Abstract
Background
The correlation between platelet function and recurrent ischemic stroke or TIA remains uncertain.
Objective
To investigate two inductive agents to detect platelet functions and assess associations with recurrent ischemic stroke/TIA.
Method
The study included 738 ischemic stroke/TIA patients. On days 0, 3, and 9 after antiplatelet therapy, platelet function tests were determined by maximum aggregation rate (MAR) using a PL-11 platelet function analyzer and phase matching reagents. Two induction agents were used: arachidonic acid (AA) and adenosine diphosphate (ADP). At 3-month follow-up, recurrence of stroke/TIA was recorded.
Result
Cut-off values of adequate platelet function inhibition were MARADP < 35% and MARAA < 35%. Data showed that antiplatelet therapy could reduce the maximum aggregation rate. More importantly, adequate platelet function inhibition of either MARADP or MARAA was not associated with the recurrence of stroke/TIA, but adequate platelet function inhibition of not only MARADP but also MARAA predicts lower recurrence (0/121 (0.00%) versus 18/459 (3.92%), P = 0.0188).
Conclusion
The platelet function tested by PL-11 demonstrated that adequate inhibition of both MARADP and MARAA could predict lower risk of ischemic stroke/TIA recurrence.
1. Introduction
Stroke is the second most common cause of death and is a major cause of disability worldwide [1, 2]. The prognosis of recurrent stroke is worse than first-ever stroke [3, 4]. Antiplatelet therapy is the cornerstone of the secondary prevention of ischemic stroke and transient ischemic attack (TIA) [5]. However, a large number of stroke/TIA patients still experience another cerebral vascular event despite sustained antiplatelet therapy. On average, the annual risk for future ischemic stroke after an initial ischemic stroke/TIA is 3% to 4% [6]. Some researchers interpret such individual difference to antiplatelet drugs as “aspirin/clopidogrel resistance” [7–9]. Therefore, appropriate platelet function measurement to predict aspirin/clopidogrel efficacy is necessary to guide the precise stroke treatment. The current study is a multicenter study designed to evaluate the platelets' function before and after antiplatelet therapy and analyze the relationship between qualified platelet inhibition and stroke recurrence in the Chinese population.
2. Methods
2.1. Study Design and Patients
The current study is a prospective trial that enrolled 738 patients from 13 stroke centers between October 2014 and December 2015 in China. Inclusion criteria were as follows: (1) age: ≥18 years old and ≤80 years old; (2) transient ischemic attack (TIA) for the first time, which was defined as a transient episode of neurological dysfunction caused by focal brain or retinal ischemia, without acute infarction symptoms; (3) ischemic stroke for the first time or recurrent patients with modified Rankin Scale ≤2 (ischemic stroke was defined as a sudden neurological deficit, and the infarct site was assessed by magnetic resonance imaging); (4) need for antiplatelet therapy: 100 mg aspirin or 75 mg clopidogrel as monoantiplatelet therapy is initiated within 24 hours after the onset; patients with the following were excluded from the study: (1) intracranial venous thrombosis, (2) cerebral hemorrhage, (3) cerebral embolism, (4) brain tumors, and (5) end-stage severe disease. The main endpoint during the 3-month follow-up was the recurrence of stroke/TIA, bleeding events including cerebral hemorrhage and gastrointestinal bleeding, and other bleeding events.
2.2. Participant Stroke Centers
The First Affiliated Hospital of Soochow University
Tianjin Huanhu Hospital
Peking Union Medical College Hospital (West)
Weihai Municipal Hospital
Changhai Hospital
Shanghai East Hospital
Lanzhou University Second Hospital
Zhuhai People's Hospital
Harrison International Peace Hospital
The Second Hospital of Tianjin Medical University
Shijiazhuang Third Hospital
Affiliated Hospital of North Sichuan Medical College
Shandong Provincial Hospital
2.3. Key Technology
PL-11 platelet function analyzer (SINNOWA Medical Science & Technology Co., Nanjing, China) is a new point-of-care apparatus for platelet function analysis via an automated impedance technique. Correlations among PL-11 and another three major assays (light transmission aggregometry (LTA), VerifyNow aspirin system, and thromboelastography (TEG)) suggested the ability of PL-11 to assess platelet function.
2.4. Treatment Protocols
All enrolled patients were given aspirin 100 mg/d or clopidogrel 75 mg/d. The medicine would not be changed during the experiment unless patients encountered hemorrhagic or ischemic events or withdrew from the experiment. At the same time, other antiplatelet medicines could not be provided, including Chinese patent medicine containing ingredients like Folium Ginkgo, Salvia Miltiorrhiza, Pseudoginseng, and so on.
2.5. Standard Protocol Approvals, Registration, and Patient Consent
The study protocol was approved by the ethics committee at each study center. Written informed consent was obtained from all participants or their proxies. This trial has been registered in the Chinese Clinical Trials Registry and the registration number is ChiCTR-OCH-14005238.
2.6. Sample Collection and Processing
Antecubital vein blood samples were collected with 3.8% sodium citrate in tubes for monitoring platelet function in all subjects on the day patients were admitted before antiplatelet treatment and 3 days and 9 days after antiplatelet therapy. Blood samples should be stored at room temperature before being tested. The whole procedure required being performed within 2 hours after sampling. Platelet aggregation was detected using PL-11 platelet function analyzer [10] (SINNOWA Medical Science & Technology Co., Nanjing, China).
The whole procedure was automatically done after transferring 500 ml of citrated blood sample into a polycarbonate tube and inserting it into the detecting position. The blood sample in the polycarbonate tube was mixed gently during the whole testing process. Platelet count was detected in duplicate at the start and the mean value of platelet count was set as the baseline. There was a short interval between each test point for system cleaning. 40 μl of adenosine diphosphate (ADP, 50 μmol/L) and arachidonic acid (AA, 2 mg/ml) were separately trickled into the blood sample after the second detecting time. The single platelet counting dropped when aggregates formed became too large to be counted as single platelets. PL-11 counted platelets several times until it detected the lowest level. The whole process was finished within 15 min (six detecting times). The system calculated the maximal platelet aggregation ratio according to the following formula:
| (1) |
The corresponding maximum aggregation rate of the platelet by each inductive agent was recorded as MARAA and MARADP.
2.7. Statistical Analysis
Baseline characteristics were compared between the ending group (recurrence of ischemic stroke/TIA) and no ending group (nonrecurrence of ischemic stroke/TIA). Continuous variables are presented as mean (standard deviation) and differences were compared using the analysis of Wilcoxon test. Categorical variables are presented as counts (proportions). Differences were compared using the Fisher test. All tests were 2-sided at a significance level of P ≤ 0.05 and were performed using SAS software, Version 9.4.
3. Results
3.1. Characteristics of the Patients
From October 2014 through December 2015, we enrolled 738 patients.
Baseline characteristics of patients by recurrent ischemic stroke/TIA at 3-month follow-up were well matched (Table 1).
Table 1.
Baseline characteristics of patients by recurrent ischemic stroke/TIA at a 3-month follow-up.
| Ending | No ending | P value | |
|---|---|---|---|
| Sex (female), number (%) | 14 (66.67) | 492 (68.62) | 0.8141 |
| Age, years, mean (SD) | 63.71 ± 9.01 | 62.54 ± 10.62 | 0.3503 |
| Smoking habit, number (%) | 7 (33.33) | 273 (38.08) | 0.8204 |
| SBP, mmHg, mean (SD) | 143.76 ± 19.91 | 147.18 ± 21.89 | 0.3506 |
| DBP, mmHg, mean (SD) | 81.67 ± 9.69 | 85.35 ± 12.75 | 0.2093 |
| FPG, mmol/l, mean (SD) | 7.21 ± 2.78 | 7.49 ± 12.97 | 0.1449 |
| TC, mmol/l, mean (SD) | 4.80 ± 1.20 | 5.99 ± 14.29 | 0.7442 |
| TG, mmol/l, mean (SD) | 1.59 ± 0.54 | 1.58 ± 1.34 | 0.1750 |
| BMI, kg/m2, mean (SD) | 23.52 ± 2.03 | 24.34 ± 2.85 | 0.1124 |
| Cr, mmol/l, mean (SD) | 72.39 ± 29.61 | 72.94 ± 37.33 | 0.8156 |
| NIHSS points, mean (SD)∗ | 6.65 ± 4.82 | 5.25 ± 3.74 | 0.2866 |
| ABCD points, mean (SD)# | 3.25 ± 1.71 | 3.15 ± 1.34 | 0.8077 |
∗Compare NIHSS points for patients of ischemic stroke. #Compare ABCD points for patients of transient ischemic attack.
3.2. Antiplatelet Therapy Reduced MAR
Compared with baseline (Figure 1), MARADP was decreased by 9.71% on day 3 (Figure 2, n = 672, 47.99% ± 20.30% versus 53.15% ± 20.72%, P < 0.0001) and by 9.48% on day 9 (Figure 3, N = 581, 48.11% ± 19.62% versus 53.15% ± 20.72%, P < 0.0001) after antiplatelet therapy. MARAA was decreased by 22.37% on day 3 (Figure 2, n = 664, 35.77% ± 21.72 versus 46.08% ± 25.29%, P < 0.0001) and by 19.34% on day 9 (Figure 3, N = 572, 37.17% ± 22.84% versus 46.08% ± 25.29%, P < 0.0001) after antiplatelet therapy.
Figure 1.
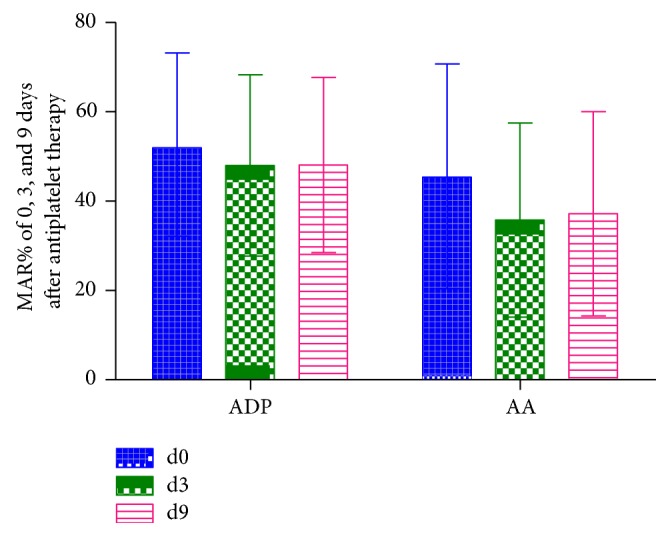
MAR% of 0, 3, and 9 days after antiplatelet therapy induced by ADP and AA.
Figure 2.
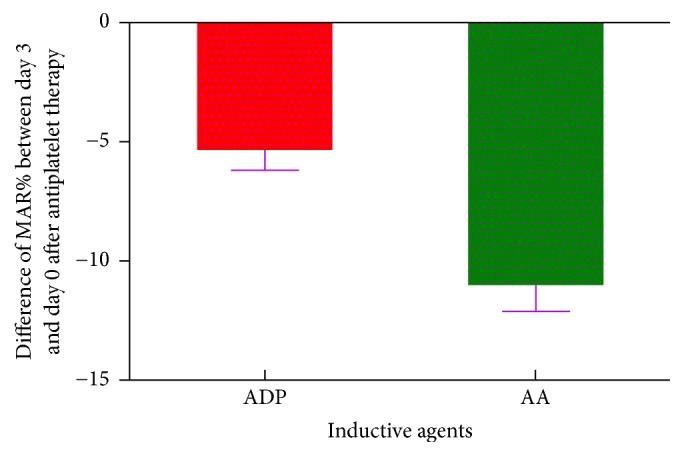
Difference of MAR% between day 3 and day 0 after antiplatelet therapy induced by ADP and AA.
Figure 3.

Difference of MAR% between day 9 and day 0 after antiplatelet therapy induced by ADP and AA.
3.3. Association between Adequate Platelet Function Inhibition and Recurrence of Stroke/TIA at 3-Month Follow-Up
The cut-off values of adequate platelet function inhibition were MARADP < 35% and MARAA < 35%. Based on these criteria, we divided patients into adequate platelet function inhibition and inadequate inhibition groups. Recurrence of ischemic stroke/TIA at 3-month follow-up was compared between the two groups. When being grouped based on MARADP, for patients with MARADP < 35%, the recurrence cases were 2 at 3-month follow-up (Table 2, 2/172 (1.16%)), while for patients with MARADP ≥ 35%, the recurrence cases were 16 at 3-month follow-up (Table 2, 16/417 (3.84%)). Although the recurrent cases of the adequate platelet function inhibition group were fewer, there was no significant difference between the two groups (P = 0.0864). When being grouped based on MARAA, the recurrence rate was not significantly different either (Table 2, MARAA < 35% versus MARAA ≥ 35%, 2.90% (10/345) versus 3.31% (8/242), P = 0.7782).
Table 2.
Association of inhibited platelet aggregation with the recurrent ischemic stroke/TIA within a 3-month follow-up.
| Inductive agent | Groups (MAR) | Ending (%) | No ending (%) | Numbers | P value |
|---|---|---|---|---|---|
| ADP | ≥35% | 16 (3.84) | 401 (96.16) | 417 | 0.0864 |
| <35% | 2 (1.16) | 170 (98.84) | 172 | ||
| AA | ≥35% | 8 (3.31) | 234 (96.69) | 242 | 0.7782 |
| <35% | 10 (2.90) | 335 (97.10) | 345 | ||
| ADP + AA | ADP ≥ 35% or AA ≥ 35% | 18 (3.92) | 441 (96.08) | 459 | 0.0188 |
| ADP < 35% and AA < 35% | 0 (0.00) | 121 (100.00) | 121 |
When setting the stricter criteria, take not only MARADP < 35% but also MARAA < 35% as adequate platelet function inhibition. Based on these stricter criteria, we divided patients into adequate platelet function inhibition and inadequate inhibition groups and compared recurrence of ischemic stroke/TIA between the two groups. At 3-month follow-up, 0.00% (0/121) of the patients experienced recurrence of stroke/TIA in the group of adequate platelet function inhibition, while for the inadequate inhibition group, 18/459 (3.92%) experienced recurrent stroke/TIA. The recurrence rate was significantly different between the two groups (Table 2, P = 0.0188).
4. Discussion
The role of antiplatelet therapy in stroke prevention was well documented, especially for secondary prevention [11–13]. But it was also confirmed that there were still a considerable number of patients with ischemic stroke/TIA recurrence even if on treatment of single antiplatelet therapy with aspirin or clopidogrel [5]. This phenomenon may be associated with platelet function. Our study showed that antiplatelet therapy could reduce both MARADP and MARAA in patients of ischemic stroke/TIA. At 3-month follow-up, neither adequate inhibition of MARADP nor MARAA was associated with the recurrence of ischemic stroke/TIA, but adequate inhibition of not only MARADP but also MARAA could predict lower recurrence of ischemic stroke/TIA.
Our study found that MARAA and MARADP significantly decreased in patients of ischemic stroke/TIA after antiplatelet therapy which was consistent with a large number of studies and clinical observations since 2002.
A number of studies had concentrated on the association between platelet function and ischemic events [14]. ARMYDA-Pro [15] and including popular research [16] which was the largest assessment of the predictive value of platelet function tests so far all showed that platelet function was significantly correlated with ischemic vascular outcome. However, several large-scale studies have denied the correlation. TRILOGY-ACS subgroup [17, 18] analysis showed that prasugrel could significantly reduce platelet aggregation but could not reduce cardiovascular mortality, nonfatal myocardial infarction, or stroke within 30 months. Translate-POPs [19] research randomly assigned ACS patients to a strategy of platelet function monitoring, with drug adjustment in patients who had poor responses to antiplatelet therapy, or to a conventional strategy without monitoring and drug adjustment. This study showed no significant improvements in clinical outcomes between the two groups. Similar negative results also had been confirmed by ARCTIC research [20]. Several reasons may account for the different results: (1) platelet function was detected by VerifyNow P2Y12 in some researches, but the method was proven to have low sensitivity [20, 21]; (2) platelets had 6-7 kinds of different receptors and all above researches only measured platelet function by ADP inductive agent, so this may not bring us accurate information [22]. Our study used MARAA and MARADP to evaluate platelet function by PL-11 platelet function analyzer, demonstrating that the single adequate inhibition of either MARADP or MARAA was not associated with the decreased risk of recurrent ischemic stroke/TIA; however, adequate inhibition of both MARADP and MARAA could predict the lower risk of ischemic stroke/TIA recurrence.
The shortcomings of this study are as follows: (1) There is a limitation of MARAA and MARADP by PL-11: the cut-off values of adequate platelet function inhibition by different inductive agents were not confirmed, requiring large cohort studies; (2) the study did not involve adjusting the antiplatelet therapy for ineffective inhibition of platelet function.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant no. 81371387).
Conflicts of Interest
All authors declare no conflicts of interest.
Authors' Contributions
Lulu Zhang and Wei Yue contributed equally to this work.
References
- 1.Feigin V. L., Lawes C. M., Bennett D. A., Barker-Collo S. L., Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. The Lancet Neurology. 2009;8(4):355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Chen H. S., Qi S. H., Shen J. G. One-compound-multi-target: combination prospect of natural compounds with thrombolytic therapy in acute ischemic stroke. Current Neuropharmacology. 2017;15(1):134–156. doi: 10.2174/1570159X14666160620102055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olindo S., Saint-Vil M., Jeannin S., et al. One-year disability, death and recurrence after first-ever stroke in a Black Afro-Caribbean population. International Journal of Stroke. 2017 doi: 10.1177/1747493016685720. [DOI] [PubMed] [Google Scholar]
- 4.Aarnio K., Haapaniemi E., Melkas S., Kaste M., Tatlisumak T., Putaala J. Long-term mortality after first-ever and recurrent stroke in young adults. Stroke. 2014;45(9):2670–2676. doi: 10.1161/STROKEAHA.114.005648. [DOI] [PubMed] [Google Scholar]
- 5.Kernan W. N., Ovbiagele B., Black H. R., et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. doi: 10.1161/str.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 6.Dhamoon M. S., Sciacca R. R., Rundek T., Sacco R. L., Elkind M. S. V. Recurrent stroke and cardiac risks after first ischemic stroke: The Northern Manhattan Study. Neurology. 2006;66(5):641–646. doi: 10.1212/01.wnl.0000201253.93811.f6. [DOI] [PubMed] [Google Scholar]
- 7.Floyd C. N., Ferro A. Antiplatelet drug resistance: molecular insights and clinical implications. Prostaglandins and Other Lipid Mediators. 2015;120:21–27. doi: 10.1016/j.prostaglandins.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Le Quellec S., Bordet J.-C., Negrier C., Dargaud Y. Comparison of current platelet functional tests for the assessment of aspirin and clopidogrel response. A review of the literature. Thrombosis and Haemostasis. 2016;116(4):638–650. doi: 10.1160/TH15-11-0870. [DOI] [PubMed] [Google Scholar]
- 9.Yi X., Wang C., Liu P., Fu C., Lin J., Chen Y. Antiplatelet drug resistance is associated with early neurological deterioration in acute minor ischemic stroke in the Chinese population. Journal of Neurology. 2016;263(8):1612–1619. doi: 10.1007/s00415-016-8181-5. [DOI] [PubMed] [Google Scholar]
- 10.Guan J., Cong Y., Ren J., et al. Comparison between a new platelet count drop method PL-11, light transmission aggregometry, VerifyNow aspirin system and thromboelastography for monitoring short-term aspirin effects in healthy individuals. Platelets. 2015;26(1):25–30. doi: 10.3109/09537104.2013.865835. [DOI] [PubMed] [Google Scholar]
- 11.Breet N. J., Van Werkum J. W., Bouman H. J., et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. Journal of the American Medical Association. 2010;303(8):754–762. doi: 10.1001/jama.2010.181. [DOI] [PubMed] [Google Scholar]
- 12.Davis K. A., Miyares M. A., Dietrich E. Dual antiplatelet therapy with clopidogrel and aspirin after ischemic stroke: a review of the evidence. American Journal of Health-System Pharmacy. 2015;72(19):1623–1629. doi: 10.2146/ajhp140804. [DOI] [PubMed] [Google Scholar]
- 13.Niewada M. Comment: the benefits of antiplatelets on stroke—more arguments to keep patients adherent. Neurology. 2015;84(11) doi: 10.1212/WNL.0000000000001380. [DOI] [PubMed] [Google Scholar]
- 14.Topçuoglu M. A., Arsava E. M., Ay H. Antiplatelet resistance in stroke. Expert Review of Neurotherapeutics. 2011;11(2):251–263. doi: 10.1586/ern.10.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patti G., Nusca A., Mangiacapra F., Gatto L., D'Ambrosio A., Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention: results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. Journal of the American College of Cardiology. 2008;52(14):1128–1133. doi: 10.1016/j.jacc.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 16.Bouman H. J., Harmsze A. M., Van Werkum J. W., et al. Variability in on-treatment platelet reactivity explained by CYP2C19*2 genotype is modest in clopidogrel pretreated patients undergoing coronary stenting. Heart. 2011;97(15):1239–1244. doi: 10.1136/hrt.2010.220509. [DOI] [PubMed] [Google Scholar]
- 17.Chin C. T., Roe M. T., Fox K. A. A., et al. Study design and rationale of a comparison of prasugrel and clopidogrel in medically managed patients with unstable angina/non-ST-segment elevation myocardial infarction: the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. American Heart Journal. 2010;160(1):16–22. doi: 10.1016/j.ahj.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Husted S., Boersma E. Case study: ticagrelor in plato and prasugrel in triton-timi 38 and trilogy-acs trials in patients with acute coronary syndromes. American Journal of Therapeutics. 2016;23(6):e1876–e1889. doi: 10.1097/MJT.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T. Y., Henry T. D., Effron M. B., et al. Cluster-randomized clinical trial examining the impact of platelet function testing on practice: The Treatment with Adenosine Diphosphate Receptor Inhibitors: Longitudinal assessment of treatment patterns and events after acute coronary syndrome prospective open label antiplatelet therapy study. Circulation: Cardiovascular Interventions. 2015;8(6) doi: 10.1161/CIRCINTERVENTIONS.114.001712.e001712 [DOI] [PubMed] [Google Scholar]
- 20.Collet J. P., Cuisset T., Rangé G., et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. The New England Journal of Medicine. 2012;367:2100–2109. doi: 10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]
- 21.Gurbel P. A., Erlinge D., Ohman E. M., et al. Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: the TRILOGY ACS platelet function substudy. Journal of the American Medical Association. 2012;308(17):1785–1794. doi: 10.1001/jama.2012.17312. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Zhao X., Lin J., et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. Journal of the American Medical Association. 2016;316(1):70–78. doi: 10.1001/jama.2016.8662. [DOI] [PubMed] [Google Scholar]


