Abstract
The present study investigated the correlations of the Tspan-1 gene expression with the clinical characteristics and survival prognoses of patients with advanced gastric cancer. A total of 150 patients with advanced gastric cancer were enrolled in the present study, of whom 84 were at stage II and 66 were at stage III according to the tumor node metastasis (TNM) staging; the immunohistochemical staining method and the semi-quantitative PCR method were used to detect the positive expression rates and mRNA relative expression levels of Tspan-1, vascular endothelial growth factor (VEGF), E-cadherin and N-cadherin. The positive expression rates of Tspan-1, VEGF, E-cadherin and N-cadherin were 58.0% (87 patients), 50.0% (75 patients), 28.0% (42 patients) and 53.3% (80 patients), respectively. The positive expressions and mRNA levels of Tspan-1, VEGF, E-cadherin and N-cadherin were not correlated with sex or age (P>0.05), but associated with the cancer state (stage II or stage III) and maximum tumor diameter (P<0.05). With the increase of stage and tumor diameter, the positive rates and mRNA levels of Tspan-1, VEGF and N-cadherin were increased, while those of E-cadherin were decreased. Among patients with stage II/III advanced gastric cancer, those with positive expression of Tspan-1, VEGF and N-cadherin had lower median survival time and survival rates than patients with negative expressions, while patients with positive expression of E-cadherin had higher median survival time and survival rate than those with negative expression (P<0.05). The high expression of Tspan-1 gene is associated with the TNM staging of advanced gastric cancer and the tumor diameter, influences the survival prognosis, and may involve the processes of angiogenesis and epithelial-mesenchymal transition.
Keywords: Tspan-1 gene, advanced gastric cancer, angiogenesis, epithelial-mesenchymal transition
Introduction
As the most common gastrointestinal malignancy, gastric cancer ranks fourth in incidence and second only to lung cancer in mortality among all malignant tumors (1). There is an absence of early specific clinical manifestations of patients with advanced gastric cancer; therefore, approximately 30%-70% of patients are newly diagnosed with advanced gastric cancer and the 3-year recurrence and metastasis rates of early gastric cancer cells after operation are approximately 40%-80% (2). The present study demonstrated that the occurrence and development of gastric cancer cells is a multi-gene involved multi-stage process, and the prognosis is closely related to tumor-node-metastasis (TNM) staging, but is different in patients with same-stage gastric cancer (3). Looking for molecular markers with high sensitivity and specificity is of great significance to improve the early diagnosis, treatment and prognosis. Tspan-1 is a member of transmembrane-4 superfamily (TM4SF) of proteins, is mainly located in the cell membrane, has a common phenomenon of glycosylation and plays important roles in intercellular adhesion, invasion and metastasis (4). Overexpression of Tspan-1 is found in ovarian cancer (5), colorectal cancer (6), hepatocellular carcinoma (7), cervical cancer (8), breast cancer (9), pancreatic cancer (10) and glioma (11), and is closely correlated with clinical features, therapeutic efficacy and survival prognoses of tumors. Tspan-1 gene in vitro can regulate and control the proliferation, differentiation, invasion, apoptosis, angiogenesis and other behaviors of tumor cells (12). A few recent studies have also pointed out that (13,14), the abnormal expression of Tspan-1 gene may be closely associated with the occurrence and development of gastric cancer cells. The present study analyzed the roles of Tspan-1 expression in the processes of angiogenesis and epithelial-mesenchymal transition (EMT), study whether it is associated with clinical features and prognoses of patients with advanced gastric cancer, to provide new targets for clinical evaluation of diagnosis, treatment and prognosis.
Patients and methods
A total of 150 patients diagnosed with advanced gastric cancer and admitted to the First Hospital of Putian from January 2013 to June 2016 were continuously selected and pathological diagnosis was confirmed. Among them were males (n=78) and females (n=72), with an average age of 62.5±15.6 years and a mean maximum tumor diameter of 3.3±1.4 cm. Patients were at stage II (n=84)and stage III (n=66), according to the TNM staging. Informed consent was obtained from the individuals who participated in the research. The present study was approved by the Ethics Committee at the First Hospital of Putian.
Study methods and observation indicators
A therapeutic regimen recommended by standard medical guidelines was used, i.e., a combination of surgical therapy, radiotherapy and chemotherapy and targeted therapy. Immunohistochemical staining method and semi-quantitative PCR method were used to detect the positive expression rates and mRNA relative expression levels of Tspan-1, vascular endothelial growth factor (VEGF), E-cadherin and N-cadherin. The follow-up time was 3.0 to 45.0 months and the median time was 25.0 months. The median survival time and survival rate were recorded. Related data were collected, entered and analyzed by a third party.
Immunohistochemical staining method
The following were purchased: Low temperature deep refrigerators (Haier Group, Qingdao, China), runner histotomes (LEICA RM2245; Leica Microsystems, Wetzlar, Germany), microscopes (Olympus BX51; Olympus Corporation, Tokio, Japan), pathology tissue bleaching and baking processors (TKY-TK; Hubei, China), electric-heated thermostatic hot air ovens (303–3; Shanghai, China), mouse anti-human Tspan-1, VEGF, E-cadherin and N-cadherin monoclonal antibodies (Beyotime Biotech, Jiangsu, Japan), rabbit anti-mouse IgG antibodies (Zhongshan Golden Bridge Biological Co., Ltd., Beijing, China), and PV-9000 second-generation general-purpose two-step immunohistochemical detection kits (Sigma, St. Louis, MO, USA).
Tissue sections were prepared through a routine fabrication method, with a thickness of 5 µm, de-waxed in xylene, rehydrated in gradient alcohol, then antigen retrieval, adding 3% H2O2 solution and incubation at 27°C for 20 min, and then normal goat serum working solution added dropwise and incubated at 27°C for 30 min. The sections were incubated with primary antibodies (1:2,000) overnight at 4°C in a humidified chamber. A negative control was designed by using normal mouse IgG instead of primary antibody. Then, the sections were added with IgG secondary antibodies dropwise (1:500) and incubated for 20 min at 27°C in the humidified chamber; horseradish-peroxidase-labeled pronase avidin (Biyuntian Science & Technology Co., Ltd., Jiangsu, China) were added dropwise and the sections were incubated for 20 min at 27°C in the humidified chamber and oscillated then washed with PBS for 5 min × 3 times. The sections were developed with DAB (diaminobenzidine), counterstained with hematoxylin, differentiated with hydrochloric alcohol, blued with ammonia, rehydrated in gradient alcohol, hyalinized in xylene, sealed with a neutral gum, dried at room temperature, and observed with an optical microscope. Result determination: the semi-quantitative method based on both the staining intensity and the proportion of stained cells was used; it was positive if the cytoplasm or nucleus was stained dark brown from yellow. The staining intensity was scored as: 0, negative; 1, weak; 2, moderate; 3, strong. The proportion of stained cells was scored as: 0, ≤5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; 4, >75%. If the product of the above two scores was 0–3, it was considered negative, and if 4–12, it was considered positive.
PCR method
Total RNA was extracted from cells according to a conventional method using TRIzol reagents. Concentration and purity were measured by using ultraviolet spectrophotometers. cDNA was synthesized by using reverse transcription kits. The primer sequences were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) based on gene bank sequences were: Tspan-1: forward, 5′-GGTTTCATCCAGGATCGAGCAGG-3′ and reverse, 5′-ACAAAGATGGTCACGGTCTGCC-3′, 445 bp; VEGF: forward, 5′-ACTACTTCTCCCGCCGCTAC-3′, and reverse, 5′-GAAATCAAACAGAGGCCGCATG-3′, 332 bp; E-cadherin: forward, 5′-ATCAAAGGTATCACGGCAAACG-3′ and reverse, 5′-CGGAGAGCTCGTCCACGTAT-3′, 479 bp; N-cadherin: forward, 5′-GTGCCATTAGCCAAGGGAATTCAGC-3′, and reverse, 5′-GCGTTCCTGTTCCACTCATAGGAG-3′, 337 bp; GAPDH forward, 5′-CGCGAGAAGATGACCCAGAT-3′, and reverse, 5′-GCACTGTGTTGGCGTACAGG-3′, 225 bp. The reaction system was 2 µl cDNA + 3 µl upper primers and 3 µl lower primers + 0.5 µl Taq polymerase + 1 µl dNTPs + 3 µl MgCl2 + 5 µl 10X buffer + 2.5 µl ddH2O2; the reaction condition was 95°C for 5 min, then 95°C for 30 sec, 58°C for 30 sec and 72°C for 60 sec, with a total of 30 cycles, and lastly, 72°C for 10 min. PCR products were identified by 2% agarose gel electrophoresis, ultraviolet spectrometry images were formed by a gel documentation and analysis system, and gray values of digital photos were analyzed. The results were expressed by using the 2−ΔΔCq method.
Statistical analysis
SPSS 20.0 software (IBM, Armonk, NY, USA) was used for statistical analysis. Measurement data were expressed as mean ± standard deviation and comparisons between groups were done by using independent sample t-test; enumeration data were indicated as case or percentage (%) and comparisons between groups were carried out by using χ2 test; Kaplan-Meier model and log-rank χ2 test were used for the median survival time; Pearson or χ2 test was used for the correlation analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Analysis of immunohistochemical results
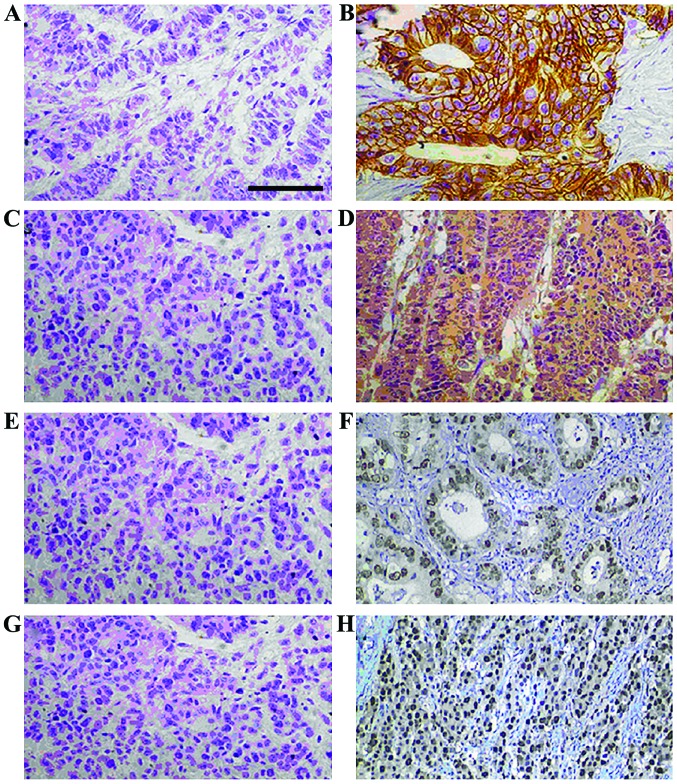
The positive expre-ssion rates of Tspan-1, VEGF, E-cadherin and N-cadherin were 58.0% (87 patients), 50.0% (75 patients), 28.0% (42 patients) and 53.3% (80 patients), respectively. The positive expressions of Tspan-1, VEGF, E-cadherin and N-cadherin was not correlated with sex or age (P>0.05), but associated with the cancer state (stage II or stage III) and maximum tumor diameter (P<0.05), that is, with the increase of stage and diameter, the positive rates of Tspan-1, VEGF and N-cadherin were increased, while that of E-cadherin was decreased (Fig. 1 and Table I).
Figure 1.
Immunohistochemical results of gastric cancer samples. (A) Tspan-1-negative, (B) Tspan-1-positive, (C) VEGF-negative, (D) VEGF-positive, (E) E-cadherin-negative, (F) E-cadherin-positive, (G) N-cadherin-negative and (H) N-cadherin-positive. Magnification, ×400; scale bar, 50 µm. VEGF, vascular endothelial growth factor.
Table I.
Analysis of immunohistochemical results.
| Tspan-1 | VEGF | E-cadherin | N-cadherin | |||||
|---|---|---|---|---|---|---|---|---|
| Item | Positive (n=87) | Negative (n=63) | Positive (n=75) | Negative (n=75) | Positive (n=42) | Negative (n=108) | Positive (n=80) | Negative (n=70) |
| Male/female | 42/45 | 36/27 | 40/35 | 38/37 | 25/17 | 53/55 | 46/34 | 32/38 |
| Age, years | 59.8±13.9 | 64.4±16.7 | 62.3±15.8 | 63.8±14.2 | 63.5±12.9 | 61.2±15.6 | 64.9±17.2 | 60.2±13.5 |
| Stage II/III | 40/47 | 44/19 | 35/40 | 49/26 | 30/12 | 54/54 | 37/43 | 47/23 |
| Maximum tumor diameter, cm | 3.9±1.6 | 3.1±1.2 | 3.8±1.7 | 3.2±1.3 | 3.1±1.3 | 3.7±1.6 | 4.1±2.1 | 2.8±1.3 |
VEGF, vascular endothelial growth factor.
Analysis of PCR results
The mRNA expression levels of Tspan-1, VEGF, E-cadherin and N-cadherin were not correlated with sex and age (P>0.05), but associated with the cancer state (stage II or stage III) and maximum tumor diameter (P<0.05). With the increase of cancer stage and diameter, the mRNA expression levels of Tspan-1, VEGF and N-cadherin were increased, while that of E-cadherin was decreased (Table II).
Table II.
Analysis of PCR results.
| Item | Tspan-1 | VEGF | E-cadherin | N-cadherin |
|---|---|---|---|---|
| Male | 0.4625±0.1325 | 0.3659±0.1324 | 0.1235±0.0685 | 0.4857±0.1526 |
| Female | 0.4526±0.1426 | 0.3529±0.1268 | 0.1325±0.0527 | 0.4759±0.1637 |
| Age, years | ||||
| <62 | 0.4429±0.1258 | 0.3652±0.1127 | 0.1426±0.0737 | 0.4659±0.1527 |
| ≥62 | 0.4725±0.1529 | 0.3528±0.1235 | 0.1258±0.0638 | 0.4925±0.1649 |
| Stage II | 0.3529±0.1123 | 0.3251±0.1426 | 0.1952±0.0859 | 0.3325±0.1323 |
| Stage III | 0.5214±0.1865 | 0.3956±0.1568 | 0.0965±0.0123 | 0.5968±0.1527 |
| Tumor diameter, cm | ||||
| <3.3 | 0.4215±0.1238 | 0.3123±0.1257 | 0.1857±0.0785 | 0.4215±0.1538 |
| ≥3.3 | 0.4968±0.1857 | 0.4214±0.1869 | 0.0865±0.0232 | 0.5263±0.2123 |
VEGF, vascular endothelial growth factor.
Correlation analysis
In immunohistochemical results, the positive rate of Tspan-1 was positively correlated with VEGF and N-cadherin (r=0.426, P=0.013; r=0.521, P=0.009), and negatively related to E-cadherin (r=0.467, P=0.011). In PCR results, the mRNA level of Tspan-1 was positively correlated with VEGF and N-cadherin (r=0.442, P=0.011; r=0.557, P=0.006), and negatively related to E-cadherin (r=0.482, P=0.008).
Analysis of survival prognosis
Among patients with stage II/III advanced gastric cancer, those with positive expression of Tspan-1, VEGF and N-cadherin had lower median survival time and survival rates than patients with negative expression, while patients with positive expression of E-cadherin had higher median survival time and survival rates than those with negative expression (P<0.05) (Table III).
Table III.
Analysis of survival prognosis.
| Tspan-1 | VEGF | E-cadherin | N-cadherin | |||||
|---|---|---|---|---|---|---|---|---|
| Item | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative |
| Stage II (n=84) | ||||||||
| Median survival time, months | 23.4 | 28.7 | 22.6 | 29.3 | 32.5 | 24.7 | 19.8 | 30.6 |
| Survival rate, % | 42.5 | 65.9 | 37.1 | 63.3 | 70.0 | 40.7 | 35.1 | 57.4 |
| Stage III (n=66) | ||||||||
| Median survival time, months | 14.6 | 21.2 | 12.9 | 20.4 | 22.3 | 14.8 | 10.7 | 19.8 |
| Survival rate, % | 25.5 | 52.6 | 30.0 | 65.4 | 58.3 | 24.1 | 27.9 | 56.5 |
VEGF, vascular endothelial growth factor.
Discussion
The present study showed that Tspan-1 plays important roles in cell signaling, adhesion regulation, metastasis, differentiation, proliferation and tumor cell immune escape (15). According to results of the study, the positive expression rates of Tspan-1, VEGF, E-cadherin and N-cadherin were 58.0, 50.0, 28.0 and 53.3%, respectively in patients with advanced gastric cancer. The positive expression and mRNA levels of Tspan-1, VEGF, E-cadherin and N-cadherin was not correlated with sex or age, but associated with the cancer state (stage II or stage III) and maximum tumor diameter. With the increase of cancer stage and tumor diameter, the positive rates and mRNA levels of Tspan-1, VEGF and N-cadherin were increased, while those of E-cadherin were decreased. It has been demonstrated that VEGF is involved in the mechanism of tumor angiogenesis. Tumor proliferation, invasion, migration, relapse and other processes are inseparable from the nutrition support of blood microcirculation. VEGF is a strong cytokine regulating angiogenesis (16). E-cadherin and N-cadherin are important molecular markers of EMT, of which E-cadherin is a marker of epithelial phenotype and N-cadherin is a marker of mesenchymal phenotype. The expression of the marker of epithelial phenotype decreased while that of the marker of mesenchymal phenotype increased. This is conssistent with the EMT occurrence and tumor metastasis. For malignant tumors of epithelial origin such as gastric cancer, the EMT occurrence plays major roles in tumor recurrence and metastasis (17).
A further correlation analysis showed that the positive rate and mRNA level of Tspan-1 were positively correlated with VEGF and N-cadherin, and negatively related to E-cadherin. Among patients with stage II and III advanced gastric cancer, those with positive expression of Tspan-1, VEGF and N-cadherin had lower median survival time and survival rates than patients with negative expressions, while patients with positive expression of E-cadherin had higher median survival time and survival rates than those with negative expression. This suggests that the high expression of Tspan-1 gene is associated with the TNM staging of patients with advanced gastric cancer and the tumor diameter influencing the survival prognosis, and may involve the processes of angiogenesis and EMT. Tspan-1 shows potential to become a target of early clinical diagnosis, intervention and prognosis evaluation. Therefore, additional sample size is needed, and the follow-up time should be extended to validate the conclusion.
References
- 1.Yung KW, Yung TT, Chung CY, Tong GT, Liu Y, Henderson J, Welbeck D, Oseni S. Principles of cancer staging. Asian Pac J Surg Oncol. 2015;1:1–16. [Google Scholar]
- 2.Zheng YF, Tan LK, Tan BH, Sterling H, Kane R. Principles of surgical oncology. Asian Pac J Surg Oncol. 2015;1:17–26. [Google Scholar]
- 3.Ghoneum M, Felo N, Nwaogu OM, Fayanju IY, Jeffe JA, Margenthaler DB. Clinical trials in surgical oncology. Asian Pac J Surg Oncol. 2015;1:73–82. [Google Scholar]
- 4.Hi G, Dong M, Sheng W, Zhou J, Yu D, Sun W. Expression and clinical significance of Tspan 1 and Integrin α6 in human pancreatic ductal adenocarcinoma. Zhonghua Wai Ke Za Zhi. 2014;52:781–786. (In Chinese) [PubMed] [Google Scholar]
- 5.Scholz CJ, Kurzeder C, Koretz K, Windisch J, Kreienberg R, Sauer G, Deissler H. Tspan-1 is a tetraspanin preferentially expressed by mucinous and endometrioid subtypes of human ovarian carcinomas. Cancer Lett. 2009;275:198–203. doi: 10.1016/j.canlet.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Zhu YY, Zhang XJ, Wang GL, Li XY, He S, Zhang JB, Zhu JW. TSPAN1 protein expression: A significant prognostic indicator for patients with colorectal adenocarcinoma. World J Gastroenterol. 2009;15:2270–2276. doi: 10.3748/wjg.15.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Yuan D, Wang GL, Wang Y, Wu YY, Zhu J. Clinicopathological significance of expression of Tspan-1, Jab1 and p27 in human hepatocellular carcinoma. J Korean Med Sci. 2010;25:1438–1442. doi: 10.3346/jkms.2010.25.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hölters S, Anacker J, Jansen L, Beer-Grondke K, Dürst M, Rubio I. Tetraspanin 1 promotes invasiveness of cervical cancer cells. Int J Oncol. 2013;43:503–512. doi: 10.3892/ijo.2013.1980. [DOI] [PubMed] [Google Scholar]
- 9.Desouki MM, Liao S, Huang H, Conroy J, Nowak NJ, Shepherd L, Gaile DP, Geradts J. Identification of metastasis-associated breast cancer genes using a high-resolution whole genome profiling approach. J Cancer Res Clin Oncol. 2011;137:795–809. doi: 10.1007/s00432-010-0937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao J, Yang JC, Ramachandran V, Arumugam T, Deng DF, Li ZS, Xu LM, Logsdon CD. TM4SF1 regulates pancreatic cancer migration and invasion in vitro and in vivo. Cell Physiol Biochem. 2016;39:740–750. doi: 10.1159/000445664. [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Bao W, Zhang G, Cui H, Shi G. Transmembrane-4-L-six-family-1, a potential predictor for poor prognosis, overexpressed in human glioma. Neuroreport. 2015;26:455–461. doi: 10.1097/WNR.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 12.Nabokina SM, Senthilkumar SR, Said HM. Tspan-1 interacts with the thiamine transporter-1 in human intestinal epithelial cells and modulates its stability. Am J Physiol Gastrointest Liver Physiol. 2011;301:G808–813. doi: 10.1152/ajpgi.00269.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, Zhang J, Huang Y. Tetraspanins in cell migration. Cell Adhes Migr. 2015;9:406–415. doi: 10.1080/19336918.2015.1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Z, Luo T, Nie M, Pang T, Zhang X, Shen X, Ma L, Bi J, Wei G, Fang G, Xue X. TSPAN1 functions as an oncogene in gastric cancer and is downregulated by miR-573. FEBS Lett. 2015;589:1988–1994. doi: 10.1016/j.febslet.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 15.Scholz CJ, Sauer G, Deissler H. Glycosylation of tetraspanin Tspan-1 at four distinct sites promotes its transition through the endoplasmic reticulum. Protein Pept Lett. 2009;16:1244–1248. doi: 10.2174/092986609789071234. [DOI] [PubMed] [Google Scholar]
- 16.Sciuto TE, Merley A, Lin CI, Richardson D, Liu Y, Li D, Dvorak AM, Dvorak HF, Jaminet SC. Intracellular distribution of TM4SF1 and internalization of TM4SF1-antibody complex in vascular endothelial cells. Biochem Biophys Res Commun. 2015;465:338–343. doi: 10.1016/j.bbrc.2015.07.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai J, Qian C, Su M, Chen M, Chen J. Gastrokine-2 suppresses epithelial mesenchymal transition through PI3K/AKT/GSK3β signaling in gastric cancer. Tumour Biol. 2016;37:12403–124010. doi: 10.1007/s13277-016-5107-x. [DOI] [PubMed] [Google Scholar]