweden). The amount of amyloid deposition in each single islet based on the viewing of one or several Congo red stained sections was estimated on a 5-graded scale (0 to 4+).
To investigate if PC2 deficiency remains in clinically transplanted islets and if PC1/3 expression is influenced we performed double immunolabeling of sections from 004, 001 and 002 with well characterized mouse monoclonal antibodies against PC2 and PC1/3 (21) together with antiinsulin antibodies produced in guinea pig. Sections used for PC2 labeling were subjected to pretreatment with hot 0.02 M sodium citrate buffer for 20 minutes prior to overnight incubation at 4°C with PC2 antibodies, diluted in tris HCI buffer, pH 7.4, containing 0.15 M NaCI and 0.001 % Tween. Sections were incubated with PC1/3 antibodies, diluted in tris HCI buffer, pH 7.4, containing 0.15 M NaCI, overnight at room temperature. PC2 and PC1/3 reactivity was detected by horseradish peroxidase labeled goat anti mouse antibodies (Dako, Glostrup, Denmark) and visualized with 3,3′-diaminobenzidine tetrahydrochloride (DAB). After extensive rinses in water and tris HCI buffer, pH 7.4, with 0.15 M NaCI, sections were incubated with guinea pig antiinsulin antibodies (Dako) overnight followed by Alexa 488 labeled goat anti guinea pig antibodies (Invitrogen). To facilitate islet detection, sections were incubated with rabbit antiglucagon antibodies(Dako) overnight and the reactivity was detected with horseradish peroxidase labeled goat antirabbit antibodies (Dako) and visualized with DAB prior to Congo staining.
Acknowledgments
The work was supported by the Swedish Research Council ((GTW-5343), (PW-5941) and (OK-12219)), the Swedish Diabetes Association (GTW), Novo Nordisk (GTW) the Family Ernfors Fund (GTW), NIH grant RO1 DK080148 (FF), Swiss National Science Foundation (SCORE grant 3232230-126233; CT), NIH-NIAID grant for the CIT network (AMJS), and through Alberta Innovates Healthcare solutions (AMJS), NIH-NIAID grant for the CIT network (2U01AI065192-06) (OK), the Juvenile Diabetes Foundation International (OK).
Abbreviations
- IAPP
Islet amyloid polypeptide
- PC2
prohormone convertase 2
- PC1/3
prohormone convertase 1/3
- Ml
Myocardial infarction
- TLM
Two layer method
- UW
University of Wisconsin solution
Footnotes
Authorship: The material and clinical data were collected at three different transplant units. Due to differences in published histopathology, we have jointly decided to perform the described work. All authors participated in the planning, implementation and compilation of the manuscript.
Contributor Information
Gunilla T. Westermark, Department of Medical Cell Biology, Uppsala University, S-75123 Uppsala, Sweden
Alberto M. Davalli, Department of Medicine and Metabolic Disease, Ospedale San Raffaele, Milan, l-20132, Italy
Antonio Secchi, Department of Medicine and Metabolic Disease, Ospedale San Raffaele, Milan, l-20132, Italy.
Franco Folli, Diabetes Division, Department of Medicine, University of Texas Health Science Center at San Antonio, San Antonio, TX 78284-3900, USA.
Tatsuya Kin, Department of Medical Microbiology and Immunology, University of Alberta, Edmonton, Alberta T6G 2S2, Canada.
Christian Toso, Department of Medical Microbiology and Immunology, University of Alberta, Edmonton, Alberta T6G 2S2, Canada; Department of Surgery, University of Geneva Hospitals, Geneva, CH-1211, Switzerland.
A. M. James Shapiro, Department of Medical Microbiology and Immunology, University of Alberta, Edmonton, Alberta T6G 2S2, Canada
Olle Korsgren, Department of Immunology, Genetics and Pathology, Uppsala University, S-75185 Uppsala, Sweden.
GunnarTufveson, Department of Surgical Sciences, Uppsala University, S-75185 Uppsala, Sweden.
Arne Andersson, Department of Medical Cell Biology, Uppsala University, S-75123 Uppsala, Sweden.
Per Westermark, Department of Immunology, Genetics and Pathology, Uppsala University, S-75185 Uppsala, Sweden.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hehng BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 3.Westermark GT, Westermark P, Berne C, Korsgren O. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359(9):977. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 4.Westermark P, Eizirik DL, Pipeleers DG, Hellerstrom C, Andersson A. Rapid deposition of amyloid in human islets transplanted into nude mice. Diabetologia. 1995;38(5):543. doi: 10.1007/BF00400722. [DOI] [PubMed] [Google Scholar]
- 5.Westermark GT, Westermark P, Nordin A, Tornelius E, Andersson A. Formation of amyloid in human pancreatic islets transplanted to the liver and spleen of nude mice. Ups J Med Sci. 2003;108(3):193. doi: 10.3109/2000-1967-113. [DOI] [PubMed] [Google Scholar]
- 6.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89(8):3629. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 7.Khemtemourian L, Killian JA, Hoppener JW, Engel MF. Recent insights in islet amyloid polypeptide-induced membrane disruption and its role in beta-cell death in type 2 diabetes mellitus. Exp Diabetes Res. 2008;2008:421287. [Google Scholar]
- 8.Davalli AM, Perego L, Bertuzzi F, et al. Disproportionate hyperproinsulinemia, beta-cell restricted prohormone convertase 2 deficiency, and cell cycle inhibitors expression by human islets transplanted into athymic nude mice: insights into nonimmune-mediated mechanisms of delayed islet graft failure. Cell Transplant. 2008;17(12):1323. doi: 10.3727/096368908787648137. [DOI] [PubMed] [Google Scholar]
- 9.Toso C, Isse K, Demetris AJ, et al. Histologic graft assessment after clinical islet transplantation. Transplantation. 2009;88(11):1286. doi: 10.1097/TP.0b013e3181bc06b0. [DOI] [PubMed] [Google Scholar]
- 10.Davalli AM, Maffi P, Socci C, et al. Insights from a successful case of intrahepatic islet transplantation into a type 1 diabetic patient. J Clin Endocrinol Metab. 2000;85(10):3847. doi: 10.1210/jcem.85.10.6877. [DOI] [PubMed] [Google Scholar]
- 11.Westermark G, Westermark P, Eizirik DL, et al. Differences in amyloid deposition in islets of transgenic mice expressing human islet amyloid polypeptide versus human islets implanted into nude mice. Metabolism. 1999;48(4):448. doi: 10.1016/s0026-0495(99)90102-6. [DOI] [PubMed] [Google Scholar]
- 12.Finzi G, Davalli A, Placidi C, et al. Morphological and ultrastructural features of human islet grafts performed in diabetic nude mice. Ultrastruct Pathol. 2005;29(6):525. doi: 10.1080/01913120500323563. [DOI] [PubMed] [Google Scholar]
- 13.Guardado-Mendoza R, Davalli AM, Chavez AO, et al. Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci USA. 2009;106(33):13992. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlsson PO. Influence of microenvironment on engraftment of transplanted beta-cells. Ups J Med Sci. 2011;116(1):1. doi: 10.3109/03009734.2010.548609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christoffersson G, Carlsson PO, Phillipson M. Intramuscular islet transplantation promotes restored islet vascularity. Islets. 2011;3(2):69. doi: 10.4161/isl.3.2.14997. [DOI] [PubMed] [Google Scholar]
- 16.Westermark P. Amyloid in the islets of Langerhans: thoughts and some historical aspects. Ups J Med Sci. 2011;116(2):81. doi: 10.3109/03009734.2011.573884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter KJ, Abedini A, Marek P, et al. Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc Natl Acad Sci U S A. 2010;107(9):4305. doi: 10.1073/pnas.0909024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulsson JF, Andersson A, Westermark P, Westermark GT. Intracellular amyloid-like deposits contain unprocessed pro-islet amyloid polypeptide (prolAPP) in beta cells of transgenic mice overexpressing the gene for human IAPP and transplanted human islets. Diabetologia. 2006;49(6):1237. doi: 10.1007/s00125-006-0206-7. [DOI] [PubMed] [Google Scholar]
- 19.Janson J, Soeller WC, Roche PC, et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93(14):7283. doi: 10.1073/pnas.93.14.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westermark GT, Johnson KH, Westermark P. Staining methods for identification of amyloid in tissue. Methods Enzymol. 1999;309:3. doi: 10.1016/s0076-6879(99)09003-5. [DOI] [PubMed] [Google Scholar]
- 21.Paulsson JF, Westermark GT. Aberrant processing of human proislet amyloid polypeptide results in increased amyloid formation. Diabetes. 2005;54(7):2117. doi: 10.2337/diabetes.54.7.2117. [DOI] [PubMed] [Google Scholar]


