Abstract
Medulloblastoma (MB) is a type of malignant brain tumor in children. Although knowledge of MB is increasing and the survival rate of patients with MB has improved in previous years, the long-term treatment-associated complications remain unfavorable. Early diagnosis and treatment is critical for patients with MB, but effective molecular markers for MB remain elusive. The Speckle-type POZ protein (SPOP) is a member of the MATH-BTB protein family and is a type of joint molecule for Cullin-3. SPOP inhibits tumor growth. However, the SPOP-like (SPOPL) gene, which is a SPOP paralog gene and shares an overall 85% sequence identity with SPOP, has not been explored in cancer studies at present. The results of the present study demonstrate that the SPOPL expression is decreased in MB cells and tissues compared with normal cells and tissues at the protein and mRNA levels. Immunohistochemical analysis revealed decreased expression of SPOPL in 42/56 (75%) paraffin-embedded archival MB biopsies, and SPOPL expression may be associated with the MB differentiation level (P=0.011). Patients with increased SPOPL expression exhibit improved survival rates compared with those with decreased SPOPL expression, and the SPOPL gene may be a potentially valuable molecular marker of MB.
Keywords: Speckle-type POZ protein, Speckle-type POZ protein-like gene, medulloblastoma, expression, prognosis
Introduction
Central nervous system (CNS) tumors are the principal cause of tumor-associated mortality in childhood (1), and the most common type of malignant brain tumor in children is medulloblastoma (MB) (2). Craniospinal radiation and chemotherapy following surgical resection are required for these patients. Although all these therapies have improved the 5-year survival rates of patients with MB (3), the complications of long-term therapy, such as developmental, neurological and neuroendocrine deficits, should not be neglected (4,5). Thus, safer therapies are needed for this disease. Previously, the consensus was that MB consists of four subgroups: WNT, SHH, group 3 and group 4 (6), and the view that the subgroups should be treated with diverse approaches is well accepted (7). However, the respective underlying molecular mechanisms of these MB subgroups have not been determined. More accurate predictors and effective therapies are required for MB.
Speckle-type POZ protein (SPOP), a type of adaptor protein which may link Cullin-3 E3 ligase to multiple protein substrates, is a member of the MATH-BTB protein family (8). Previously, multiple studies have suggested that SPOP inhibits tumors by identifying that the gene copies of SPOP are lost in certain types of human tumor (9,10). Multiple studies have also demonstrated that SPOP directly targets oncogenic proteins, including the Polycomb complex protein (11), pancreatic and duodenal homeobox protein 1 (12), apoptosis factor death domain-associated protein 6 (13), and Hedgehog signaling transcription factors zinc finger proteins GLI2 and GLI3 (14). Notably, the SPOP-like (SPOPL) gene has been identified as a SPOP paralog gene (15), sharing an overall 85% sequence identity with SPOP. The distinct difference between these genes is that SPOPL exhibits 18 more amino acid residues compared with SPOP (16). With the exception of a case report indicating that SPOPL was one of the deleted genes in a young male with unexplained somatopsychic illness through array comparative genomic hybridization (17), there is little knowledge of SPOPL in tumor generation and development. The high similarity of SPOPL to SPOP may indicate that SPOPL also functions as a tumor suppressor. However, a previous study of Cullin-RING ubiquitin ligase demonstrated that SPOP self-assembly and E3 ubiquitin ligase activity were inhibited by SPOPL in a dose-dependent manner (16). Therefore, the role of SPOPL in human malignancy is worth evaluating.
In the present study, the expression of SPOPL in MB tissue specimens and cell lines was first detected, and it focused on determining whether SPOPL is associated with the tumor suppression of MBs, and whether it would be a feasible indicator for estimating the prognosis of MBs following treatment.
Materials and methods
Tissue samples
A total of 58 formalin-fixed paraffin-embedded MB samples and 4 fresh MB surgical samples and matched adjacent normal human cerebellums were gathered from the Neurosurgery Department of the First Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China) between June 2002 and March 2014. The inclusion of patients in the study was unbiased and depended exclusively on the availability of sufficient tumor material and clinical follow-up data. Table I summarizes the clinical information corresponding to the samples. The following information was assessed: General information (age and sex) and residual tumor size. The MB patients were divided into two groups on the basis of the metastatic phase, age and extent of resection. Infants (≤3 years of age), patients with residual tumor (≥1.5 cm2) following neurosurgery and patients with leptomeningeal dissemination at presentation belonged to the high-risk group; otherwise, patients belonged to the standard-risk group (18). The World Health Organization (WHO) histological subtype (19), metastatic status and differentiation level was evaluated by a pathologist following hematoxylin and eosin staining using the following procedure: 4-µm thick serial sections were fixed for 20 min at 98°C with sodium citrate buffer (10 mM sodium citrate, 0.05% Tween-20, pH 6.0), and then stained with hematoxylin (Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) for 45 sec and 1% eosin for 10 sec at room temperature. The evaluation was performed with a CX31 microscope (abbe condenser with blue filter) and corresponding Image Analysis system software (CellSens Standard, version1.16; both from Olympus Corporation, Tokyo, Japan). A follow-up investigation was performed for all 58 patients, and December 2014 was the final date of follow-up for the present study. The present study was approved by the Ethics Committee of Sun Yat-Sen University and written informed consent was obtained from all subjects.
Table I.
Clinicopathological characteristics of patient samples and expression of SPOPL in medulloblastoma.
| Clinicopathological characteristic | No. of cases (%) |
|---|---|
| Age, years | |
| ≤3 | 5 (8.6) |
| >3 | 53 (91.4) |
| Sex | |
| Male | 46 (79.3) |
| Female | 12 (20.7) |
| WHO histological subtype | |
| Classic | 44 (75.9) |
| Desmoplastic | 14 (24.1) |
| Residual tumor size, cm2 | |
| <1.5 | 44 (75.9) |
| ≥1.5 | 14 (24.1) |
| Metastatic status | |
| M0 | 25 (43.1) |
| M1 | 33 (56.9) |
| Tumor risk | |
| Standard | 17 (29.3) |
| High | 41 (70.7) |
| Differentiation level | |
| Undifferentiated | 19 (32.8) |
| Differentiated | 39 (67.2) |
| Expression of SPOPL | |
| Negative | 2 (3.4) |
| Positive | 56 (96.6) |
| Low | 42 (75) |
| High | 14 (25) |
WHO, World Health Organization; SPOPL, Speckle-type POZ protein-like.
Immunohistochemical staining and scoring
Each tissue block was cut into 4-µm thick serial sections on aminopropyltriethoxysilane-coated glass slides, and fixed as described above. Then the sections were incubated with anti-SPOPL antibody (1:50 dilution; catalog no. 191175; Abcam, Cambridge, UK) overnight at 4°C in a humidified chamber. Next, the slides were processed using a ChemMate EnVision/horseradish peroxidase kit (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) for 30 min at room temperature, which was followed by processing with diaminobenzidine for visualization. All sections were counterstained with Mayer's hematoxylin (Beijing Dingguo Changsheng Biotechnology Co., Ltd.) for 30 sec at room temperature. Normal human cerebellum was used as a control.
Immunohistochemical staining evaluation was performed based on the proportion and intensity of positively stained tumor cells (20,21). The scoring was as follows: 0, 0–5; 1, 6–25; 2, 26–50; 3, 51–75; and 4, >75%. The staining intensity was scored as one of the following four grades: 0, negative; 1, weak; 2, moderate; and 3, strong. The final score for each section was the product of the percentage and intensity score. The SPOPL protein expression level was categorized as lower (final score <4) and higher (final score ≥4). Two pathologists separately scored all immunohistochemical staining. The pathologists were not familiar with the patient information. The mean scores were the final score in each case. All evaluation was performed with the microscope and image analysis software as described above.
Cell culture
The normal human astrocytes (NHAs) were obtained from the American Type Culture Collection (Manassas, VA, USA) and human medulloblastoma Daoy, D283 and D341 cell lines were from the Institute of Basic Medical Sciences of the Chinese Academy of Medical Sciences (Beijing, China). The Daoy and D283 cell lines were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The D341 cell line was cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.), and NHA was cultured in Astrocyte medium (#1801; ScienCell Research Laboratories, Carlsbad, CA, USA) with astrocyte growth supplement and 10% fetal bovine serum (Thermo Fisher Scientific, Inc.). All cells were incubated at 37°C in a humidified incubator in the presence of 5% CO2.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. cDNA was generated using a First-Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). Real-time PCR was performed with SYBR-Green qPCR kit on the OpenArray® Real-Time PCR Platform (both from Thermo Fisher Scientific, Inc.), and expression levels of the SPOPL gene were calculated by the 2−ΔΔCq method (22) and normalized to GAPDH (internal control). The PCR program was: 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C and 60 sec at 60°C. Relative mRNA levels of SPOPL were measured in relation to NHA and normal human cerebellum as positive control. Primers were designed using the Primer Express software (version 2.0 software; Applied Biosystems; Thermo Fisher Scientific, Inc.), and the primer sequences were as follows: SPOPL forward, 5′-GCTGGAGTCGTAACTCGGAAG-3′ and reverse, 5′-CCCTATCTCCCGCTCCTAAAC-3′; GAPDH forward, 5′-GACTCATGACCACAGTCCATGC-3′ and reverse, 5′-AGAGGCAGGGATGATGTTCTG-3′. The RT-qPCR analysis was performed at least three times.
Western blot analysis
The NHA, Daoy, D283 and D341 cells were lysed in lysis buffer (catalog no. P0013B; Beyotime Institute of Biotechnology, Haimen, China) on ice and then centrifuged at 12,000 × g for 10 min at 4°C. The protein contents in the supernatants were determined using a Bicinchoninic Acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of protein lysates (50 mg) were separated by SDS-PAGE (10% gels) and transferred on to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 1% bovine serum albumin for 2 h at room temperature, and then incubated with anti-SPOPL (catalog no. 191175; dilution, 1:1,000; Abcam, Cambridge, UK) and anti-β-actin primary antibody (catalog no. AF7018; dilution, 1:1,000; EarthOx Life Sciences, Millbrae, CA, USA) overnight at 4°C. Then, horseradish peroxidase-conjugated secondary antibody was added for 2 h at room temperature. Enhanced chemiluminescence by ECL substrate (Pierce; Thermo Fisher Scientific, Inc.) was used for detection. Experiments were performed at least twice.
Statistical analysis
SPSS software (version 16.0 for Windows; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A χ2 test was used for comparisons between groups. P<0.05 was considered to indicate a statistically significant difference. The overall survival (OS) time was measured (months) from the date of diagnosis to the date of mortality or the last follow-up prior to study termination. The Kaplan-Meier estimator method and Cox's regression were used for survival analysis.
Results
Downregulation of SPOPL mRNA and protein levels in MB cell lines and primary MB tumors
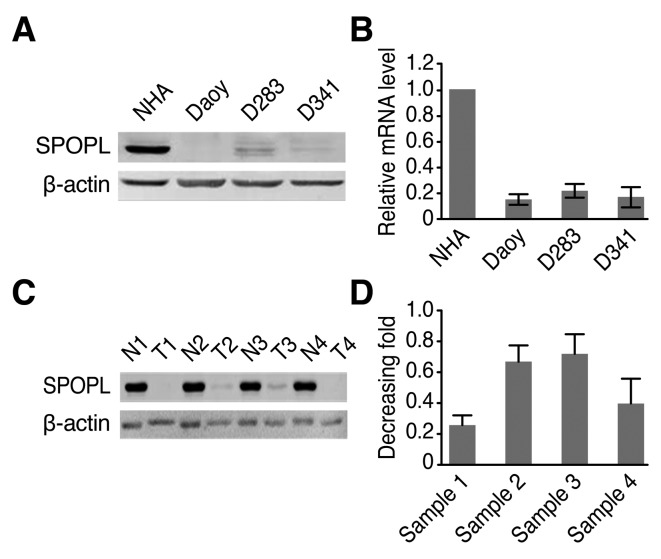
Western blotting results demonstrated that the SPOPL protein level was significantly decreased in the MB cell lines compared with the NHA cells (Fig. 1A). RT-qPCR results additionally confirmed that the SPOPL mRNA level was also decreased in the MB cell lines compared with the NHA cells (Fig. 1B). A total of four pairs of MB samples and normal human cerebellum were detected by western blotting to explore whether they exhibited similar tendencies. The present study demonstrated that SPOPL was differentially expressed in all four human MB samples compared with the normal human cerebellum, as presented in Fig. 1C. This result is similar to the results obtained for the mRNA level. As demonstrated in Fig. 1D, the tumor/normal ratio of SPOPL signals exhibited an 0.25–0.7-fold difference in the four tissue pairs. These results indicate decreased SPOPL expression in cancer lesions.
Figure 1.
SPOPL protein and mRNA expression in established cell lines and paired clinical MB samples. (A) SPOPL protein expression in NHA and cultured MB Daoy, D283 and D341 cells. (B) SPOPL mRNA expression was quantified by RT-qPCR. The expression levels were normalized for GAPDH. Data are presented as the mean ± standard deviation. (C) SPOPL protein expression in each of the primary MB T and N samples based on Western blotting. (D) The average tumor/normal (T/N) ratios of SPOPL expression were quantified by RT-qPCR. The expression levels were normalized for GAPDH. Data are presented as the mean ± standard deviation. SPOPL, Speckle-type POZ protein-like; NHA, normal human astrocyte; MB, medulloblastoma; N, normal human cerebellum sample; T, tumor sample; RT-qPCR, quantitative reverse transcription polymerase chain reaction.
Decreased SPOPL expression in archived MB tissues
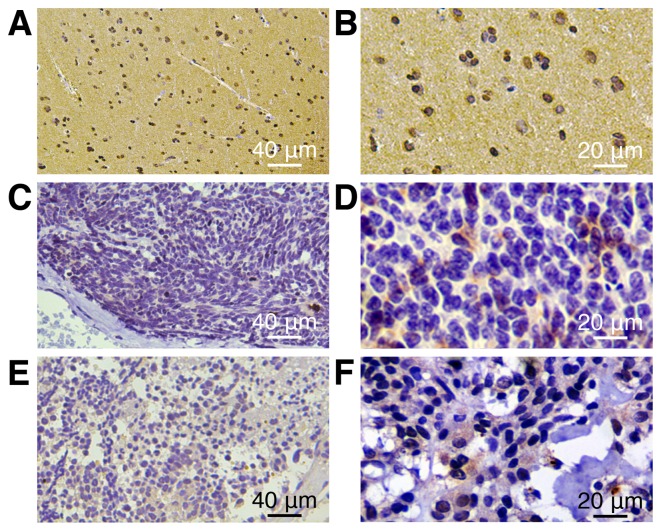
On the basis of the aforementioned results, whether SPOPL exhibits decreased expression levels in a larger cohort of clinical samples was assessed. In total, 58 archived MB tissues were examined. SPOPL was detected in 56/58 (96.7%) cases. According to immunohistochemical staining evaluation, the 56 cases were separated into two groups: 42 cases with decreased expression (75%) and 14 cases with increased expression (25%). In contrast, more intense SPOPL staining was observed in the normal human cerebellum (Fig. 2). Statistical analyses were performed to explore the association between SPOPL and the clinicopathological characteristics of MB. As summarized in Table II, SPOPL expression was markedly associated with the tumor differentiation level (P=0.011), whereas no association was identified with the patient age, WHO histological subtype or tumor risk with MB.
Figure 2.
SPOPL protein is expressed at diverse levels in MB tumors and normal human cerebellum histopathological sections, as examined by immunohistochemistry. Expression of SPOPL in normal human cerebellum was almost completely detectable. (A) Magnification, ×200. (B) Magnification, ×400. SPOPL expression in the primary lesion of MB tumors was highly detectable. (C) Magnification, ×200. (D) Magnification, ×400. Expression of SPOPL in the MB tumors was only marginally detectable. (E) Magnification, ×200. (F) Magnification, ×400. SPOPL, Speckle-type POZ protein-like; MB, medulloblastoma.
Table II.
Association between SPOPL and the clinicopathological characteristics of patients with MB.
| SPOPL | |||
|---|---|---|---|
| Characteristic | Low or none | High | χ2 (P-value) |
| Age, years | |||
| ≤3 | 4 | 1 | 0.821 |
| >3 | 40 | 13 | |
| Sex | |||
| Male | 34 | 12 | 0.764 |
| Female | 10 | 2 | |
| WHO histological subtype | |||
| Classic | 34 | 10 | 0.931 |
| Desmoplastic | 10 | 4 | |
| Residual tumor size, cm2 | |||
| <1.5 | 41 | 14 | 0.756 |
| ≥1.5 | 3 | 0 | |
| Metastatic status | |||
| M0 | 19 | 6 | 0.983 |
| M1 | 25 | 8 | |
| Tumor risk | |||
| Standard | 16 | 5 | 0.965 |
| High | 28 | 9 | |
| Differentiation level | |||
| Undifferentiated | 10 | 9 | 0.011 |
| Differentiated | 34 | 5 | |
WHO, World Health Organization; SPOPL, Speckle-type POZ protein-like.
SPOPL expression is associated with the prognosis of patients with MB
SPOPL expression in patients with MB was significantly associated with the survival time of patients (P<0.05) at a coefficient of 0.187, which manifested in increased SPOPL expression and survival time in patients with MB (Table III). The effects of SPOPL and classic clinicopathological characteristics (including age, sex, WHO histological subtype, residual tumor size, metastatic status and differentiation level) on the survival rates by Kaplan-Meier estimator analysis and the log-rank test were calculated. The results indicated that the survival time significantly differed between the low and high SPOPL expression groups (P<0.05). As presented in Fig. 3, the cumulative 5-year survival rate for the low SPOPL expression group was only 35.1% [95% confidence interval (CI) 0.439–0.720], whereas it was 83.6% in the high SPOPL expression group (95% CI 0.668–0.940). Furthermore, multivariate survival analysis was performed to test the SPOPL expression level, age, sex, WHO histological subtype, metastatic status and differentiation level to determine whether SPOPL was an independent prognostic factor of the outcome of patients with MB. The residual tumor size and SPOPL expression were independent prognostic factors (Table IV). Thus, it was concluded that the SPOPL gene is associated with MB prognosis.
Table III.
Kaplan-Meier estimator analysis for overall survival rate of patients with medulloblastoma.
| Clinicopathological characteristics | STQ2 (STQ1, STQ3), months | P-valuea |
|---|---|---|
| Age, years | ||
| ≤3 | 20 (13, 21) | 0.161 |
| >3 | 41 (20.5, 55) | |
| Sex | ||
| Male | 41 (19, 53) | 0.585 |
| Female | 40 (24, 56) | |
| WHO histological subtype | ||
| Classic | 40 (19.75, 55) | 0.820 |
| Desmoplastic | 37.5 (17.75, 60.75) | |
| Residual tumor size, cm2 | ||
| <1.5 | 40 (20, 54) | 0.001 |
| ≥1.5 | 19 (11, 25) | |
| Metastatic status | ||
| M0 | 32.5 (14.75, 53.25) | 0.562 |
| M1 | 41.5 (20, 55) | |
| Tumor risk | ||
| Standard | 40 (19.5, 53.5) | 0.352 |
| High | 26 (19, 57) | |
| Differentiation level | ||
| Undifferentiated | 38 (14, 53.5) | 0.273 |
| Differentiated | 41 (24, 55) | |
| SPOPL expression | ||
| Low | 36.5 (19, 53.5) | 0.026 |
| High | 41.5 (33.25, 57) |
Log-rank test. ST, survival time; Q, interquartile range; WHO, World Health Organization; SPOPL, Speckle-type POZ protein-like.
Figure 3.

Kaplan-Meier estimator curves with univariate analyses (log-rank) for patients with low vs. high SPOPL expression tumors. The cumulative 5-year survival rate was 83.6% in the high SPOPL protein group (n=14; unbroken line) and 35.1% in the low SPOPL protein group (n=42; broken line). SPOPL, Speckle-type POZ protein-like.
Table IV.
Cox regression model for multivariate analyses of prognostic factor in medulloblastoma.
| Variable | Hazard ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Age, years (≤3 vs. >3) | 4.443 | 0.451–43.78 | 0.201 |
| Sex (male vs. female) | 1.779 | 0.550–5.755 | 0.336 |
| WHO histological subtype (classic vs. desmoplastic) | 1.454 | 0.507–4.169 | 0.486 |
| Metastatic status (M0 vs. M1) | 1.089 | 0.083–14.29 | 0.949 |
| Residual tumor size, cm2 (<1.5 vs. ≥1.5) | 7.639 | 1.663–35.09 | 0.009 |
| Tumor risk (standard vs. high) | 0.906 | 0.055–14.84 | 0.945 |
| Differentiation (differentiated vs. undifferentiated) | 0.571 | 0.211–1.546 | 0.270 |
| Speckle-type POZ protein-like expression (low vs. high) | 0.218 | 0.048–0.982 | 0.047 |
Discussion
To the best of our knowledge, the present study is the first to demonstrate that decreased SPOPL expression is associated with the decreased survival time of patients with MB. SPOPL expression is decreased in MB cell lines at the mRNA and protein levels, which is in contrast with NHAs. Additionally, MB lesions and normal human cerebellum tissues express SPOPL at different levels, and MB tissues exhibit markedly decreased expression of SPOPL at the mRNA and protein levels. Furthermore, immunostaining demonstrated that the SPOPL expression in histological sections was significantly associated with the tumor differentiation level (P=0.011), and increased survival time of patients with MB. Taken together, these results indicate that SPOPL potentially represents a novel marker for determining the prognosis of MB.
As indicated by previous studies, SPOP regulates signaling pathways that control numerous types of cellular response that are essential to tumor progression, including proliferation and differentiation (23,24). Currently, SPOP is considered important in numerous tumor types, including breast, prostate, liver, gastric and colorectal cancer (25,26). In brain tumors, Ding et al (27) identified that decreased expression of SPOP is associated with a poor prognosis in glioma. All these results indicate that SPOP serves a role in tumor suppression. As it shares a high sequence identity with SPOP, SPOPL may also exhibit similar functions to those of SPOP, including tumor suppression. Notably, Errington et al (16) identified that SPOPL could interact with SPOP and inhibit its self-assembly and further affect E3 ubiquitin ligase activity, which indicated that SPOPL may serve the opposite role in tumorigenesis compared with SPOP. The present study provided evidence that SPOPL reduction may serve a role in MB progression, suggesting that SPOPL serves the same role in tumorigenesis as SPOP
As identified by immunohistochemical detection, 42/56 (75%) paraffin-embedded archival MB biopsies revealed weak SPOPL staining, whereas strong SPOPL staining was observed in normal human cerebellum tissues, indicating that SPOPL loss may accelerate the development and progression of MB. The present study of the association between SPOPL and clinical characteristics demonstrated a marked association between SPOPL and differentiation level of MB cells, suggesting that SPOPL may be used as a possible valuable marker for identifying patients with MB. The 35.1% 5-year survival rate of patients with low SPOPL expression, which was decreased compared with the 83.6% rate in the high SPOPL expression group, indicates that SPOPL may be used as a prognosis and survival predictor for patients with MB. Additional studies are required to confirm these data, and to verify the significance of SPOPL. In addition, more functional analyses are also required to elucidate the role of SPOPL in MB.
In conclusion, the present study assessed the possibility of using SPOPL as a prognostic marker for MB. Additionally, SPOPL may be regarded as a novel MB biomarker for evaluating therapeutic strategies and developing treatment standards. Therefore, additional studies on the mechanism of SPOPL and more clinical patients with MB are required.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81370072 and 81572477), the Natural Science Foundation of Guangdong Province for Distinguished Young Scholars (grant no. S2013050014535), the Key Project Supported by the Science and Technology Planning Project of Guangdong Province (grant no. S2012020006147), the Scientific Research Project of Guangdong Provincial Science and Technology (grant no. 2013B021800111), the Pearl River New Star Science and Technology Program of Guangzhou City (grant no. 2013J2200022) and the Science and Technology Program of Guangzhou City (grant no. 2013J4100060).
References
- 1.Pui CH, Gajjar AJ, Kane JR, Qaddoumi IA, Pappo AS. Challenging issues in pediatric oncology. Nat Rev Clin Oncol. 2011;8:540–549. doi: 10.1038/nrclinonc.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller S, Chang S. Pediatric brain tumors: Current treatment strategies and future therapeutic approaches. Neurotherapeutics. 2009;6:570–586. doi: 10.1016/j.nurt.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: Therapy at a crossroads. Arch Neurol. 2008;65:1419–1424. doi: 10.1001/archneur.65.11.1419. [DOI] [PubMed] [Google Scholar]
- 4.Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, Pomeroy SL, Korshunov A, Lichter P, Taylor MD, Pfister SM. Medulloblastomics: The end of the beginning. Nat Rev Cancer. 2012;12:818–834. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 6.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samkari A, White JC, Packer RJ. Medulloblastoma: Toward biologically based management. Semin Pediatr Neurol. 2015;22:6–13. doi: 10.1016/j.spen.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang M, Calabrese MF, Liu J, Waddell MB, Nourse A, Hammel M, Miller DJ, Walden H, Duda DM, Seyedin SN, et al. Structures of SPOP-substrate complexes: Insights into molecular architectures of BTB-Cul3 ubiquitin ligases. Mol Cell. 2009;36:39–50. doi: 10.1016/j.molcel.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, Sboner A, Esgueva R, Pflueger D, Sougnez C, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 11.Hernández-Muñoz I, Lund AH, van der Stoop P, Boutsma E, Muijrers I, Verhoeven E, Nusinow DA, Panning B, Marahrens Y, van Lohuizen M. Stable X chromosome inactivation involves the PRC1 Polycomb complex and requires histone MACROH2A1 and the CULLIN3/SPOP ubiquitin E3 ligase; Proc Natl Acad Sci USA; 2005; pp. 7635–7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu A, Desai BM, Stoffers DA. Identification of PCIF1, a POZ domain protein that inhibits PDX-1 (MODY4) transcriptional activity. Mol Cell Biol. 2004;24:4372–4383. doi: 10.1128/MCB.24.10.4372-4383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon JE, La M, Oh KH, Oh YM, Kim GR, Seol JH, Baek SH, Chiba T, Tanaka K, Bang OS, et al. BTB domain-containing speckle-type POZ protein (SPOP) serves as an adaptor of Daxx for ubiquitination by Cul3-based ubiquitin ligase. J Biol Chem. 2006;281:12664–12672. doi: 10.1074/jbc.M600204200. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Pan Y, Wang B. Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors. Development. 2010;137:2001–2009. doi: 10.1242/dev.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choo KB, Chuang TJ, Lin WY, Chang CM, Tsai YH, Huang CJ. Evolutionary expansion of SPOP and associated TD/POZ gene family: Impact of evolutionary route on gene expression pattern. Gene. 2010;460:39–47. doi: 10.1016/j.gene.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Errington WJ, Khan MQ, Bueler SA, Rubinstein JL, Chakrabartty A, Privé GG. Adaptor protein self-assembly drives the control of a cullin-RING ubiquitin ligase. Structure. 2012;20:1141–1153. doi: 10.1016/j.str.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Mulatinho MV, de Carvalho Serao CL, Scalco F, Hardekopf D, Pekova S, Mrasek K, Liehr T, Weise A, Rao N, Llerena JC., Jr Severe intellectual disability, omphalocele, hypospadia and high blood pressure associated to a deletion at 2q22.1q22.3: Case report. Mol Cytogenet. 2012;5:30. doi: 10.1186/1755-8166-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellison DW, Clifford SC, Gajjar A, Gilbertson RJ. What's new in neuro-oncology? Recent advances in medulloblastoma. Eur J Paediatr Neurol. 2003;7:53–66. doi: 10.1016/S1090-3798(03)00014-X. [DOI] [PubMed] [Google Scholar]
- 19.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song LB, Liao WT, Mai HQ, Zhang HZ, Zhang L, Li MZ, Hou JH, Fu LW, Huang WL, Zeng YX, Zeng MS. The clinical significance of twist expression in nasopharyngeal carcinoma. Cancer Lett. 2006;242:258–265. doi: 10.1016/j.canlet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Fukuoka J, Fujii T, Shih JH, Dracheva T, Meerzaman D, Player A, Hong K, Settnek S, Gupta A, Buetow K, et al. Chromatin remodeling factors and BRM/BRG1 expression as prognostic indicators in non-small cell lung cancer. Clin Cancer Res. 2004;10:4314–4324. doi: 10.1158/1078-0432.CCR-03-0489. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression using real time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Wang H, Sun M, Yang J, Zhang W, Han S, Xu B. Speckle-type POZ protein, SPOP, is involved in the DNA damage response. Carcinogenesis. 2014;35:1691–1697. doi: 10.1093/carcin/bgu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mani RS. The emerging role of speckle-type POZ protein (SPOP) in cancer development. Drug Discov Today. 2014;19:1498–1502. doi: 10.1016/j.drudis.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MS, Je EM, Oh JE, Yoo NJ, Lee SH. Mutational and expressional analyses of SPOP, a candidate tumor suppressor gene, in prostate, gastric and colorectal cancers. APMIS. 2013;121:626–633. doi: 10.1111/apm.12030. [DOI] [PubMed] [Google Scholar]
- 26.Kim MS, Kim MS, Yoo NJ, Lee SH. Somatic mutation of SPOP tumor suppressor gene is rare in breast, lung, liver cancers, and acute leukemias. APMIS. 2014;122:164–166. doi: 10.1111/apm.12108. [DOI] [PubMed] [Google Scholar]
- 27.Ding D, Song T, Jun W, Tan Z, Fang J. Decreased expression of the SPOP gene is associated with poor prognosis in glioma. Int J Oncol. 2015;46:333–341. doi: 10.3892/ijo.2014.2729. [DOI] [PubMed] [Google Scholar]