Abstract
The aim of the present study was to investigate the regulation of Wilms Tumor 1 (WT1) by serine protease high-temperature requirement protein A2 (HtrA2), a member of the Htr family, in K562 cells. In addition, the study aimed to observe the effect of this regulation on cell biological functions and its associated mechanisms. Expression of WT1 and HtrA2 mRNA, and proteins following imatinib and the HtrA2 inhibitor 5-[5-(2-nitrophenyl) furfuryl iodine]-1, 3-diphenyl-2-thiobarbituric acid (UCF-101) treatment was detected with reverse transcription-quantitative polymerase chain reaction and western blot analysis. Subsequent to treatment with drugs and UCF-101, the proliferative function of K562 cells was detected using MTT assays, and the rate of apoptosis was detected using Annexin V with propidium iodide flow cytometry in K562 cells. The protein levels in the signaling pathway were analyzed using western blotting following treatment with imatinib and UCF-101. In K562 cells, imatinib treatment activated HtrA2 gene at a transcription level, while the WT1 gene was simultaneously downregulated. Following HtrA2 inhibitor (UCF-101) treatment, the downregulation of WT1 increased gradually. At the protein level, imatinib induced the increase in HtrA2 protein level and concomitantly downregulated WT1 protein level. Subsequent to HtrA2 inhibition by UCF-101, the WT1 protein level decreased temporarily, but eventually increased. Imatinib induced apoptosis in K562 cells, but this effect was attenuated by the HtrA2 inhibitor UCF-101, resulting in the upregulation of the WT1 protein level. However; UCF-101 did not markedly change the proliferation inhibition caused by imatinib. Imatinib activated the p38 mitogen activated protein kinase (p38 MAPK) signaling pathway in K562 cells, and UCF-101 affected the activation of imatinib in the p38 MAPK signaling pathway. Imatinib inhibited the extracellular signal-related kinase (ERK1/2) pathway markedly and persistently, but UCF-101 exhibited no notable effect on the inhibition of the ERK1/2 pathway. HtrA2 and its regulatory effect on WT1 may affect the sensitivity of BCR/ABL(+) cell lines to target therapy drugs through different mechanisms. Regulation of WT1 by HtrA2 occurs in K562 cells, and the regulation may affect the apoptosis of K562 cells under the stress caused by chemotherapeutic treatment. The p38 MAPK signaling pathway, which serves an important role in cell apoptosis, is a downstream pathway of this regulation.
Keywords: serine protease high-temperature requirement protein A2, mitochondrial, Wilms Tumor 1, imatinib, mitogen-activated protein kinase signal pathway, chronic myelocytic leukemia
Introduction
Chronic myelocytic leukemia (CML) is a type of hematopoietic stem-cell disease, which is characterized by the presence of the Philadelphia chromosome, t(9;22)(q34;q11) and generation of the breakpoint cluster region protein/Abelson murine leukemia viral oncogene homolog 1 (BCR/ABL) fusion gene. Treatment with tyrosine kinase inhibitors (TKIs) has significantly improved the prognosis of patients with CML, although certain patients are resistant to TKIs, leading to treatment failure. CML resistance involves multifaceted and complex mechanisms, including BCR/ABL-dependent mechanisms, such as ABL kinase domain mutations, BCR/ABL overexpression and various BCR/ABL-independent mechanisms (1,2).
The Wilms Tumor 1 (WT1) gene, which encodes a regulatory molecule that is important in the process of cell growth and development, is located on chromosome 11p13, and is a bispecific gene with antioncogenic and oncogenic properties (3). Radich et al (4) compared the gene expression profiles of patients with CML in different phases of the disease state (chronic, accelerated and blast crisis). The results of the aforementioned study revealed changes to gene expression in the early accelerated phase, in which the WT1 gene ranked fifth among the top 10 differentially expressed genes exhibiting upregulation/downregulation during disease progression. Furthermore, certain studies have demonstrated that WT1 overexpression in the K562 cell line (BCR/ABL-positive) results in resistance to the TKI imatinib (5–7). These observations suggest that the WT1 gene serves an important role in CML resistance and progression.
The WT1 gene serves primarily as an oncogene in hematological malignancies and regulates the expression of downstream genes. WT1 target genes may be classified according to their functions, among which the most notable are those associated with the mitogen-activated protein kinase (MAPK) and Wnt signaling pathways (8–10). Conversely, WT1 gene expression may be regulated by upstream genes. Previous in vivo and in vitro studies revealed that the high-temperature requirement family (Htr)A family member, HtrA2 serves as an upstream regulator of WT1 by binding specifically to the WT1 inhibition domain (11,12). HtrA2 possesses serine protease activity and degrades WT1 at multiple loci on the N- and C-termini (11,12).
The present study aimed to investigate the regulatory role of HtrA2 on WT1 and the effects of imatinib in K562 cells. In addition, the effects of its regulation on cell function and changes in the downstream signaling pathway were explored.
Materials and methods
Cells and experimental drugs
The K562 cell line (American Type Culture Collection, Manassas, VA, USA) used in the present study was derived from a patient in the acute transformation phase of CML and was preserved in the State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin, China. The primary drugs used were: Imatinib and the HtrA2 inhibitor 5-[5-(2-nitrophenyl) furfuryl iodine]-1, 3-diphenyl-2-thiobarbituric acid (UCF-101; both from Calbiochem; Merck KGaA, Darmstadt, Germany).
Design and synthesis of primers
All primers were synthesized and purified by Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The upstream and downstream primers were designed using Primer Premier 5.0 (Premier Biosoft International, Palo Alto, CA, USA), and their amplification specificities were validated using the Basic Local Alignment Search Tool (https://blast.ncbi.nlm.nih.gov). All primers were dissolved in deionized water to a concentration of 10 µM and stored at −20°C for subsequent experiments. The sequences of the primers were as follows: WT1 forward, 5′-CACGAGGAGCAGTGCCTGAG-3′ and reverse, 5′-AACCCTGATTGCGAATAGCG-3′; HtrA2 forward, 5′-AGACATCGCAACGCTGAGGATT-3′ and reverse, 5′-GGACGCTGAGCAGAGCTAACAA-3′; BCR/ABL-p210 forward, 5′-GGGCTCTATGGGTTTCTGAATG-3′ and reverse, 5′-CGCTGAAGGGCTTTTGAACT-3′; Internal reference gene GAPDH forward, 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′.
Analysis of protein expression
K562 cells (1×106 cells/system) were treated with half maximal inhibitory concentrations (IC50) of imatinib (1 µM), and cells were collected and counted at 0, 3, 6, 12, 24 and 48 h following drug application. Cells in the drugs+UCF-101 group were pretreated with UCF-101 (final concentration, 2 µM) for 2 h. Protein levels, changes in the location of WT1 and HtrA2 expression, and changes in phosphorylation of components of MAPK-associated signaling pathways following drug treatment were determined by western blot analysis using the following antibodies: Anti-WT1 rabbit mAb (cat. no. ab89901; 1:1,000; Abcam, Cambridge, UK), anti-HtrA2 rabbit mAb (cat. no. ab75982; 1:2,000; Abcam), horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (cat. no. ab6721; 1:5,000; Abcam), HRP-labeled goat anti-rabbit IgG (cat. no. ab6789; 1:5,000; Abcam), anti-poly ADP-ribose polymerase (PARP) rabbit mAb (cat. no. 9532; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-Histone H3 rabbit mAb (cat. no. 4499; 1:2,000; Cell Signaling Technology, Inc.), anti-phospho-p38 MAPK (Thr180/Tyr182) rabbit mAb (cat. no. 4511; 1:1,000; Cell Signaling Technology, Inc.), anti-p38 MAPK rabbit mAb (cat. no. 8690; 1:1,000; Cell Signaling Technology, Inc.), anti-phospho-p44/42 extracellular signal-related kinase (ERK; Thr202/Tyr204) rabbit mAb (cat. no. 8544; 1:1,000; Cell Signaling Technology, Inc.), anti-p44/42 ERK rabbit mAb (cat. no. 4695; 1:1,000; Cell Signaling Technology, Inc.), and anti-β-actin mouse mAb (cat. no. SC8432; 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). In the western blot analysis, the present study used RIPA as the protein extraction buffer (Beyotime Institute of Biotechnology, Haimen, China). The protein determination method was the BCA method and 20 ug protein were loaded per lane. The present study used a 10% gel to perform SDS-PAGE for the protein. The blocking step was performed in 5% BSA buffer at room temperature for 2 h. In the antibody incubation step, the primary antibodies were incubated at 4°C overnight and the secondary antibodies were incubated at room temperature for 1 h. The HRP-goat anti-mouse/rabbit immunoglobulin G antibodies were supplied by Abcam (1:5,000). The type of membrane used was nitrocellulose. The HRP-enhanced chemiluminescence method was used for visualization. Image J version 2 software was used for result analysis (National Institutes of Health, Bethesda, MD, USA). β-actin was used as the control.
Cell proliferation
Cells in the control group, imatinib group, UCF-10 group and imatinib+UCF-101 group were seeded in a 96-well plate (2×104 cells/well) in 100 µl RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (HyClone Company; GE Healthcare, Chicago, IL, USA); three wells were set up for each group. Then, 10 µl MTT (5 mg/ml) was added at 0, 24, 48 and 72 h following seeding. Subsequent to incubation for an additional 4 h, 100 µl 10% SDS/0.01 M HCL was added to each well. Plates were incubated at 37°C overnight and agitated using an oscillator for 10 min. The optical density (OD) at 546 nm was measured using a micro-plate reader. The OD value at 0 h in each group was set arbitrarily as 1, and the relative OD values at the other time points were calculated to construct the proliferation curves for comparison of the proliferation rates among different groups.
Cell apoptosis
K562 cells were collected and analyzed for apoptosis with an Annexin V-FITC kit (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer's protocol, and membrane integrity was simultaneously assessed with propidium iodide (PI) exclusion (BD Biosciences). The concentration of cells was 1×106 cells/ml, and in each system there were 1×105 cells. The cells were collected using centrifugation at 140 × g for 5 min at room temperature.
Statistical analysis
Statistical analyses were performed using SPSS19.0 software IBM Corp., Armonk, NY, USA). Measurement data were first subjected to normality testing using the single-sample Kolmogorow-Smirnov test. Normally distributed data were analyzed using a paired t-test, or one-way analysis of variance followed by the Student-Newman-Keuls method. Non-normally distributed data were analyzed using the rank sum test. P<0.05 was considered to indicate a statistically significant difference.
Results
Detection of WT1 and HtrA2 mRNA in K562 cells treated with imatinib and UCF-101 using reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
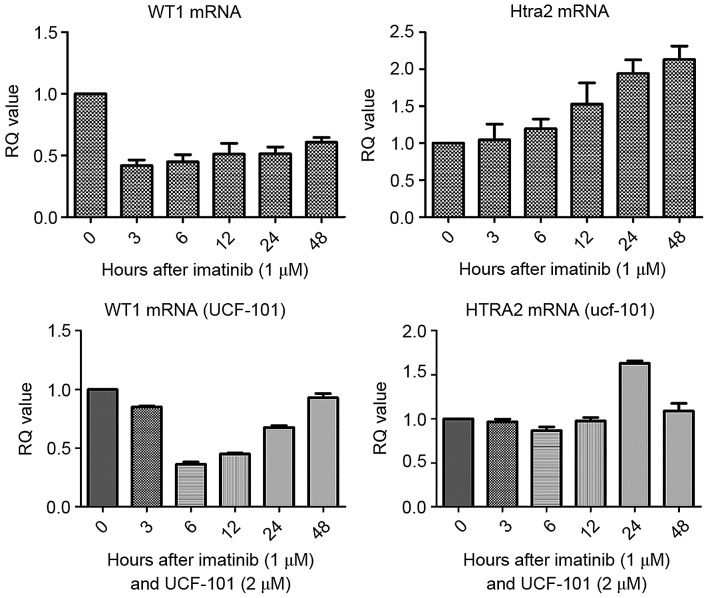
Firstly, the IC50 of imatinib for the K562 cell line was determined. Following treatment of K562 cells for 48 h, the IC50 value for imatinib was 1.18±0.2 µM. In subsequent experiments, 1.0 µM imatinib was used. Cells were collected following treatment with imatinib (± UCF-101) for 0, 3, 6, 12, 24 and 48 h. Total RNA was extracted and reverse transcribed to obtain cDNA, and the changes of WT1 and HtrA2 mRNA in K562 cells were analyzed by qPCR. As presented in Fig. 1, compared with the control group, WT1 mRNA levels were downregulated in cells treated with imatinib. In contrast, HtrA2 mRNA levels were gradually upregulated, reaching a 2-fold increase at 48 h compared with the levels in the control group. Following pretreatment with UCF-101, the downregulation of WT1 mRNA induced by imatinib was delayed, measuring at its lowest level at 6 h, and was restored to the levels of the control at 48 h. However, no significant changes in HtrA2 expression were observed.
Figure 1.
Effects of imatinib and UCF-101 treatment on WT1 and HtrA2 mRNA levels. WT1, Wilms Tumor 1; HtrA2, high-temperature requirement protein A2; RQ, relative quantity.
Effects of imatinib
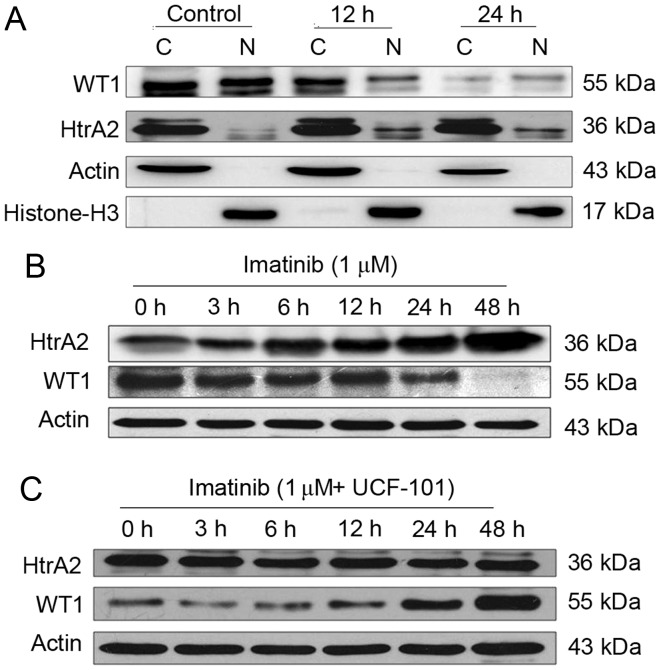
Following treatment of the K562 cells with imatinib for 12 and 24 h, cells were collected and counted. Cytoplasmic and nuclear proteins were analyzed by western blotting using β-actin, and histone H3 as internal controls for the cytoplasmic and nuclear proteins, respectively. WT1 and HtrA2 were expressed in the cytoplasm and nuclei, with HtrA2 located primarily in the cytoplasm (Fig. 2A). Subsequent to treatment with imatinib, WT1 protein levels were downregulated markedly in the cytoplasm and nuclei. WT1 protein levels were markedly reduced in the nuclei at 12 h following imatinib treatment, while HtrA2 protein levels were upregulated in the cytoplasm and, more evidently, in the nuclei.
Figure 2.
Effect of imatinib (or with UCF-101) on the expression and location of WT1 and HtrA2 protein in K562 cells. (A) Changes in the cellular localization of WT1 and HtrA2 expression following treatment with imatinib in K562 cells. (B) Changes in HtrA2 and WT1 expression following treatment of K562 cells with imatinib. (C) Changes of HtrA2 and WT1 expression following treatment with imatinib and UCF-101 in K562 cells. C, cytoplasm; N, nuclei; WT1, Wilms Tumor 1; HtrA2, high-temperature requirement protein A2.
Effects of imatinib and UCF-101
Subsequent to treatment with imatinib (with and without UCF-101 pretreatment) for up to 48 h, K562 cells were collected and counted. Cells were lysed with radioimmunoprecipitation assay buffer, and total proteins were collected for western blot analysis. The results are presented in Fig. 2B and C. Following prolonged treatment of K562 cells with imatinib in the absence of UCF-101 pretreatment, HtrA2 expression was upregulated, and the WT1 level was decreased. However, no significant HtrA2 variation was observed in cells with UCF-101 pretreatment, while WT1 was slightly downregulated and rapidly restored to a level higher compared with the baseline. These data suggest that imatinib induces the upregulation of HtrA2 protein and downregulation of the WT1 protein, that HtrA2 is an upstream regulatory factor of WT1 and that it is activated by imatinib.
Effect of HtrA2 regulation of WT1 on the biological function of K562 cells
Cells were treated with imatinib, and divided into control, UCF-101, imatinib and imatinib+UCF-101 groups. Cells were then collected at 24 and 48 h. Apoptosis was detected by flow cytometry following staining with Annexin V and PI and cell proliferation was measured using the MTT method. Digestion of PARP was detected by western blotting.
Effect of imatinib and UCF-101 on K562 cell apoptosis
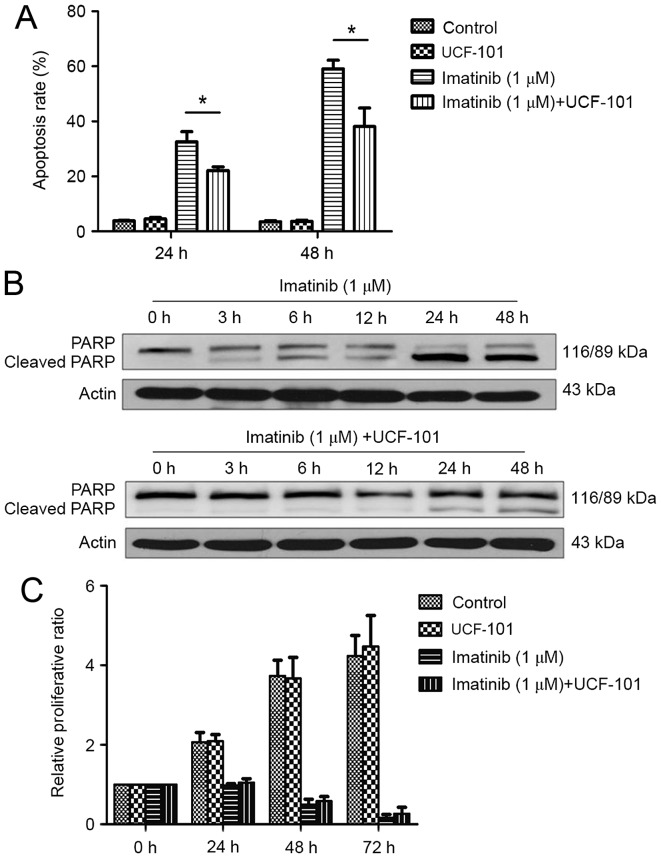
As demonstrated in Fig. 3A, significant increases in apoptosis rates were observed at 24 and 48 h following treatment with imatinib; this effect was increased with prolonged duration. However, imatinib-induced apoptosis was significantly reduced by pretreatment with UCF-101, with significant differences observed between the two groups at 24 and 48 h (both P<0.05). Concomitantly, western blot analysis demonstrated that PARP digestion was markedly increased following treatment with imatinib alone, suggesting increased apoptosis (Fig. 3B). In contrast, imatinib-induced PARP digestion was remarkably decreased by pretreatment with UCF-101, which was consistent with the effects observed on apoptosis (Fig. 3B).
Figure 3.
Effect of imatinib (or with UCF-101) on apoptosis and proliferation of K562 cells. (A) Effect of imatinib and UCF-101 on K562 cell apoptosis. *P<0.05. (B) PARP digestion following treatment of K562 cells with imatinib and UCF-101. (C) Effect of imatinib and UCF-101 on K562 cell proliferation. PARP, poly ADP-ribose polymerase; RQ, relative quantity.
Effect of imatinib and UCF-101 on K562 cell proliferation
Imatinib suppressed K562 cell proliferation significantly and persistently, while UCF-101 pretreatment exhibited no significant effect on K562 cell proliferation. Compared with the imatinib group, the proliferation rate was slightly increased in the imatinib+UCF-101 group, suggesting that UCF-101 suppressed the imatinib-induced inhibition of cell proliferation, although this effect did not reach the level of statistical significance (Fig. 3C).
Effect of HtrA2 regulation of WT1 on its downstream signaling pathways
WT1 possesses extensive targets for downstream signaling, of which the MAPK signaling pathway is particularly notable. The present study demonstrated that the regulatory effects of HtrA2 on WT1 affect the apoptosis and proliferation of K562 cells. In this section, to assess the downstream mechanism of the functional changes induced by the regulatory effects of HtrA2 on WT1, K562 cells were treated with imatinib, and changes in the phosphorylation of the MAPK signaling pathway members ERK1/2 and p38 were investigated by western blot analysis.
Effect of imatinib and UCF-101 on signaling pathways
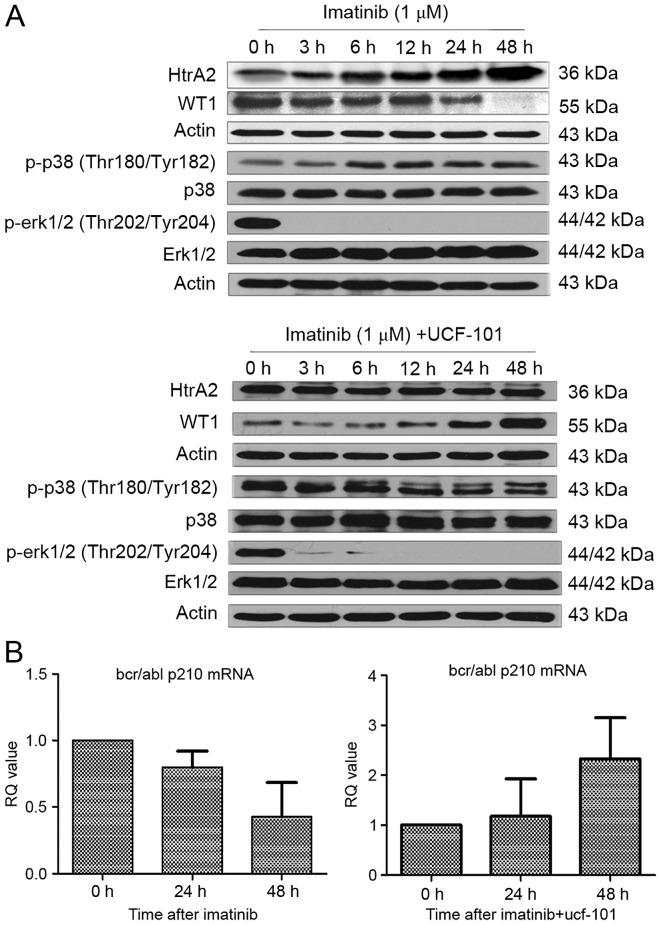
As aforementioned, HtrA2 expression was upregulated and WT1 expression was downregulated following treatment with imatinib in K562 cells. However, the downregulation of WT1 protein expression was reversed subsequent to pretreatment with UCF-101 to suppress HtrA2 function. Under similar conditions and time points, it was identified that p38-MAPK phosphorylation began to increase following treatment with imatinib for 6 h, and was sustained to 48 h (Fig. 4A). Concurrently, the ERK1/2 phosphorylation started to decrease significantly from 3 h following imatinib treatment and was sustained to 48 h without restoration. In cells pretreated with UCF-101, p38-MAPK phosphorylation was not upregulated and ERK1/2 phosphorylation remained at a low level (Fig. 4A).
Figure 4.
The effect of imatinib or UCF-101 on the MAPK signaling pathway and BCR/ABL expression. (A) Effect of imatinib and UCF-101 on the mitogen-activated protein kinase pathway. (B) Effect of imatinib and UCF-101 on BCR/ABL p210 expression levels. WT1, Wilms Tumor 1; HtrA2, high-temperature requirement protein A2; p, phosphorylated; p38, mitogen-activated protein kinase 14; ERK, extracellular signal-related kinase; Thr, threonine; Tyr, tyrosine; BCR/ABL p120, breakpoint cluster region/tyrosine-protein kinase ABL1 p210 catenin; MAPK, mitogen activated protein kinase.
Association between HtrA2 regulation of WT1 and BCR/ABL expression
Expression of the BCR/ABL-p210 fusion gene is a feature of K562 cell lines. To investigate the association of HtrA2 regulation on WT1 with the expression of BCR/ABL-p210 fusion gene, RNA was extracted from cells treated with imatinib ±UCF-101. cDNA was reverse transcribed, and alterations to BCR/ABL-p210 fusion gene expression were determined by qPCR. Imatinib downregulated expression of the BCR/ABL-p210 fusion gene significantly, while UCF-101 pretreatment resulted in a gradual upregulation of BCR/ABL-p210 fusion gene expression, which was consistent with the variation observed in the expression of the WT1 gene under similar conditions (Fig. 4B).
Discussion
In previous years, extensive studies of CML resistance have demonstrated that this phenomenon involves multi-faceted and complex mechanisms, including BCR/ABL-dependent mechanisms and a variety of non-BCR/ABL-dependent mechanisms, such as ABCB1 and OCT1-mediated intake and efflux of drugs, clonal cell evolution, and bone marrow stroma-mediated resistance in addition to resistance mechanisms associated with CML stem cells (1,2). Otahalova et al (6) identified that the sensitivity to imatinib was predictable based on the WT1 expression level in peripheral blood lymphocytes in patients with CML following in vitro culture and treatment with imatinib. In addition, specific studies revealed that the overexpression of WT1 protein following gene transfection of K562 cell lines induced imatinibresistance (7). These studies indicate that the WT1 gene serves an important role in the progression of CML and TKI-resistance.
As a transcription factor, WT1 possesses extensive downstream targets, thereby regulating the biological behavior of cells (3). Certain studies functionally classified the target genes of WT1 in the Wilms cell line CCG99-11 using the CHIP-CHIP method; the most important genes were identified to be associated with the MAPK and Wnt pathways (8). Our previous studies on the K562 cell line also indicated that WT1 target genes involved a variety of MAKP and Wnt/b-catenin signaling pathway genes, including MAPK6, MAPK7, Wnt2b and Wnt11 (9,10). However, the WT1 upstream regulatory factors have rarely been studied. It is currently unknown whether HtrA2 regulates WT1 in BCR/ABL-positive cells, including K562 cells. In the present study, the classical CML-targeted therapy drug imatinib caused upregulation of HtrA2 protein expression and downregulation of the WT1. UCF-101, which is a specific inhibitor of HtrA2 and competitively inhibits the activity of the HtrA2 protease, was used to determine their regulatory association in K562 cell lines (13). When cells were pretreated with UCF-101 to suppress HtrA2 activity, the drug-induced downregulation of WT1 protein expression was reversed, and WT1 expression was maintained at a high level. These results indicate that drug stimulation induced the HtrA2 protein upregulation and increased WT1 protein degradation in K562 cell lines.
Furthermore, it was identified that imatinib upregulated HtrA2 expression and downregulated WT1 expression at the transcriptional level, while UCF-101 pretreatment reversed this effect. These results suggest that HtrA2 exerted a regulatory effect on WT1 at the protein level (protein degradation) and at the transcriptional level, although the mechanism remains to be elucidated. Regulation of HtrA2 may ultimately lead to a downregulation of WT1 protein expression, which may affect the binding of WT1 with the promoters, and may lead to changes in gene regulation. Therefore, HtrA2 functions as a regulatory factor of WT1 under the effects of drug stimulation.
Our previous studies revealed that the WT1 protein was expressed in the cytoplasm and nuclei of K562 cells, with higher expression in the cytoplasm (9). However, HtrA2 protein is expressed as a 45-kDa precursor protein, which is translated and translocated to the mitochondria, where it is lysed to form a 36-kDa mature protein located in the inner mitochondrial membrane region, and partly located in the nuclei. In the present study, following treatment with imatinib, the WT1 protein level was decreased in the cytoplasm and nuclei of K562 cells. This effect was enhanced with prolonged imatinib exposure, and the reduction was more rapid in the nuclei compared with that observed in the cytoplasm. Conversely, under similar conditions, HtrA2 protein expression was identified to increase in the cytoplasm and nuclei. These results indicate that HtrA2 protein activation is increased in the cytoplasm under drug stimulation, leading to WT1 protein degradation in the cytoplasm, and effects on WT1 localization and transcriptional regulation. Alternatively, it is possible that HtrA2 protein migrates to the nuclei under the effect of external stimulation, leading to WT1 protein degradation in the nuclei, therefore affecting its transcriptional regulation.
WT1 is an important transcription regulation factor involved in maintaining cell growth and self-renewal (9). It is unknown whether the regulation of HtrA2 on WT1 affects the biological behavior of cells. In the present study, K562 cell apoptosis and proliferation as investigated under HtrA2 regulation, and it was identified that the proportion of apoptotic cells was decreased significantly (P<0.05) by pretreatment with UCF-101 to suppress the imatinib-induced HtrA2 activation. This was also verified by the results of PARP digestion analysis, suggesting that the regulation of HtrA2 on WT1 affects K562 cell apoptosis. Under the effects of external apoptotic stimulation (imatinib), HtrA2 was activated and upregulated, while WT1 protein expression was downregulated, affecting its transcriptional regulation of downstream genes, and promoting the occurrence of apoptosis. Conversely, inhibition of HtrA2 activation may affect its regulatory effect on WT1, leading to sustained WT1 expression and an anti-apoptotic effect on cells. Therefore, HtrA2 regulation of WT1 affects cell apoptosis, where a loss of this regulatory ability prevents apoptosis. It may be hypothesized that this effect represents one of the non-BCR/ABL-dependent mechanisms for the treatment of CML.
The mechanism investigations of the present study demonstrated that imatinib activated the p38 MAPK pathway, leading to upregulation of p38 MAPK phosphorylation. However, activation of the p38 MAPK phosphorylation was inhibited by pretreatment of cells with UCF-101. The changes in p38 MAPK phosphorylation caused by HtrA2 regulation of WT1 were partially consistent with the changes in K562 cell apoptosis under drug treatment, indicating that the p38 MAPK signaling pathway is a downstream target pathway of WT1 and its activation is indirectly affected by HtrA2 regulation of WT1, which in turn affects the biological function of cells.
The ERK-MAPK signaling pathway is the most prevalent MAPK signaling pathway, and it serves a significant role in cell proliferation. The present study demonstrated that imatinib downregulated ERK1/2 phosphorylation significantly and persistently. However, UCF-101 pretreatment failed to reverse p-ERK1/2 downregulation. This was identical to the features of cell proliferation under imatinib treatment with/without UCF-101 pretreatment. Furthermore, no significant association was observed between the phosphorylation levels of the ERK1/2-MAPK pathway and WT1 protein level, indicating that the ERK1/2 pathway is not the primary downstream target of WT1. Therefore, the imatinib-induced downregulation of ERK1/2 phosphorylation may be regulated primarily by other upstream factors.
In the present study, it was also observed that imatinib led to the downregulation of BCR/ABL p210 fusion gene expression, which was reversed by UCF-101-mediated suppression of HtrA2, and even exhibited a trend of upregulation. These observations suggest that the pattern of BCR/ABL p210 fusion gene expression is consistent with that of WT1 under the effects of HtrA2. This indicates that HtrA2 and its regulatory effect on WT1 may affect the sensitivity of BCR/ABL-positive cell lines to target therapy drugs through different mechanisms, whereby BCR/ABL-dependent and -independent mechanisms may be involved.
The results of the present study indicate that HtrA2 functions as an upstream regulatory factor of WT1, and affects imatinib-induced K562 cell apoptosis. These data provide an insight into novel targets for treatment of CML in the future.
Acknowledgements
The present study was supported by the National Science and Technology Pillar Program (grant no., 2014BAI09B12), the National Natural Science Foundation of China (grant no., 30870913) and the Tianjin Research Program of Application Foundation and Advanced Technology (grant no. 15JCZDJC36400).
References
- 1.Apperley JF. Part I: Mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8:1018–1029. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- 2.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Han Y, Suarez Saiz F, Minden MD. A tumor suppressor and oncogene: The WT1 story. Leukemia. 2007;21:868–876. doi: 10.1038/sj.leu.2404624. [DOI] [PubMed] [Google Scholar]
- 4.Radich J, Dai H, Mao M, Oehler V, Schelter J, Druker B, Sawyers C, Shah N, Stock W, Willman CL, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci USA. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varma N, Anand MS, Varma S, Juneja SS. Role of hTERT and WT1 gene expression in disease progression and imatinib responsiveness of patients with BCR-ABL positive chronic myeloid leukemia. Leuk Lymphoma. 2011;52:687–693. doi: 10.3109/10428194.2010.550978. [DOI] [PubMed] [Google Scholar]
- 6.Otahalova E, Ullmannova-Benson V, Klamova H, Haskovec C. WT1 expression in peripheral leukocytes of patients with chronic myeloid leukemia serves for the prediction of Imatinib resistance. Neoplasma. 2009;56:393–397. doi: 10.4149/neo_2009_05_393. [DOI] [PubMed] [Google Scholar]
- 7.Svensson E, Vidovic K, Lassen C, Richter J, Olofsson T, Fioretos T, Gullberg U. Deregulation of the Wilms' tumour gene 1 protein (WT1) by BCR/ABL1 mediates resistance to imatinib in human leukaemia cells. Leukemia. 2007;21:2485–2494. doi: 10.1038/sj.leu.2404924. [DOI] [PubMed] [Google Scholar]
- 8.Kim MK, McGarry TJ, Broin OP, Flatow JM, Golden AA, Licht JD. An integrated genome screen identifies the Wnt signaling pathway as a major target of WT1. Proc Natl Acad Sci USA. 2009;106:11154–11159. doi: 10.1073/pnas.0901591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Wang J, Li X, Jia Y, Huai L, He K, Yu P, Wang M, Xing H, Rao Q, et al. Role of the Wilms' tumor 1 gene in the aberrant biological behavior of leukemic cells and the related mechanisms. Oncol Rep. 2014;32:2680–2686. doi: 10.3892/or.2014.3529. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Li Y, Yuan T, Zhang Q, Jia Y, Li Q, Huai L, Yu P, Tian Z, Tang K, et al. Exogenous expression of WT1 gene influences U937 cell biological behaviors and activates MAPK and JAK-STAT signaling pathways. Leuk Res. 2014;38:931–939. doi: 10.1016/j.leukres.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Hartkamp J, Carpenter B, Roberts SG. The Wilms' tumor suppressor protein WT1 is processed by the serine protease HtrA2/Omi. Mol Cell. 2010;37:159–171. doi: 10.1016/j.molcel.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartkamp J, Roberts SG. HtrA2, taming the oncogenic activities of WT1. Cell Cycle. 2010;9:2508–2514. doi: 10.4161/cc.9.13.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klupsch K, Downward J. The protease inhibitor Ucf-101 induces cellular responses independently of its known target, HtrA2/Omi. Cell Death Differ. 2006;13:2157–2159. doi: 10.1038/sj.cdd.4401955. [DOI] [PubMed] [Google Scholar]