Abstract
Pancreatic cancer is characterized by increased hyperplasia of fibrotic tissue, termed desmoplasia, and lymph node metastasis is an independent prognostic factor in this disease. However, there are no reports focused on desmoplasia in pancreatic cancer lymph node metastases. The present study evaluated a range of factors and investigated their association with poor prognosis in pancreatic cancer cases with lymph node metastasis, including the degree of desmoplasia in lesions. To identify the poor prognostic factors associated with lymph node metastasis, the present study retrospectively reviewed the clinical data of 65 patients with lymph node metastases that underwent surgical pancreatic cancer resection between 2007 and 2012 at a single institution. The investigation focused on the degree of fibrosis in metastatic lesions in 216 lymph nodes, and investigated associations with prognosis or clinicopathological findings. The ratios of the fibrotic area in metastatic lymph node lesions were evaluated and classified into three categories, high (≥70%), moderate (10–70%) and low (<10%). Desmoplasia was not observed in cancer-free lymph nodes. The size of metastatic lymph node lesions was additionally measured, and a significant association between metastatic lesion size and the degree of desmoplasia was observed (P<0.001). The degree of desmoplasia was additionally associated with local extranodal invasion. In the analysis of 65 pancreatic cancer patients with metastatic lymph nodes, the presence of multiple metastatic lymph nodes with moderate or high desmoplasia was significantly associated with poor survival (high, P=0.0048; moderate/high, P=0.0075). Of several clinicopathological factors, the presence of multiple metastatic lymph nodes with high or moderate desmoplasia was associated with overall survival in univariate (P=0.0098) and multivariate (P=0.0466) analyses. The degree of desmoplasia in metastatic lymph nodes is associated with lesion size, and the presence of multiple metastatic lymph nodes with desmoplasia is an independent poor prognostic factor, suggesting that the desmoplasia may have an important role in the malignant progression of lymph node metastases.
Keywords: pancreatic cancer, lymph node metastasis, desmoplasia, locally extranodal invasion, prognostic factor
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths, with a 5-year survival rate less than 7% (1). The only curative treatment for this malignancy is complete resection, which is possible in only 10–20% of patients (1–3). Pancreatic cancer rapidly metastasizes to the liver, lungs, lymph nodes and peritoneum, and the majority of patients eventually succumb with widespread metastatic lesions. Metastasis to regional lymph nodes is one of the key indicators of aggressive tumors. Lymph node status is a powerful predictor of patient survival and one of the key parameters used for staging tumors (4). These data suggest that there are significant prognostic implications of lymph node metastasis in pancreatic cancer.
Pancreatic cancer is characterized by abundant desmoplastic stroma with activated pancreatic stellate cells and an excessive accumulation of extracellular matrix (5). Interactions between cancer cells and various stromal cells promote malignancy and inhibit drug penetration into tumors and drug uptake by cancer cells (6). In previous studies, it has been reported that for resectable pancreatic cancer, high α-smooth muscle actin (αSMA) expression in the primary tumor was a poor prognostic factor, while high stroma density was a favorable prognostic factor (7–9). Furthermore, a recent report showed that distant pancreatic cancer metastases have highly fibrotic stroma, similar to the primary tumors, and there was no significant difference in the degree of desmoplasia between primary tumors and metastatic lesions (10). In papillary thyroid tumors, lymph node metastasis with desmoplasia was correlated with reduced disease-free survival times (11). However, there are no reports investigating desmoplasia in metastatic lymph nodes in pancreatic cancer patients. Therefore, the precise contribution of desmoplasia to the malignant progression of lymph node metastases in pancreatic cancer remains unclear.
In this study, we evaluated the degree of desmoplasia in metastatic lymph node lesions from pancreatic cancer patients using hematoxylin and eosin (H&E) and immunohistochemical staining for cytokeratin 19 (CK19) and αSMA. In addition, we measured the size of lymph node metastases and investigated potential correlations between the degree of desmoplasia and the size of lymph node metastases as well as evaluating its clinical significance based on clinicopathological findings and prognoses.
Materials and methods
Case selection
We investigated 216 metastatic lymph nodes and 10 lymph nodes without cancer cells dissected from 65 patients with invasive pancreatic ductal carcinomas, which were macroscopically curatively resected at our institution between August 2007 and February 2012. Pancreatectomy was performed in three broad categories, standard pancreaticoduodenectomy, distal pancreatectomy and total pancreatectomy. The lymph node dissection technique at our institution has been standardized to D2 lymph node dissection, according to the general rules for the study of pancreatic cancer by Japan Pancreas Society (12,13). Unusual differentiation variants of ductal carcinoma, such as intraductal mucinous neoplasms with invasive carcinoma, were excluded from the study.
Clinical and pathologic data were retrospectively obtained from surgical and pathologic reports. The clinical data included age, sex, neoadjuvant chemotherapy and postoperative chemotherapy; the pathologic data included T, N and R status according to the 7th AJCC/UICC TNM classification system, histologic grade and invasion factors (vascular, lymphatic, perineural, or extranodal) (14). Overall survival was measured as the time from pancreatic resection to death. Survival analyses were performed in August 2016. Overall survival was analyzed based on the number of metastatic lymph nodes and the degree of desmoplasia in the metastatic lesions.
The study protocol was approved by the Ethics Committee of Kyushu University, Fukuoka, Japan (approval nos. 24–222 and 25–117) and conformed to the Ethical Guidelines for Human Genome/Gene Research enacted by the Japanese Government and the Declaration of Helsinki. All patients provided signed informed consent, approving the use of their personal health information for unspecified research purposes.
Immunohistochemical analysis
Metastatic lymph node tissues from primary pancreatic cancer patients were evaluated by H&E, and CK19 and αSMA immunohistochemistry. Tissues were sliced 4-µm thick and incubated with mouse anti-CK19 antibody (1:500; #sc-376126; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or mouse anti-αSMA antibody (1:500; #M0851; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) overnight at 4°C. Staining was performed on serial sections. The percentage of fibrous components in the metastatic lymph node lesions were classified into three categories, high (≥70%), moderate (10–70%), and low (<10%). The optimal cut off value in degree of desmoplasia was determined according to our previous report (7). The sizes of the lesions were also classified into three categories, 0–999, 1,000–1,999, and ≥2,000 µm. When the several status of the degree of desmoplasia in lymph nodes was observed in one patient, the status of the degree of desmoplasia was defined based on the highest degree of desmoplasia in all lymph nodes from such patient. Images were acquired using a confocal laser-scanning microscope (BZ-X700; Keyence, Osaka, Japan).
Statistical analysis
Results are presented as means ± SD. Patient characteristics were analyzed using the χ2 and Student's t-tests. Statistical significance was defined as P<0.05. Survival analyses were conducted using the Kaplan-Meier method, and the curves were compared using the log-rank test. All statistical analyses were performed using JMP 12 software (SAS Institute, Cary, NC, USA).
Results
We investigated 226 lymph nodes from 65 patients with invasive pancreatic ductal carcinomas; 216 had metastatic involvement and 10 were cancer-free. The mean age of the patients was 66 years (range, 36–85 years), and 35% were females (n=23). The median tumor size was 37 mm (range, 13–80 mm). The median number of examined lymph nodes was 32 (range, 7–75), and the median number of metastatic lymph nodes was 5 (range, 1–25). At the last follow-up assessment, 56 patients (86%) had died and 9 (14%) were alive; median survival was 22 months. No patients underwent neoadjuvant chemotherapy prior to resection, but postoperative chemotherapy was given to 58 patients (89%). The clinicopathological characteristics of the study cohort are summarized in Table I.
Table I.
Clinicopathological features of the institutional cohort.
| Characteristic | Institutional cohort n=65 (%) |
|---|---|
| Sex | |
| Female | 23 (35) |
| Male | 42 (65) |
| Age | |
| Years | 66.1 (36–85) |
| Tumor size (median, mm) | 37 (13–80) |
| T stage | |
| T1 | 1 (1.5) |
| T2 | 2 (3.1) |
| T3 | 59 (90.8) |
| T4 | 3 (4.6) |
| Median number of LNs examined (range) | 32 (7–75) |
| Median number of LNs with metastasis (range) | 5 (1–25) |
| Outcome | |
| Dead | 56 (86) |
| Alive | 9 (14) |
| Median survival (months) | 22 |
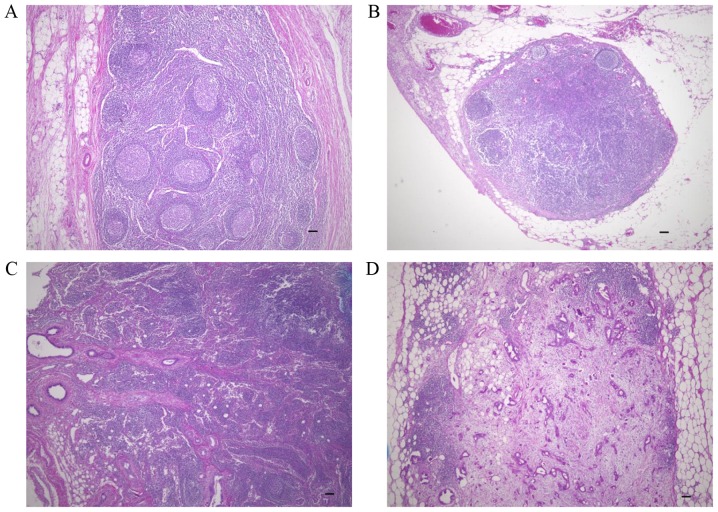
To investigate desmoplasia in lymph node metastases, we assessed the extent of fibrotic components in the 216 lymph nodes with a variety of sizes of metastatic lesions and the ten cancer-free lymph nodes (Fig. 1). No desmoplasia was observed in lymph nodes without metastases (Fig. 1A) or in lymph nodes with small metastatic lesions (<100 µm) composed of few cancer cells (Fig. 1B). In lymph nodes with >100 µm metastatic lesions, desmoplasia was observed in some, but not all cases (Fig. 1C). In the lymph nodes with >2,000 µm metastatic lesions, desmoplasia was frequently observed (Fig. 1D).
Figure 1.
Histology of patient-derived lymph nodes. (A) Non-metastatic lymph node. (B) Metastatic lymph node without a fibrotic component. (C) Metastatic lymph node with a fibrotic component. (D) Metastatic lymph node with a desmoplastic fibrotic component. Original magnification, ×40; scale bar, 100 µm.
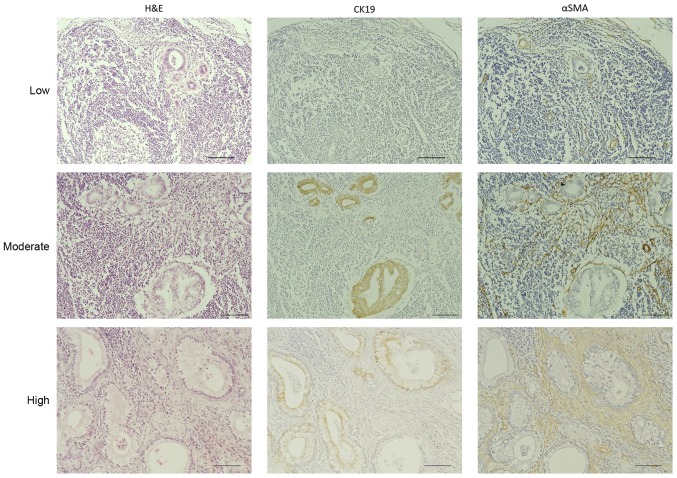
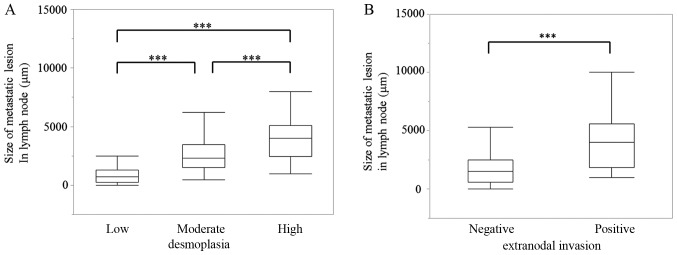
To investigate the clinical significance of desmoplasia in pancreatic cancer lymph node metastases, we stained the 216 metastatic lymph nodes with H&E, anti-CK19, which is a marker for cancer cells, and anti-αSMA, which is a marker for cancer-associated fibroblasts. Then, we evaluated the area covered by the fibrotic components in the samples. We classified these metastatic lesions into three categories according to the percentage of the total area of the metastatic lesions that were histologically fibrotic, high (≥70%), moderate (10–70%), and low (<10%) (Fig. 2). Based on this classification, 37 (56.9%), 16 (24.6%) and 12 (18.5%) of the 65 evaluable cases fell into the high, moderate and low degree of desmoplasia categories, respectively. Furthermore, 107 lymph nodes (49.54%) of the 216 evaluable samples had a high degree of desmoplasia, 64 (29.63%) had a moderate degree and 45 (20.83%) had a low degree (Table II). Next, we measured the lesion size of these 216 metastatic lymph nodes to investigate possible correlations between the degree of desmoplasia and lesion size. These results showed that 95.7% of the metastases smaller than 1,000 µm (69/216, 32.0%) were in the low desmoplasia group. Among the metastases larger than 1,000 µm (52/216, 24.1%), 44.2% showed moderate or high degrees of desmoplasia, and among the metastases larger than 2,000 µm (95/216, 44.0 %), 87.3% showed moderate or high degrees of desmoplasia (Table II). There was a significant correlation between metastatic lesion size and the degree of desmoplasia in these patient-derived samples (P<0.001; Fig. 3A). Additionally, there was a significant correlation between metastatic lesion size and locally extranodal invasion in these samples (P<0.001; Fig. 3B).
Figure 2.
Immunohistochemical assessment of desmoplasia in metastatic lymph nodes. The ratios of the area of the fibrotic components to the total metastatic lesion area were grouped into three categories, high (≥70%), moderate (10–70%), and low (<10%). Metastatic lymph nodes from pancreatic cancer patients were evaluated by H&E, CK19 and αSMA immunohistochemical staining. Original magnification, ×200; scale bar, 100 µm. H&E, hematoxylin and eosin; CK19, cytokeratin 19; αSMA, α-smooth muscle actin.
Table II.
Relationship between desmoplasia and metastatic lesion size.
| Size of metastatic lesion in lymph node | ||||
|---|---|---|---|---|
| Desmoplasia | 0-999 µm | 1,000–1,999 µm | 2,000 µm | Total (%) |
| High | 0 | 7 | 38 | 45 (21) |
| Moderate | 3 | 16 | 45 | 64 (30) |
| Low | 66 | 29 | 12 | 107 (49) |
| Total | 69 (32%) | 52 (24%) | 95 (44%) | 216 |
Figure 3.
Correlations between the size of metastatic lesions and the degree of desmoplasia, and locally extranodal invasion. (A) Correlation between the size of metastatic lesions and the degree of desmoplasia. (B) Correlation between the size of metastatic lesions and locally extranodal invasion. ***P<0.001.
We also investigated possible correlations between clinicopathological factors and the degree of desmoplasia. As shown in Table III, the degree of desmoplasia in metastatic lesions was not correlated with most clinicopathological factors, such as pT category and lymphatic invasion. Number of metastatic lymph node and locally extranodal invasion were significantly correlated with the degree of desmoplasia in the metastatic lesions.
Table III.
Association between the degree of desmoplasia and clinicopathological factors.
| Desmoplasia | ||||
|---|---|---|---|---|
| Characteristics | High n=37 (%) | Moderate n=16 (%) | Low n=12 (%) | P-value |
| Age (years) | ||||
| ≥65 | 21 (57) | 11 (69) | 6 (50) | 0.5732 |
| <65 | 16 (43) | 5 (31) | 6 (50) | |
| pT category | ||||
| pT1-3 | 37 (100) | 14 (87.5) | 11 (92) | 0.0681 |
| pT4 | 0 (0) | 2 (12.5) | 1 (8) | |
| Histologic grade | ||||
| G1/G2 | 15 (41) | 5 (31) | 6 (50) | 0.6003 |
| G3 | 22 (59) | 11 (69) | 6 (50) | |
| Pathologic margin | ||||
| Negative | 25 (68) | 12 (75) | 8 (67) | 0.8420 |
| Positive | 12 (32) | 4 (25) | 4 (33) | |
| Lymphatic invasion | ||||
| No | 5 (14) | 2 (12) | 3 (25) | 0.6210 |
| Yes | 32 (86) | 14 (88) | 9 (70) | |
| Vascular invasion | ||||
| No | 9 (24) | 5 (31) | 7 (58) | 0.1022 |
| Yes | 28 (76) | 11 (69) | 5 (42) | |
| Perineural invasion | ||||
| No | 3 (8) | 0 (0) | 1 (8) | 0.3095 |
| Yes | 34 (92) | 16 (100) | 11 (92) | |
| Extranodal invasion | ||||
| No | 18 (48.65) | 15 (93.75) | 12 (100) | <0.0001a |
| Yes | 19 (51.35) | 1 (6.25) | 0 (0) | |
| Number of metastatic lymph node | ||||
| 1 | 1 (3) | 2 (12.5) | 5 (42) | 0.0042a |
| ≥2 | 36 (97) | 14 (87.5) | 7 (58) | |
P<0.05.
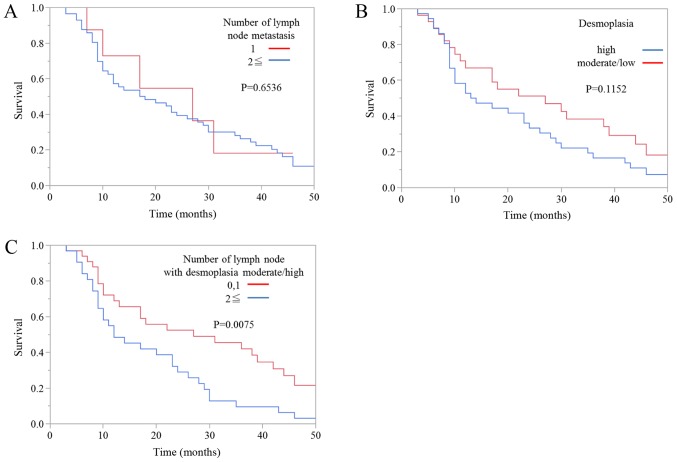
Next, we evaluated the association between prognosis and the degree of desmoplasia in metastatic lymph node lesions. Kaplan-Meier survival curves showed that there was no significant difference in overall survival between patients with one metastatic lymph node and patients with more than one metastatic lymph node (Fig. 4A). Moreover, there was no significant difference in overall survival between patients with a high degree of metastatic lymph node desmoplasia and patients with moderate or low degrees of desmoplasia (Fig. 4B). However, the prognosis of patients with one or zero metastatic lymph nodes with high or moderate degrees of desmoplasia was also significantly better than patients with two or more metastatic lymph nodes with high or moderate degrees of desmoplasia (Fig. 4C).
Figure 4.
Kaplan-Meier survival curves for the 65 patients with metastatic lymph nodes according to (A) the number of metastatic lymph nodes, (B) the degree of desmoplasia in the metastatic lymph nodes, (C) the number of metastatic lymph nodes with high or moderate degrees of desmoplasia.
Finally, we evaluated the clinicopathological findings, including the degree of desmoplasia in metastatic lymph node lesions, to identify prognostic factors for overall survival in pancreatic cancer patients with lymph node metastasis using univariate and multivariate analyses (Table IV). In the univariate analysis, we found sex (P=0.0203), locally extranodal invasion (P=0.0051) and multiple metastatic lymph nodes with high or moderate desmoplasia (P=0.0098) to be prognostic factors. In multivariate analysis, the presence of multiple metastatic lymph nodes with high or moderate desmoplasia was also a prognostic factor (P=0.0466). Because we analyzed only patients with lymph node metastases who underwent surgical resection for pancreatic cancer in this study, patients with pT4 were exactly the same group as the patients with UICC Stage III or IV. Therefore, we did not include UICC stage in Tables III and IV.
Table IV.
Prognostic factors for overall survival in univariate and multivariate analyses.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Characteristics | N | HR (95%) CI | P-value | HR (95%) CI | P-value |
| Age, ≥65/<65 | 38/27 | 1.11 (0.64–1.93) | 0.7110 | ||
| Sex, male/female | 42/23 | 0.51 (0.27–0.90) | 0.0203a | 0.66 (0.34–1.21) | 0.1855 |
| pT category, pT1-3/T4 | 62/3 | 2.36 (0.57–6.57) | 0.2048 | ||
| Histologic grade, G1-G2/G3 | 26/39 | 1.25 (0.73–2.20) | 0.4224 | ||
| Pathologic margin, −/+ | 45/20 | 1.60 (0.86–2.84) | 0.1305 | 1.93 (1.02–3.52) | 0.0437a |
| Lymphatic invasion, −/+ | 10/45 | 1.04 (0.53–2.27) | 0.9235 | ||
| Vascular invasion, −/+ | 21/44 | 1.58 (0.89–2.93) | 0.1173 | 1.45 (0.79–2.78) | 0.2312 |
| Perineural invasion, −/+ | 4/61 | 0.44 (0.18–1.46) | 0.1586 | 0.39 (0.15–1.37) | 0.1302 |
| Extranodal invasion, −/+ | 45/20 | 2.39 (1.31–4.31) | 0.0051a | 1.63 (0.82–3.22) | 0.1632 |
| Number of lymph node with desmoplasia high or moderate, 0,1/≥2 | 33/32 | 2.07 (1.19–3.63) | 0.0098a | 1.90 (1.01–3.61) | 0.0466a |
P<0.05.
Discussion
The main findings of this study were: i) the degree of desmoplasia in metastatic lymph node lesions from pancreatic cancer patients was significantly correlated with lesion size; ii) the degree of desmoplasia in metastatic lymph nodes was significantly correlated with locally extranodal invasion; and iii) the presence of multiple lymph nodes with high or moderate desmoplasia was correlated with poor prognosis.
In this study, the median number of regional lymph nodes examined per case was 32, and the median number of lymph nodes with metastases was 5. These median values are higher than most values reported in the literature (4,15–17). Also, the percentage of T4 cases in our institutional cohort was higher, possibly due to the fact that this study only included cases with lymph node metastases. The male to female ratio was also higher in our study, but this is a recognized tendency in previous Japanese studies (18). The median age and tumor size were similar to previous reports. These data suggested that our cohort was representative of the general group of patients with resectable pancreatic cancer, except for there being more advanced cancers and a greater number of metastatic lymph nodes, which was due to our study including only lymph node metastasis cases.
A recent report showed that there were significant prognostic implications of lymph node metastasis in pancreatic cancer (4). In this study, we focused on desmoplasia, a characteristic pathological finding in primary pancreatic cancer lesions, in metastatic lymph node lesions. Our data showed that there were significant correlations between the size of metastatic lesions and the degree of desmoplasia in the lesions. We also found no increase in the fibrotic component of cancer-free lymph nodes in patients with other metastatic lymph nodes, suggesting that there was no induction of stromal cells as a pre-metastatic niche in lymph nodes without metastases. Furthermore, these findings also suggested that desmoplasia in lymph node lesions was not present in the early stages of metastasis, but was induced and/or increased with the growth of the lesions. Also, we found that the number of metastatic lymph node was significantly correlated with the degree of desmoplasia. In cases with multiple lymph node metastases, a high degree of desmoplasia was frequently observed. These findings also suggest that desmoplasia in lymph node lesions was induced and/or increased with the progression of lymph node metastasis.
Finally, we also investigated correlations between clinicopathological factors and the degree of desmoplasia in metastatic lymph nodes, finding that locally extranodal invasion was significantly correlated with the degree of desmoplasia. In addition, locally extranodal invasion was significantly correlated with metastatic lesion size. These results suggest the possibility that locally extranodal invasion in lymph nodes can be controlled by suppressing desmoplasia, and treatment for desmoplasia may improve the prognosis of pancreatic cancer patients with lymph node metastasis accompanied by desmoplasia or locally extranodal invasion. The locally extranodal invasion of lymph nodes is a histological feature that has been considered a prognostic factor in several cancers (19–21), and recent reports have shown that locally extranodal invasion of lymph node metastases in pancreatic cancer and cancer of the papilla of Vater was common in these tumors, leading to a significantly increased risk for all-cause mortality (22). Although the nonrandomized nature and small number of patients in this study limit generalizable conclusions, the results regarding locally extranodal invasion raise important issues that should be investigated in future studies.
In conclusion, these data demonstrated that the presence of multiple metastatic lymph nodes with high or moderate degrees of desmoplasia was correlated with overall survival in univariate and multivariate analyses. We performed multivariate analysis with six factors (10% of sample size) as shown in Table IV because the number of samples in the present study was small. We also confirmed that the results obtained in multivariate analysis including all factors showed the similar results (number of lymph node with desmoplasia high or moderate, 0, 1/≥2; P=0.0087) and didn't alter the interpretation of our findings and the conclusions of the present study. Locally extranodal invasion and metastatic lesion size were also correlated with the degree of desmoplasia in metastatic lymph nodes. To further determine the contribution of desmoplasia to lymph node metastases, we should clarify the functional role of desmoplasia in the establishment and/or growth of lymph node metastases in the future.
Acknowledgements
The authors thank E. Manabe, S. Sadatomi and M. Ohmori (Department of Surgery and Oncology, Kyushu University Hospital, Fukuoka, Japan). The present study was supported in part by a Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (B) and (C) and Scientific Research on Innovative Areas (grant nos. 26293305, 25713050, 16K15621, 16K10601, 16K10600, 16H05417, 15H04933, 15K15498 and 16H05418).
Glossary
Abbreviations
- H&E
hematoxylin and eosin
- CK19
cytokeratin 19
- αSMA
α-smooth muscle actin
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malvezzi M, Carioli G, Bertuccio P, Rosso T, Boffetta P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann Oncol. 2016;27:725–731. doi: 10.1093/annonc/mdw022. [DOI] [PubMed] [Google Scholar]
- 4.Basturk O, Saka B, Balci S, Postlewait LM, Knight J, Goodman M, Kooby D, Sarmiento JM, El-Rayes B, Choi H, et al. Substaging of lymph node status in resected pancreatic ductal adenocarcinoma has strong prognostic correlations: Proposal for a revised N classification for TNM staging. Ann Surg Oncol. 2015;22:1187–1195. doi: 10.1245/s10434-015-4861-0. (Suppl 3) [DOI] [PubMed] [Google Scholar]
- 5.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen MF, Mortensen MB, Detlefsen S. Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells. World J Gastroenterol. 2016;22:2678–2700. doi: 10.3748/wjg.v22.i9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita H, Ohuchida K, Mizumoto K, Nakata K, Yu J, Kayashima T, Cui L, Manabe T, Ohtsuka T, Tanaka M. Alpha-smooth muscle actin expressing stroma promotes an aggressive tumor biology in pancreatic ductal adenocarcinoma. Pancreas. 2010;39:1254–1262. doi: 10.1097/MPA.0b013e3181dbf647. [DOI] [PubMed] [Google Scholar]
- 8.Sinn M, Denkert C, Striefler JK, Pelzer U, Stieler JM, Bahra M, Lohneis P, Dörken B, Oettle H, Riess H, Sinn BV. α-Smooth muscle actin expression and desmoplastic stromal reaction in pancreatic cancer: Results from the CONKO-001 study. Br J Cancer. 2014;111:1917–1923. doi: 10.1038/bjc.2014.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang LM, Silva MA, D'Costa Z, Bockelmann R, Soonawalla Z, Liu S, O'Neill E, Mukherjee S, McKenna WG, Muschel R, Fokas E. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7:4183–4194. doi: 10.18632/oncotarget.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C, Hostetter G, Shepard HM, Von Hoff DD, Han H. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res. 2015;21:3561–3568. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho SY, Lee TH, Ku YH, Kim HI, Lee GH, Kim MJ. Central lymph node metastasis in papillary thyroid microcarcinoma can be stratified according to the number, the size of metastatic foci, and the presence of desmoplasia. Surgery. 2015;157:111–118. doi: 10.1016/j.surg.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Japan Pancreas Society, corp-author. Classification of Pancreatic Carcinoma. 2nd English. Kanehara & Co.; Tokyo, Japan: 2003. [Google Scholar]
- 13.Japan Pancreas Society, corp-author. Classification of Pancreatic Carcinoma. 1st English. Kanehara & Co.; Tokyo, Japan: 2009. [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1419. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 15.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huebner M, Kendrick M, Reid-Lombardo KM, Que F, Therneau T, Qin R, Donohue J, Nagorney D, Farnell M, Sarr M. Number of lymph nodes evaluated: Prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:920–926. doi: 10.1007/s11605-012-1853-2. [DOI] [PubMed] [Google Scholar]
- 17.Strobel O, Hinz U, Gluth A, Hank T, Hackert T, Bergmann F, Werner J, Büchler MW. Pancreatic Adenocarcinoma: Number of positive nodes allows to distinguish several N categories. Ann Surg. 2015;261:961–969. doi: 10.1097/SLA.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 18.Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, Miyagawa S, Yamaguchi A, Ishiyama S, Takeda Y, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: Long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci. 2012;19:230–241. doi: 10.1007/s00534-011-0466-6. [DOI] [PubMed] [Google Scholar]
- 19.Veronese N, Nottegar A, Pea A, Solmi M, Stubbs B, Capelli P, Sergi G, Manzato E, Fassan M, Wood LD, et al. Prognostic impact and implications of extra-capsular lymph node involvement in colorectal cancer: A systematic review with meta-analysis. Ann Oncol. 2016;27:42–48. doi: 10.1093/annonc/mdv494. [DOI] [PubMed] [Google Scholar]
- 20.Luchini C, Nottegar A, Solmi M, Sergi G, Manzato E, Capelli P, Scarpa A, Veronese N. Prognostic implications of extranodal extension in node-positive squamous cell carcinoma of the vulva: A systematic review and meta-analysis. Surg Oncol. 2016;25:60–65. doi: 10.1016/j.suronc.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Veronese N, Luchini C, Nottegar A, Kaneko T, Sergi G, Manzato E, Solmi M, Scarpa A. Prognostic impact of extra-nodal extension in thyroid cancer: A meta-analysis. J Surg Oncol. 2015;112:828–833. doi: 10.1002/jso.24070. [DOI] [PubMed] [Google Scholar]
- 22.Luchini C, Veronese N, Pea A, Sergi G, Manzato E, Nottegar A, Solmi M, Capelli P, Scarpa A. Extranodal extension in N1-adenocarcinoma of the pancreas and papilla of Vater: A systematic review and meta-analysis of its prognostic significance. Eur J Gastroenterol Hepatol. 2016;28:205–209. doi: 10.1097/MEG.0000000000000520. [DOI] [PubMed] [Google Scholar]