Abstract
In our previous studies, a functionally unknown gene, family with sequence similarity 172, member A (FAM172A), was identified. High levels of FAM172A suppressed the cell cycle process, arresting HepG2 cells in G1/S and inhibiting cell proliferation. The present study aimed to confirm the expression levels of FAM172A and nucleotide-binding protein 1 (NUBP1) in colorectal cancer (CRC) tissues and normal colorectal tissues. The impact of FAM172A and NUBP1 on the prognosis of patients with CRC was also analyzed. Immunohistochemical staining for FAM172A and NUBP1 was performed on 180 cancerous tissues and 60 normal paraffin-embedded tissues from patients with CRC. In total, 85 and 83% of 180 patients revealed positive expression of FAM172A and NUBP1, respectively. FAM172A expression level was associated with Tumor-Node-Metastasis (TNM) staging (P<0.001), the levels of serum carcinoembryonic antigen (CEA; P=0.023) and carbohydrate antigen 19–9 (CA19-9; P=0.016), lymph node involvement (P=0.004), tissue type (P=0.016), Dukes' staging (P<0.001) and NUBP1 (P=0.026). Furthermore, the expression level of NUBP1 was also markedly associated with the levels of serum CEA (P=0.006) and CA19-9 (P=0.001), TNM staging (P<0.001), lymph node involvement (P=0.005), histological typing (P=0.024) and Dukes' stage (P<0.001). Results of the univariate analysis demonstrated that there was a negative correlation between the expression level of FAM172A and overall survival (OS) and relapse-free survival (RFS) (P=0.013 and P=0.012, respectively), and there was also a negative correlation between NUBP1 expression level and OS and RFS (P<0.001 and P<0.001, respectively). With regards to OS and RFS, multivariate analysis revealed that expression levels of FAM172A and NUBP1 and tumor stage may be independent prognostic factors Thus, the present study suggested that FAM172A and NUBP1 may be prognostic makers for CRC.
Keywords: family with sequence similarity 172, member A, nucleotide-binding protein 1, expression, poor prognosis, colorectal carcinoma
Introduction
As a major malignant tumor of the digestive system, the morbidity and mortality rates of colorectal cancer (CRC) are increasing globally (1). Similar to other types of solid tumors, the high mortality rate of CRC is due to metastasis, a complicated process that is often associated with alterations in the extracellular matrix to enhance cell motility and the ability of cells to grow at a remote location (2). The molecular mechanisms underlying this process remain to be elucidated (3). Despite progress in improving the early diagnosis and treatment of CRC, patients with advanced cancer have a poor prognosis, and their 5-year survival rate is only 45% (4,5). Patients with CRC do not exhibit many early symptoms and a large portion are diagnosed at the mid-late stage, thus the 5-year survival rate is low (6,7). Currently, the pathogenesis of CRC remains ill-defined, thus there is an urgent requirement to investigate novel treatments.
The growth of CRC involves various genes. In a previous study, a functionally unknown gene, family with sequence similarity 172, member A (FAM172A), was identified (8). The association between the presence of tumors and FAM172A has been investigated in our previous studies. It was revealed that overexpressed FAM172A inhibited proliferation of the HepG2 cell line, as assessed by reverse transcription-quantitative polymerase chain reaction and western blotting (9). HepG2 cells demonstrated cell cycle period arrest in the S phase, and their proliferation was signally inhibited when transfected with high concentrations of FAM172A recombinant protein (9). An online software program, CELLO 2.5 (http://cello.life.nctu.edu.tw/), was used to predict the subcellular localization of FAM172A (10), and the finding revealed that it was generally positioned in the cytoplasm.
Nucleotide-binding protein 1 (NUBP1) is a protein coding gene and a member of the NUBP/multidrug resistance-associated protein subfamily of adenosine triphosphate-binding proteins (11,12). Among its associated pathways are metabolism and cytosolic iron-sulfur cluster assembly, and Gene Ontology annotations regarding this gene include nucleotide-binding and 4 iron, 4 sulfur cluster-binding. NUBP1 is involved in the regulation of centrosome duplication and the assembly of cytosolic protein (13,14). NUBP1 is a component of an iron-sulfur scaffold complex, which mediates the assembly of an iron-sulfur cluster (15).
Taken together, these two proteins may be associated with regulating the cell cycle. As the association between the clinical activity and the combined expression levels of NUBP1 and FAM172A in CRC have not yet been investigated, the present study examined the association between the expression level and prognostic impact of NUBP1 and FAM172A in patients with CRC, as assessed by immunohistochemical staining.
Materials and methods
Samples and patients
A total of 180 patients who underwent surgery between October 2012 and October 2014 at the Department of Pathology, Nanfang Hospital and Zhujiang Hospital, which are affiliated with Southern Medical University (Guangzhou, China) were enrolled. The study consisted of 83 males and 97 females, with a median age of 57 years (range, 38–77 years). A total of 180 paraffin-embedded specimens of CRC were included in the present study. In accordance with the World Health Organization criteria, the data of all patients were examined and regraded (16), and pathological stage was distinguished in the light of the Tumor-Node-Metastasis (TNM) stage system (17). Furthermore, 60 cases of normal colorectal tissues were also included for the evaluation of the FAM172A and NUBP1 expression levels in the non-cancerous colorectal mucosa. The patients did not receive biotherapy, chemotherapy, radiotherapy or any other treatment strategies pre-operatively. The pathological diagnosis was formed by three blinded experts from the Department of Pathology of Nanfang Hospital and Zhujiang Hospital. The 180 patients were grouped according to patient age and sex, serum levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19-9), tumor staging, the identification of lymph node (LN) metastasis, presence of distant metastasis and type of histology according to the World Health Organization classification. Written informed consent was obtained from all patients prior to enrollment in the present study. The study was approved by the Ethics Committee of Nanfang Hospital. According to the Declaration of Helsinki, written informed consent was obtained from all patients prior to enrollment in the present study.
Main reagents
Rabbit anti-human FAM172A monoclonal antibody (cat. no. ab121364; Abcam Biotechnology, Inc., Cambridge, UK), mouse anti-human NUBP1 polyclonal antibody (cat. no. ab88622; Abcam Biotechnology, Inc., Abcam, Cambridge, UK), SP immunohistochemical kit (cat. no. SP-9000; Beijing Zhongshan Golden Bridge Biotechnology, Inc., Beijing, China) and DAB color-developing reagent (catalog no. ZLI-9017; Beijing Zhongshan Golden Bridge Biotechnology, Inc.) were used in the present study.
Immunohistochemical staining and scoring
Firstly, tissue samples were cut into 4-µm thick slices. The antibodies that were used for immunohistochemistry were the FAM172A monoclonal antibody clone and NUBP1 monoclonal antibody clone. The tissue sections were stained according to the manufacturer's instructions from the SP immunohistochemical kit and DAB color-developing reagent.
Briefly, the tissues were deparaffinized in xylene at room temperature for 10 min and rehydrated in an ethanol gradient. The antigen of paraffin slices was retrieved by citric acid buffer for 10 min at 95°C (pH=6.0). Hydrogen peroxide solution (0.3%) was used to block the activation of endogenous peroxidase for 18 min at room temperature. Incubation was performed overnight at 4°C and the primary antibodies for FAM172A and NUBP1 were applied at a dilution of 1:200. At room temperature, the tissue samples were washed with PBS and incubated with the enzyme horseradish peroxidase-labeled polymer rabbit or mouse antibodies for 16 min (cat. no. SP-9000; Beijing Zhongshan Golden Bridge Biotechnology, Inc.). Subsequently, the tissue samples were incubated with DAB at room temperature for 5 min in order to visualize the expression levels. Counterstaining was performed using hematoxylin and the tissues were dehydrated using a series of ethanol and xylene.
The scoring of the immunohistochemical staining was performed independently by two researchers from the Department of Vascular Surgery, Nanfang Hospital, Southern Medical University, who were not familiar with the clinicopathological data. PBS, which was used as the negative control, was a substitute for the primary antibody. Observations were conducted in 10 random high-power fields under a light microscope (magnification, ×400) according to the count and staining intensity of positive cells (CX31-LV320; Olympus, Inc., Tokyo, Japan).
The H-score method was used in the present study. The staining intensity of positive cells was scored as follows: 0, no color; 1, light yellow; 2, light brown; or 3, brown. The quantity of positive cells was scored as follows: 0, 0%; 1, <10%; 2, 10–35%; 3, 35–75%; or 4, >75%. The percentage score was multiplied with the staining intensity score. The score of each tissue sample was classified into one of four grades: 0–2 (−), 2–4 (+), 4–6 (++) and >6 (+++). When the score was ≥4 for a tissue sample, the protein expression level was considered to be positive.
Statistical analysis
Overall survival (OS) and relapse-free survival (RFS) were the endpoints of interest, and the endpoint of follow-up was the date of the last contact or mortality between 2000 and 2013. The paired-samples t-test was designed to compare expression levels in CRC tissues with normal tissues. The period of time from surgery to mortality or last contact was used as the OS time, and patients that survived to the last contact period were considered as censored. The period of time from surgery to recurrence, last contact or mortality was defined as RFS time, and patients were regarded as censored if they survived and experienced no relapse. For prognosis, Pearson's χ2 test was used for analyzing correlations between overall scores and potentially meaningful categorical variables. For clinicopathological factors and FAM172A and NUBP1, the present study applied univariate and multivariate cox proportional hazards regression analysis to evaluate their effect on OS and RFS. Kaplan-Meier survival curves were constructed to estimate the OS of patients and further analyze the impact of FAM172A and NUBP1 expression using the log-rank test. SPSS software (version 20.0; IBM Corp., Armonk, NY, USA) was used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of FAM172A and NUBP1 are associated with certain clinicopathological features of patients with CRC
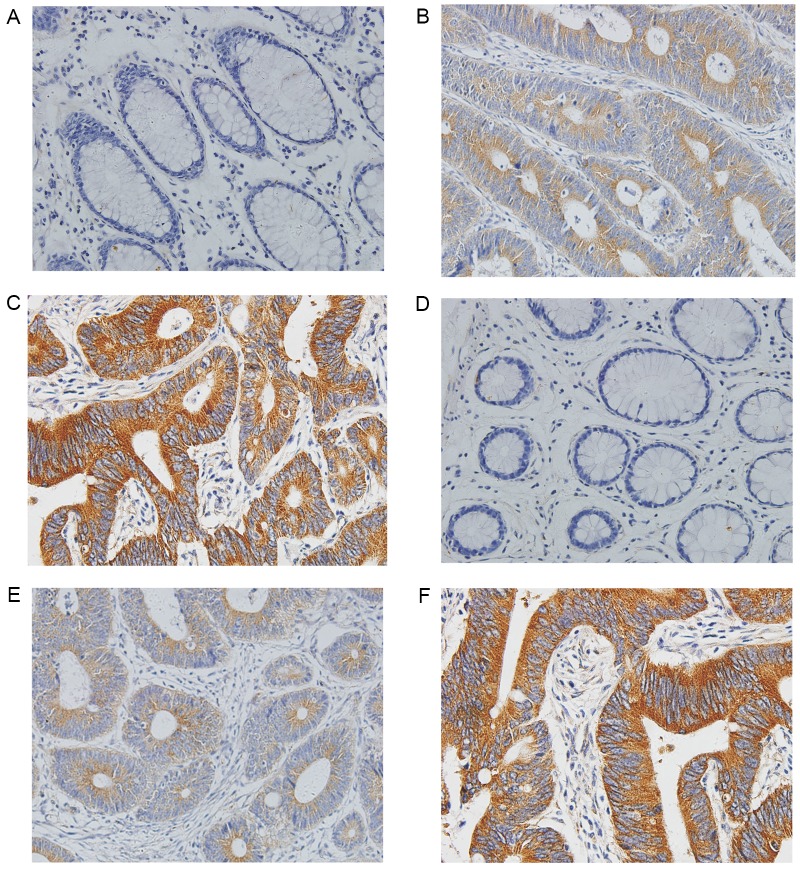
The clinicopathological characteristics are outlined in Table I. Associations between FAM172A and NUBP1 expression levels in normal colorectal mucosa and CRC tissues are presented in Table II. The present study revealed that immunoreactivity of FAM172A and NUBP1 existed primarily in the cytoplasm (Fig. 1). Weak nuclear staining was identified in 11 cases for FAM172A and 10 cases for NUBP1, and positive expression levels of FAM172A and NUBP1 were absent in 60 samples of normal colorectal mucosa. However, positive expression levels of FAM172A and NUBP1 were observed in 85% (153/180) and 83% (149/180) of patients with CRC, respectively.
Table I.
Association between expression levels of FAM172A and NUBP1 and various clinicopathological parameters.
| FAM172A | NUBP1 | ||||
|---|---|---|---|---|---|
| Characteristics | No. of patients | Positive, n (%) | P-value | Positive, n (%) | P-value |
| Patient age, years | 0.084 | 0.944 | |||
| <40 | 60 | 42 (70) | 39 (65) | ||
| 40–60 | 58 | 34 (59) | 38 (66) | ||
| >60 | 62 | 48 (77) | 42 (68) | ||
| Sex | 0.851 | 0.924 | |||
| Female | 97 | 82 (85) | 80 (82) | ||
| Male | 83 | 71 (86) | 68 (82) | ||
| CEA | 0.023 | 0.006 | |||
| Normal | 66 | 26 (39) | 23 (35) | ||
| Elevated | 114 | 65 (57) | 64 (56) | ||
| CA19-9 | 0.016 | 0.001 | |||
| Normal | 71 | 32 (45) | 37 (52) | ||
| Elevated | 109 | 66 (61) | 75 (69) | ||
| TNM stage | <0.001 | <0.001 | |||
| I and II | 101 | 50 (50) | 47 (47) | ||
| III and IV | 79 | 72 (91) | 75 (95) | ||
| LN metastasis | 0.004 | 0.005 | |||
| Absence | 109 | 83 (76) | 81 (74) | ||
| Presence | 71 | 66 (93) | 65 (92) | ||
| Distant metastasis | 0.071 | 0.079 | |||
| Absence | 138 | 77 (56) | 81 (59) | ||
| Presence | 42 | 30 (72) | 31 (74) | ||
| Differentiation | 0.016 | 0.024 | |||
| WD | 44 | 20 (45) | 22 (50) | ||
| MD | 121 | 85 (70) | 88 (73) | ||
| PD | 15 | 11 (73) | 10 (67) | ||
| Tumor magnitude, cm | 0.068 | 0.064 | |||
| <2 | 26 | 10 (38) | 12 (46) | ||
| 2–4 | 109 | 59 (54) | 64 (59) | ||
| >4 | 45 | 30 (67) | 33 (73) | ||
| Dukes' stage | <0.001 | <0.001 | |||
| A and B | 102 | 50 (49) | 48 (39) | ||
| C and D | 78 | 74 (95) | 71 (73) | ||
| NUBP1 | 0.026 | ||||
| Positive | 149 | 144 (92) | |||
| Negative | 31 | 27 (16) | |||
LN, lymph node; WD, well-differentiated; MD, moderately differentiated; PD, poorly differentiated; NUBP1, nucleotide-binding protein 1; FAM172A, family with sequence similarity 172 member A; TNM, Tumor-Node-Metastasis.
Table II.
Comparison of FAM172A and NUBP1 positive expression levels with colorectal cancer and normal colorectal tissues.
| Colorectal tissue | n | FAM172A-positive, n (%) | P-value | NUBP1-positive, n (%) | P-value |
|---|---|---|---|---|---|
| Cancer | 60 | 58 (85.0) | 0.022 | 59 (98.3) | 0.012 |
| Normal | 60 | 11 (18.3) | 10 (20.0) |
FAM172A, the family with sequence similarity 172, member A; NUBP1, nucleotide-binding protein 1.
Figure 1.
Immunohistochemical staining in CRC. (A) Normal colorectal mucosa was negative for FAM172A expression. (B) Low FAM172A expression level in CRC tissue samples. (C) High FAM172A expression level in CRC tissue samples. (D) Normal colorectal mucosa was negative for NUBP1 expression. (E) Low NUBP1 expression level in CRC tissue samples. (F) High NUBP1 expression level in CRC tissue samples (magnifications, ×200). NUBP1, nucleotide-binding protein 1; FAM172A, family with sequence similarity 172 member A; CRC, colorectal cancer.
The expression level of FAM172A was markedly associated with the expression levels of serum CEA (P=0.023) and CA19-9 (P=0.016), TNM staging (P<0.001), LN metastasis (P=0.004), histological grade (P=0.01), Dukes' staging (P<0.001) and NUBP1 expression level (P=0.026). Furthermore, the expression level of NUBP1 was markedly associated with the serum CEA (P=0.006) and CA19-9 (P=0.001) expression levels, TNM staging (P<0.001), LN metastasis (P=0.005), histological type (P=0.024) and Dukes' staging (P<0.001), as presented in Table I.
The present study used univariate cox regression analysis to investigate the association between clinicopathological characteristics plus the expression levels of the two proteins and RFS and OS (Table III). Results of univariate analysis revealed lower TNM staging and the existence of LN metastasis were associated with longer OS and RFS times, and that there were negative associations between the expression level of FAM172A and OS and RFS (P=0.013 and P=0.012, respectively) and there was also a negative correlation between NUBP1 expression level and OS and RFS (P<0.001 and P<0.001, respectively).
Table III.
Univariate cox proportional hazards regression analysis of clinicopathological characteristics and their effect on RFS and OS.
| OS | RFS | ||||
|---|---|---|---|---|---|
| Characteristics | No. of patients | HR (95% CI) | P-value | HR (95% CI) | P-value |
| CEA | |||||
| Normal | 66 | 1.000 | 1.000 | ||
| Elevated | 114 | 1.499 (1.703–5.008) | 0.217 | 1.473 (0.775–2.802) | 0.237 |
| TNM stage | |||||
| I and II | 101 | 1.000 | 1.000 | ||
| III and IV | 79 | 6.391 (3.790–10.744) | <0.001 | 5.940 (3.551–9.937) | <0.001 |
| LN metastasis | |||||
| Absence | 109 | 1.000 | 1.000 | ||
| Presence | 71 | 2.816 (1.641–4.831) | <0.001 | 2.758 (1.609–4.726) | <0.001 |
| NUBP1 | |||||
| Negative | 31 | 1.000 | 1.000 | ||
| Positive | 149 | 4.293 (1.967–9.373) | <0.001 | 1.612 (1.946–9.268) | <0.001 |
| FAM172A | |||||
| Negative | 29 | 1.000 | 1.000 | ||
| Positive | 145 | 4.200 (1.816–9.714) | 0.01 | 4.018 (1.740–9.275) | 0.012 |
RFS, relapse-free survival; OS, overall survival; CEA, carcinoembryonic antigen; TNM, Tumor-Node-Metastasis; LN, lymph node; FAM172A, the family with sequence similarity 172, member A; NUBP1, nucleotide-binding protein 1; CI, confidence interval; HR, hazard ratio.
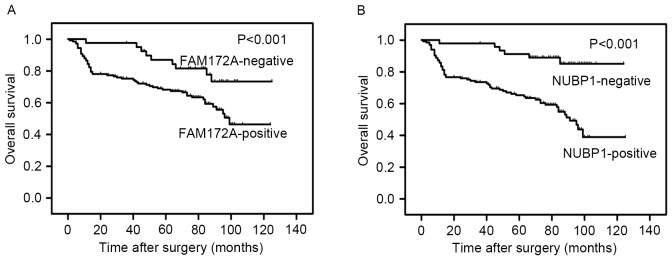
Multivariate analysis was performed according to the total data of 180 patients for the considered variables, including the serum expression levels of CEA and CA19-9, TNM staging and Dukes' staging, the presence of LN metastasis and distant metastasis, and FAM172A and NUBP1 expression levels. The results of the present study suggested that only the expression levels of FAM172A and NUBP1 and tumor staging may be independent prognostic makers for OS and RFS of patients with CRC (Table IV). Patients with positive FAM172A expression had a 2.726-fold [95% confidence interval (95% CI), 1.121–6.630] increase in the mortality risk and a 2.478-fold (95% CI, 1.027–5.982) increase in the risk of disease. Patients with positive expression of NUBP1 had a 3.029-fold (95% CI, 1.286–7.130) higher risk of mortality and a 3.101-fold (95% CI, 1.318–7.296) higher risk for recurrence of disease. Kaplan-Meier survival curves were constructed to estimate the OS of patients and further analyze the impact of FAM172A and NUBP1 expression using the log-rank test (Fig. 2). Higher expression levels of FAM172A and NUBP1 were associated with a significantly shorter survival and higher relapse rate in colorectal carcinoma patients (P<0.001 and P<0.001, respectively).
Table IV.
Multivariate Cox regression analysis for RFS and OS.
| OS | RFS | |||
|---|---|---|---|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value |
| FAM172A | ||||
| Negative | 1.000 | 1.000 | ||
| Positive | 2.726 (1.121–6.630) | 0.027 | 2.478 (1.027–5.982) | 0.03 |
| NUBP1 | ||||
| Negative | 1.000 | 1.000 | ||
| Positive | 3.029 (1.286–7.130) | 0.011 | 3.101 (1.318–7.296) | 0.01 |
| TNM stage | ||||
| I and II | 1.000 | 1.000 | ||
| III and IV | 4.472 (2.403–8.323) | <0.001 | 4.083 (2.216–7.523) | <0.001 |
RFS, relapse-free survival; OS, overall survival; TNM, Tumor-Node-Metastasis; FAM172A, the family with sequence similarity 172, member A; NUBP1, nucleotide-binding protein 1; CI, confidence interval; HR, hazard ratio.
Figure 2.
Association between overall survival and expression levels of FAM172A and NUBP1. (A) Association between FAM172A-negative and FAM172A-positive expression and overall survival in patients with CRC. (B) Association between NUBP1-negative and NUBP1-positive expression and overall survival in patients with CRC. NUBP1, nucleotide-binding protein 1; FAM172A, family with sequence similarity 172 member A; CRC, colorectal cancer.
Discussion
The development of novel tumor markers may contribute to improving the early diagnosis of CRC and the 5-year patient survival rate. Firstly, the present study performed immunohistochemical staining to investigate the expression levels of FAM172A and NUBP1 in human CRC tissues. Secondly, analysis of the association between the expression levels of FAM172A and NUBP1 and the corresponding prognostic significance was performed. To the best of our knowledge, the present study demonstrated the following for the first time: i) A high proportion of CRC tissue samples expressed FAM172A and NUBP1; ii) expression levels of FAM172A and NUBP1 were associated with unfavorable clinicopathological variables, including high serum level of CEA, high clinical stage (stage III and IV) and the existence of LN metastasis; and iii) higher expression levels of FAM172A and NUBP1 were associated with a significantly shorter survival time and higher relapse rate in patients with CRC. Additionally, NUBP1 and FAM172A were revealed to be independent prognostic factors of CRC by multivariate analysis. Taken together, the results of the present study demonstrated that FAM172A and NUBP1 may participate in the progression of CRC and be independent indicators of poor prognoses for patients with CRC.
Numerous previous studies have revealed that NUBP1 was involved in regulating centrosome duplication in mammalian cells (14,18). Other previous studies have suggested that NUBP1 protein may be involved in chaperonin-containing TCP-1/TCP-1 ring complex chaperone activity in ciliogenesis (12,19) and that the C-terminal region of NUBP1 may be crucial for nuclear transfer (18). NUBP1 was primarily believed to be a nuclear protein (20–22); however, little is known about the association between NUBP1 and CRC.
Cytoplasmic localization of FAM172A was observed by the present study. Our previous study demonstrated that complete cell cycle arrest was exhibited in the S phase of HepG2 cells at a high concentration when HepG2 cells were co-cultured with the FAM172A recombinant protein (9). Evaluation of cytoplasmic FAM172A expression levels revealed that 144/180 cases in the present study exhibited cytoplasmic FAM172A expression. However, the association between CRC and the expression of FAM172A remains unknown.
In the present study, FAM172A and NUBP1 were demonstrated to be expressed in normal colorectal mucosa and CRC tissues, and CRC tissues exhibited higher expression levels. Thus, the results revealed that FAM172A and NUBP1 genes may act as important and novel cancer-associated genes, and the deficiency or variation of these two genes may result in the development of CRC.
However, when considering the anti-oncogenic role of FAM172A in hepatocellular carcinoma development and progression in our previous study (9), FAM172A may also serve an anti-oncogenic role in CRC. Therefore, it has been suggested that loss of FAM172A may result in the inhibition of cell death and promote tumorigenesis. In addition, the present study indicated that there was a positive association between the expression levels of FAM172A and NUBP1, and that the FAM172A and NUBP1 expression levels were significantly associated with advanced clinicopathological factors and a poor prognosis for patients with CRC.
Although our previous study demonstrated that FAM172A was essential to the cell cycle regulation and proliferation of HepG2 cells (9), the reason the high expression level of FAM172A is indicated as a tumor suppressor and is associated with advanced cancer characteristics remains unknown. This may be due to an accumulation of immunohistochemically detectable mutant FAM172A or due to downstream functional defects, despite the presence of normal FAM172A protein expression. The results of the present study indicated that FAM172A expression was significantly associated with NUBP1 expression level, which supports the contention that NUBP1 is upregulated to modulate FAM172A activity. Therefore, the present study assumed that the expression levels of FAM172A and NUBP1 serve important roles. They may make a difference in regulating cell survival in CRC, and the poor prognosis of patients with CRC may result from decreased or low expression levels of FAM172A. However, studies investigating the exact role of FAM172A and NUBP1 have previously been limited. Therefore, further analysis of NUBP1 and FAM172A expression levels in CRC is required in order to identify their mechanism of action and to determine the role of NUBP1 and FAM172A in carcinogenesis.
In conclusion, a high proportion of CRC tissues demonstrated expression of FAM172A and NUBP1, and the expression was associated with unfavorable CRC characteristics and a poor outcome for patients with CRC. Although the role of FAM172A and NUBP1 in tumorigenesis remains unclear, the results of the present study suggested that their expression levels may be a diagnostic and prognostic index with prospective clinical function for patients with CRC.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81172383), the Guangdong Natural Science Foundation (grant no. 2014A030313324) and the Key Clinical Specialty Discipline Construction Program.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12:89–96. doi: 10.1006/scbi.2001.0416. [DOI] [PubMed] [Google Scholar]
- 4.Lin L, Piao J, Gao W, Piao Y, Jin G, Ma Y, Li J, Lin Z. DEK over expression as an independent biomarker for poor prognosis in colorectal cancer. BMC Cancer. 2013;13:366. doi: 10.1186/1471-2407-13-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vodicka J, Spidlen V, Treska V, Fichtl J, Simanek V, Safranek J, Vejvodova S, Mukensnabl P, Topolcan O. Surgical treatment of colorectal cancer pulmonary metastases: 12-year results. Anticancer Res. 2014;34:4239–4245. [PubMed] [Google Scholar]
- 6.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 7.Cortéa H, Manceau G, Blons H, Laurent-Puiga P. MicroRNA and colorectal cancer. Dig Liver Dis. 2012;44:195–200. doi: 10.1016/j.dld.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Li LX, Tao Z, Dong XH, Liang WC, Yang ZH, Mou B, Bao YQ, Wang C, Jia WP, Hu RM. Molecular cloning of a novel gene, C5orf21 gene and its roles in diabetic macroangiopathy. Zhonghua Yi Xue Za Zhi. 2009;89:2574–2577. (In Chinese) [PubMed] [Google Scholar]
- 9.Feng Z, Li H, Liu S, Cheng J, Xiang G, Zhang J. FAM172A induces S phase arrest of HepG2 cells via Notch 3. Oncol Rep. 2013;29:1154–1160. doi: 10.3892/or.2013.2235. [DOI] [PubMed] [Google Scholar]
- 10.Yu CS, Chen YC, Lu CH, Hwang JK. Prediction of protein subcellular localization. Proteins. 2006;64:643–651. doi: 10.1002/prot.21018. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima H, Grahovac MJ, Mazzarella R, Fujiwara H, Kitchen JR, Threat TA, Ko MS. Two novel mouse genes-Nubp2, mapped to the t-complex on chromosome 17, and Nubp1, mapped to chromosome 16-establish a new gene family of nucleotide-binding proteins in eukaryotes. Genomics. 1999;60:152–160. doi: 10.1006/geno.1999.5898. [DOI] [PubMed] [Google Scholar]
- 12.Kypri E, Christodoulou A, Maimaris G, Lethan M, Markaki M, Lysandrou C, Lederer CW, Tavernarakis N, Geimer S, Pedersen LB, Santama N. The nucleotide-binding proteins Nubp1 and Nubp2 are negative regulators of ciliogenesis. Cell Mol Life Sci. 2014;71:517–538. doi: 10.1007/s00018-013-1401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kupke T, Di Cecco L, Müller HM, Neuner A, Adolf F, Wieland F, Nickel W, Schiebel E. Targeting of Nbp1 to the inner nuclear membrane is essential for spindle pole body duplication. EMBO J. 2011;30:3337–3352. doi: 10.1038/emboj.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christodoulou A, Lederer CW, Surrey T, Vernos I, Santama N. Motor protein KIFC5A interacts with Nubp1 and Nubp2, and is implicated in the regulation of centrosome duplication. J Cell Sci. 2006;119:2035–2047. doi: 10.1242/jcs.02922. [DOI] [PubMed] [Google Scholar]
- 15.Stehling O, Netz DJ, Niggemeyer B, Rösser R, Eisenstein RS, Puccio H, Pierik AJ, Lill R. Human Nbp35 is essential for both cytosolic iron-sulfur protein assembly and iron homeostasis. Mol Cell Biol. 2008;28:5517–5528. doi: 10.1128/MCB.00545-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton SR, Aaltonen LA, editors. Pathology and Genetics of Tumours of the Digestive System. IARC Press; Lyon: 2000. World Health Organization classification of tumours. [Google Scholar]
- 17.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 18.Ververis A, Christodoulou A, Christoforou M, Kamilari C, Lederer CW, Santama N. A novel family of katanin-like 2 protein isoforms (KATNAL2), interacting with nucleotide-binding proteins Nubp1 and Nubp2, are key regulators of different MT-based processes in mammalian cells. Cell Mol Life Sci. 2016;73:163–184. doi: 10.1007/s00018-015-1980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kupke T, Di Cecco L, Müller HM, Neuner A, Adolf F, Wieland F, Nickel W, Schiebel E. Targeting of Nbp1 to the inner nuclear membrane is essential for spindle pole body duplication. EMBO J. 2011;30:3337–3352. doi: 10.1038/emboj.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuno T, Yamabayashi H, Kogure K. Comparison of intracellular localization of Nubp1 and Nubp2 using GFP fusion proteins. Mol Biol Rep. 2010;37:1165–1168. doi: 10.1007/s11033-009-9477-7. [DOI] [PubMed] [Google Scholar]
- 21.Araki Y, Lau CK, Maekawa H, Jaspersen SL, Giddings TH, Jr, Schiebel E, Winey M. The Saccharomyces cerevisiae spindle pole body (SPB) component Nbp1p is required for SPB membrane insertion and interacts with the integral membrane proteins Ndc1p and Mps2p. Mol Biol Cell. 2006;17:1959–1970. doi: 10.1091/mbc.E05-07-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu Y, Akashi T, Okuda A, Kikuchi A, Fukui K. NBP1 (Nap1 binding protein 1), an essential gene for G2/M transition of Saccharomyces cerevisiae, encodes a protein of distinct sub-nuclear localization. Gene. 2000;246:395–404. doi: 10.1016/S0378-1119(00)00067-6. [DOI] [PubMed] [Google Scholar]