Abstract
The aim of the present study was to investigate the effects of epigallocatechin-3-gallate (EGCG) on the apoptosis of the human MCF-7 cancer cell line and the underlying mechanism. MCF-7 cells were divided into the control group and EGCG groups. The proliferation of MCF-7 cells in the two groups was determined using MTT and apoptosis was examined using flow cytometry. Western blot analysis and qRT-PCR were used to analyze P53 and Bcl-2 expression levels. The silencing effect of specific siRNA was evaluated using RT-PCR and western blot analysis. P53 and Bcl-2 expression levels were determined using western blot analysis in the si-P53, EGCG and EGCG-combined si-P53 groups. EGCG inhibited the proliferation of MCF-7 cells in a concentration-dependent manner and IC50 was 37.681 mol/l. The apoptotic rates were 1.37 and 5.83% (t=8.9, p=0.0124) in the blank control and treatment groups after treatment with 30 µmol/l EGCG. The RT-qPCR and western blot results demonstrated that the effect of siRNA interference was evident. The expression of P53 in the EGCG-combined si-P53 group was higher than that of the si-P53 group, but lower than the EGCG group. The Bcl-2 expression level in the EGCG-combined si-P53 group was lower than that of the si-P53 group and higher than that of the EGCG group. In conclusion, EGCG suppressed the proliferation of human MCF-7 breast cancer cells and promoted apoptosis. In addition, the underlying mechanism may be related to the P53/Bcl-2 signaling pathway.
Keywords: EGCG, MCF-7, apoptosis, P53, Bcl-2
Introduction
Green tea is one of the main tea species in China. It contains tea polyphenols, catechins, chlorophyll, caffeine, amino acids, vitamins and other active ingredients (1). Epigallocatechin-3-gallate (EGCG) content is very high in green tea (1). EGCG has anti-aging, sterilization, anti-inflammatory and anticancer properties, and it also can inhibit the growth of a variety of malignant cells and induce apoptosis (2–4). It can also inhibit the angiogenesis, invasion and metastasis of malignant tumor cells, in various types of cancer (5).
Breast cancer is one of the common malignancies in women. It is crucial to identify new effective drugs with minimal side effects to treat breast cancer. Liang et al (6) identified that EGCG inhibited the proliferation of breast cancer cells by repressing cyclin-dependent protein activity and also inhibited the growth and metastasis of breast cancer cells by downregulating the expression of VEGF and MMP-9 (7–9). However, the mechanism by which EGCG promoted breast cancer apoptosis has rarely been studied.
The present study provides a theoretical reference for the development of breast cancer target by investigating the mechanism of apoptosis of EGCG on breast cancer.
Materials and methods
Materials
EGCG and thiazolyl blue were purchased from Sigma (St. Louis, MO, USA). RPMI-1640 basal medium and fetal bovine serum (FBS) was produced by HyClone (Logan, UT, USA). RPMI-1640 basal medium and EGCG were used to prepare a 10 mmol/l storage solution. It was sterilized by filtration (0.22 µm) and stored at −80°C in the dark. MTT was dissolved in phosphate-buffered saline (PBS) solution at 5 mg/ml, and was stored at −20°C in the dark after sterilization. Mouse anti-human P53, Bcl-2 and GAPDH were all purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and the RT-PCR kit was purchased from Takara (Otsu, Shiga, Japan). P53, Bcl-2 and GAPDH primers were produced by Invitrogen (Carlsbad, CA, USA) (Table I) and si-P53 was supplied by Gemma (Shanghai, China).
Table I.
Primers of P53, Bcl-2 and GAPDH used in RT-qPCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| P53 | 5′-TTCCCTGGATTGGCCAGACT-3′ | 5′-ACCATCGCTATCTGAGCAGC-3′ |
| Bcl-2 | 5′-CGACTTCGCCGAGATGCCAGCCAG-3′ | 5′-ACTTGTGGCCCAGATAGGCACCCAG-3′ |
| GAPDH | 5′-AGAAGGCTGGGGCTCATTTG-3′ | 5′-AGGGGCCATCCACAGTCTTC-3′ |
Cell line
MCF-7 cells were purchased from the Shanghai ATCC cell bank. The cells were cultured in RPMI-1640 medium containing 10% FBS (with 100 U/ml green-streptomycin) and incubated under 5% CO2 at 37°C.
Methods
MTT detection of the sensitivity of MCF-7 cells to EGCG
The EGCG concentration gradient was 0, 2, 4, 8, 16, 32, 64 and 128 µmol/l. MCF-7 cells (2×103) were seeded in a 96-well plate with blank control wells. After cells adhered to the walls, EGCG of the concentration gradient of 0, 2, 4, 8, 16, 32, 64 and 128 µmol/l was added. After incubation for 72 h, the supernatant was discarded and 20 µl of MTT was added to each well. After incubation for 4 h, the liquid was discarded and 150 µl of dimethyl sulfoxide (DMSO) was added to each well of the EGCG. The optical density (OD) of each well at 490 nm was measured by a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The inhibition rate was calculated as: (control group OD - experimental group OD/control group OD) × 100%.
Effect of EGCG on MCF-7 cell apoptosis measured by flow cytometry (FCM)
MCF-7 cells were seeded in a 6-well cell culture plate. After adherence, 30 µmol/l EGCG was added and the cells were stained after 48 h with Annexin V-FITC kit. The change in apoptotic rate was measured, and the experiment was repeated three times.
RT-qPCR and western blot analysis determine how EGCG treatment on MCF-7 affects the mRNA and protein levels of P53 and Bcl-2
The experiment was conducted on the control and EGCG groups. EGCG was added to fresh MCF-7 cells in the experimental group after cell adhesion (EGCG at a final concentration of 30 µmol/l), but the control group was not treated. After 72 h of incubation, total RNA was extracted using TRIzol reagent. The RT-qPCR assay was performed using the Takara kit, and the GAPDH gene was used as an internal control. The conditions for RT-qPCR were: Preheating at 95°C for 2 min, denaturation at 95°C for 30 sec, annealing at 58°C for 30 sec and extension at 72°C for 40 sec. The experiment was repeated three times. The results were expressed as 2−ΔΔCq. MCF-7 cells were treated with 30 µmol/l EGCG for 48 h. The cells were then rinsed three times with PBS and 100 µl of RIPA lysate was added. The cells were placed on ice for 30 min and centrifuged at 12,000 × g for 20 min at 4°C. The supernatant was collected and stored at −80°C. Protein concentration was determined using the BCA protein method. The protein extract was subjected to SDS-PAGE (10%) and transferred to a PVDF membrane. The membrane was blocked with 3% BSA solution for 2 h. Then, primary rabbit monoclonal P53 antibody (dilution, 1:500; cat. no. ab32049), rabbit polyclonal Bcl-2 antibody (dilution, 1:500; cat. no. ab59348) and rabbit polyclonal GAPDH antiboody (dilution, 1:500; cat. no. ab37168), purchased from Abcam (Cambridge, MA, USA), were added at 1:1,000 and incubated at 4°C overnight. The membrane was washed with PBS three times (5 min each time). Then secondary goat anti-rabbit (HRP) IgG antibody (dilution, 1:2,000; cat. no. ab6721) was added followed by 2-h incubation and washing with PBS three times (5 min each time). Finally, it was placed on the ECL for exposure and development.
RT-qPCR and western blot analysis of si-P53 interference effect
The cells were divided into the si-P53, si-NC and NC groups (si-P53 was added with small fragments of interference P53, with si-NC as a positive control and NC as a negative control). Cells in the logarithmic growth phase were inoculated into the culture flask and transiently transfected according to the protocol of the Lipofectamine™ 2000 kit. After 48 h, the RNA was extracted and purified according to the kit protocol, and RNA purity and concentration was determined by a spectrophotometer (Hitachi, Tokyo, Japan). The P53 mRNA expression was evaluated using RT-qPCR, and the P53 protein expression level was measured using western blot analysis. The three groups were compared and the transfection efficiency was calculated.
Alterations in the expression of P53 and Bcl-2 proteins in MCF-7 cells transfected with si-P53 detected by western blot analysis
The cells were divided into the si-P53, EGCG and EGCG + si-P53, (si-P53 plus interference P53 small fragments, si-P53 for the positive control, NC for the negative control) groups. The P53 and Bcl-2 protein expression levels in the three groups were evaluated using western blot analysis using the same method explained before.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA) and SPSS 20.0 (Chicago, IL, USA) statistical software. The measurement data were presented as mean ± standard deviation. Comparison between two groups was conducted using a t-test. The one-way analysis of variance (ANOVA) test was used for comparison among three groups. P<0.05 was considered to indicate a statistically significant difference.
Results
MTT detection of the sensitivity of MCF-7 cells to EGCG
The sensitivity of EGCG to MCF-7 was evaluated using MTT assay. As shown in Fig. 1, 10 µmol/l EGCG significantly inhibited the proliferation of MCF-7 cells and the inhibition was enhanced with the increase of drug concentration. The IC50 was 37.684 µmol/l. We selected 30 µmol/l as the follow-up dose (Table II).
Figure 1.

Different concentrations of EGCG of the inhibitory rate of MCF-7 cells using MTT. EGCG, epigallocatechin-3-gallate.
Table II.
Different concentrations of EGCG and the inhibitory rate of MCF-7 cells using MTT.
| Concentration | OD (mean ± SD) | Inhibition rate (%) |
|---|---|---|
| 0 | 1.187±0.049 | 0 |
| 10 | 0.979±0.035 | 17.52 |
| 20 | 0.726±0.021 | 38.85 |
| 40 | 0.589±0.027 | 50.39 |
| 60 | 0.427±0.012 | 64 |
| 80 | 0.309±0.017 | 73.92 |
| 100 | 0.219±0.016 | 81.53 |
| 120 | 0.116±0.011 | 90.23 |
EGCG, epigallocatechin-3-gallate.
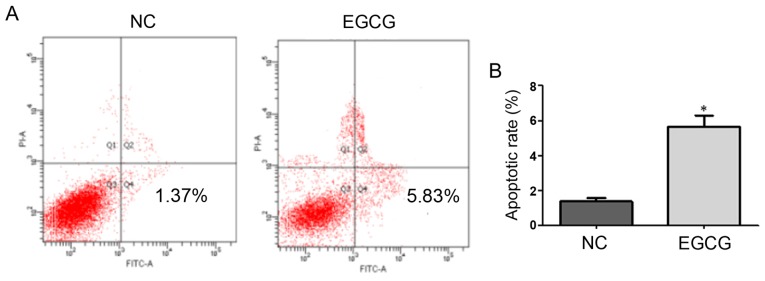
Effect of EGCG on MCF-7 cell apoptosis measured using FCM
As shown in Fig. 2, the apoptotic rates of the EGCG and control groups were 1.37 and 5.83%, respectively, with a significant difference (t=8.9, p=0.0124). These results suggested that EGCG promoted apoptosis in MCF-7 cells (Table III).
Figure 2.
(A) The effect of 30 µmol/l EGCG on the apoptosis rate of MCF-7 after 48 h was detected by flow cytometer. (B) Statistics of apoptosis rate. *P<0.05, compared to the NC group. EGCG, epigallocatechin-3-gallate; NC, negative control.
Table III.
The effect of 30 µmol/l EGCG on the apoptotic rate of MCF-7 after 48 h was detected using a flow cytometer.
| Group | Apoptosis rate (%) (mean ± SD) | t | P-value |
|---|---|---|---|
| NC | 1.38±0.19 | ||
| EGCG | 5.65±0.64 | 8.9 | 0.0124 |
EGCG, epigallocatechin-3-gallate; NC, negative control.
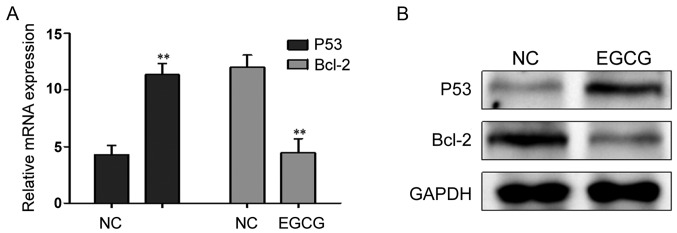
Effects of EGCG treatment on the expression of P53 and Bcl-2
After treatment with 30 µmol/l EGCG, P53 mRNA and protein expression levels were significantly higher, while the Bcl-2 mRNA and protein expression levels decreased significantly (Fig. 3; Table IV).
Figure 3.
(A) The effect of 30 µmol/l EGCG on the mRNA expression of P53 and Bcl-2 in MCF-7 cells. **P<0.001 compared to the NC group. (B) The effect of 30 µmol/l EGCG on the protein expression of P53 and Bcl-2 in MCF-7 cells. EGCG, epigallocatechin-3-gallate; NC, negative control.
Table IV.
The effect of 30 µmol/l EGCG on the Cq and F value of P53 and Bcl-2 in MCF-7 cells.
| 2−ΔΔCq (mean ± SD) | ||||
|---|---|---|---|---|
| Gene | NC | EGCG | t | P-value |
| P53 | 4.289±0.831 | 11.352±0.992 | 9.451 | <0.001 |
| Bcl-2 | 12.013±1.082 | 4.465±1.228 | 7.985 | <0.001 |
EGCG, epigallocatechin-3-gallate; NC, negative control.
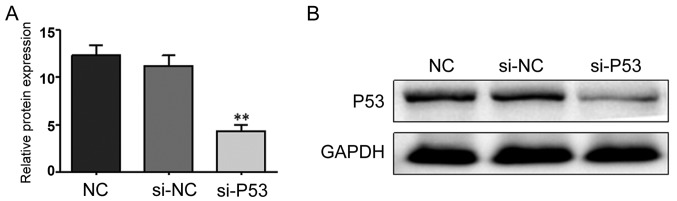
si-P53 interference effect
The RNA interference technique was used to silence P53 expression. The interference effect was studied using RT-qPCR and western blot analysis. As shown in Fig. 4, the interference effect was significant and could be further tested.
Figure 4.
(A) The Cq value of P53 in the MCF-7 cells after transfection with si-P53 was measured using RT-qPCR. (B) The P53 protein expression in the MCF-7 cells after transfection with si-P53 was detected by western blot analysis. **P<0.001 compared to the NC group. NC, negative control.
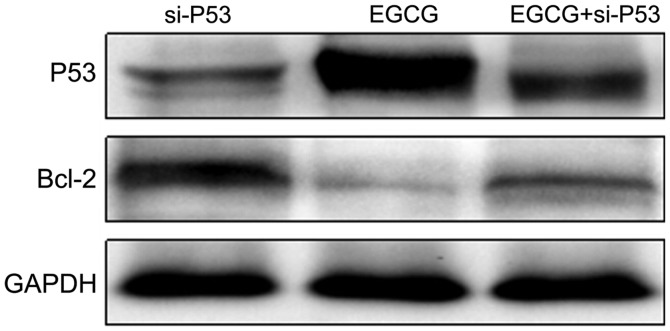
Changes in expression levels of P53 and Bcl-2 proteins in MCF-7 cells transfected with si-P53
P53 and Bcl-2 expression levels in si-P53-transfected MCF-7 cells treated with EGCG were detected using western blot analysis. As shown in Fig. 5, the expression of P53 in the EGCG + si-P53 group was higher than that in the si-P53 group but lower than that in the EGCG group. The expression of Bcl-2 in the EGCG + si-P53 group was lower than that in the si-P53 group but higher than that in the EGCG group. The results suggested that EGCG promoted the expression of P53 (Table V).
Figure 5.
Protein expression of P53 and Bcl-2 was examined using western blot analysis on MCF-7 cells treated with EGCG and transfected with si-P53. EGCG, epigallocatechin-3-gallate.
Table V.
The Cq value of P53 was detected by RT-qPCR after transfection with si-P53.
| Group | 2−ΔΔCq (mean ± SD) | F | P-value |
|---|---|---|---|
| NC | 12.332±1.063 | ||
| si-NC | 11.219±1.097 | ||
| si-P53 | 4.329±0.658 | 61.450 | <0.001 |
NC, negative control.
Discussion
P53 is an important tumor suppressor gene. It is involved in tumor occurrence and development through DNA repair, cell cycle regulation, angiogenesis inhibition and cell apoptosis (10). The P53 gene has been found to be mutated in more than 50% of malignant tumors (11). Mutations and deletions of the P53 gene have been reported to be closely related to breast cancer (12–15). Apoptosis is a spontaneous cell death process regulated by a series of genes. The proto-oncogene Bcl-2 is a member of the Bcl-2 family. The oncoproteins located in the nuclear membrane, part of the endoplasmic reticulum and mitochondrial outer membrane, can negatively regulate cell apoptosis and prolong cell life (16). Previous findings have shown that P53 can inhibit the expression of Bcl-2 protein (17,18).
EGCG is the most active substance in green tea, which has great developmental value, and its anticancer effect has been widely studied. Previous results have shown that EGCG can promote apoptosis in a variety of malignant tumor cells, including pancreatic and gastric cancer cells (18,19). In the present study, we showed that the apoptotic rate in MCF-7 cells treated with EGCG was significantly higher than that of the control group. In order to explore the mechanism of apoptosis, P53 and Bcl-2 expression levels were determined. The RT-qPCR and western blot results showed that EGCG induced the expression of P53 but inhibited the expression of Bcl-2. The EGCG + si-P53 group had a higher P53 expression level compared with the si-P53 group. The P53 expression level was lower than that of the EGCG group, while the Bcl-2 expression was lower than that in the si-P53 group but higher than that in the EGCG group. These results suggest that EGCG inhibited the expression of Bcl-2 and promoted the expression of P53.
In conclusion, the findings show that EGCG inhibited the proliferation of MCF-7 cells and promoted apoptosis in these cells. The underlying mechanism involved may be related to the P53/Bcl-2 signaling pathway, which can provide a theoretical basis for EGCG to be used in the new target drug development for breast cancer.
References
- 1.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 2.Tan XH, Zhou DY, Zhang YL. Advances in research on anticancer mechanism of tea. Foreign Medical Oncology. 1998;25:4. [Google Scholar]
- 3.Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–616. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89:1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 5.Zhang G, Wang Y, Zhang Y, Wan X, Li J, Liu K, Wang F, Liu K, Liu Q, Yang C, et al. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Curr Mol Med. 2012;12:163–176. doi: 10.2174/156652412798889063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang YC, Lin-Shiau SY, Chen CF, Lin JK. Inhibition of cyclin-dependent kinases 2 and 4 activities as well as induction of Cdk inhibitors p21 and p27 during growth arrest of human breast carcinoma cells by (−)-epigallocatechin-3-gallate. J Cell Biochem. 1999;75:1–12. doi: 10.1002/(SICI)1097-4644(19991001)75:1<1::AID-JCB1>3.3.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132:2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- 8.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 9.Farabegoli F, Papi A, Orlandi M. (−)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9 and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant cells. Biosci Rep. 2011;31:99–108. doi: 10.1042/BSR20090143. [DOI] [PubMed] [Google Scholar]
- 10.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 11.Pakos EE, Kyzas PA, Ioannidis JP. Prognostic significance for TP53 tumor suppressor gene expression and mutations in human motations in human osteosarcoma: A meta-analysis. Clin Cancer Res. 2004;10:6208–6214. doi: 10.1158/1078-0432.CCR-04-0246. [DOI] [PubMed] [Google Scholar]
- 12.Barnes DM, Dublin EA, Fisher CJ, Levison DA, Millis RR. Immunohistochemical detection of p53 protein in mammary carcinoma: An important new independent indicator of prognosis? Hum Pathol. 1993;24:469–476. doi: 10.1016/0046-8177(93)90158-D. [DOI] [PubMed] [Google Scholar]
- 13.Dastjerdi MN, Rarani MZ, Valiani A, Mahmoudieh M. The effect of adenosine A1 receptor agonist and antagonist on p53 and caspase 3, 8, and 9 expression and apoptosis rate in MCF-7 breast cancer cell line. Res Pharm Sci. 2016;11:303–310. doi: 10.4103/1735-5362.189301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Synnott NC, Murray A, McGowan PM, Kiely M, Kiely PA, O'Donovan N, O'Connor DP, Gallagher WM, Crown J, Duffy MJ. Mutant p53: A novel target for the treatment of patients with triple-negative breast cancer? Int J Cancer. 2017;140:234–246. doi: 10.1002/ijc.30425. [DOI] [PubMed] [Google Scholar]
- 15.Darb-Esfahani S, Denkert C, Stenzinger A, Salat C, Sinn B, Schem C, Endris V, Klare P, Schmitt W, Blohmer JU, et al. Role of TP53 mutations in triple negative and HER2-positive breast cancer treated with neoadjuvant anthracycline/taxane-based chemotherapy. Oncotarget. 2016;7:67686–67698. doi: 10.18632/oncotarget.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schinzel A, Kaufmann T, Borner C. Bcl-2 family members: Integrators of survival and death signals in physiology and pathology. Biochim Biophys Acta. 2004;1644:95–105. doi: 10.1016/j.bbamcr.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/S1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhu BH, Zhan WH, Li ZR, Wang Z, He YL, Peng JS, Cai SR, Ma JP, Zhang CH. (−)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J Gastroenterol. 2007;13:1162–1169. doi: 10.3748/wjg.v13.i8.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qanungo S, Das M, Haldar S, Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. 2005;26:958–967. doi: 10.1093/carcin/bgi040. [DOI] [PubMed] [Google Scholar]