Abstract
Current therapy for rheumatoid arthritis (RA) relies on global suppression of the immune response or specific blockade of inflammatory cytokines. However, it is unclear how immunosuppressants affect patients with cancer. Therefore, in the present study, the effect of three biological agents, tofacitinib, anti-mouse IL-6 receptor antibody (MR16-1) and etanercept, which are used for the treatment of RA diseases, on a tumor-bearing mouse model was investigated. The effect of the three agents was examined using a mouse lung-metastasis model with the murine colon 26 cancer cell line. Lymphocyte subsets and natural killer (NK) cells in peripheral blood and spleen were analyzed using fluorescence-activated cell sorting, and the number of lung surface nodules was examined. In the continuous tofacitinib administration (15 mg/kg/day) group, the number of lung surface nodules was significantly increased compared with that of the vehicle-treated group (vehicle, 1.20±0.58; tofacitinib, 35.6±10.81; P<0.01). NK cell number in the blood and spleen of tofacitinib-treated mice was decreased 10-fold, and the percentage of cluster of differentiation (CD)11+CD27− NK cells was significantly reduced. MR16-1 [8 mg/mouse; once a week; intraperitoneal (i.p.)] or etanercept (1 mg/mouse; 3 times a week; i.p.) treatment did not affect the number of NK cells or lung metastasis. In the present study, immunosuppressants that target cytokines, including tofacitinib, were demonstrated to inhibit the proliferation and differentiation of NK cells, and exhibit the potential to promote cancer metastasis using a mouse model of lung metastasis.
Keywords: cancer metastasis, etanercept, interleukin-6, natural killer cell, tofacitinib
Introduction
Immune cells are associated with carcinogenesis, tumor growth, invasion and metastasis. Natural killer (NK) cells in particular serve an important role in immune surveillance, and are generally accepted as a beneficial cell population for anti-tumor immunity (1). Several studies have reported that depletion of NK cells causes increased survival of circulating tumor cells, resulting in enhanced cancer metastasis (2–5). In addition, it has been suggested that a favorable prognosis is associated with the extent of NK cell infiltration into the tumor in patients with gastric cancer or colorectal cancer (6,7). Therefore, inhibition of NK cell activity may promote cancer metastasis through a decrease in the number of NK cells.
In addition, cluster of differentiation (CD)4+ and CD8+ T cells, which are specific for tumor-associated antigens, serve important roles in antitumor immunity (8,9). CD4+ T cells serve an important role in generating effective immune responses by stimulating CD8+ T cell proliferation and establishing long-lived functional T cell memory (8). It has been reported that CD4+ T cell can enhance CD8+ T cell recruitment and infiltration into tumors (8). Similarly, several reports have suggested that the infiltration of CD8+ T cells is associated with a better prognosis in colon cancer (10).
Several inflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-6, serve important roles in the development and progression of rheumatoid arthritis (RA) (11,12). Thus, TNF-α inhibitors, including etanercept and the anti-IL-6 receptor (IL-6R) antibody (Ab) tocilizumab are efficacious RA treatments (13,14). Additionally, the novel small-molecule Janus kinase (JAK) inhibitor tofacitinib, suppresses several cytokine signals, including IL-2, −4, −6, −7 and −15. Therefore, it is also effective for the treatment of RA (15,16).
There are concerns about the potential increase in cancer risk associated with certain RA drug treatments, but these possibilities remain to be demonstrated. Therefore, in the present study, the effect of tofacitinib, the anti-mouse IL-6R Ab MR16-1 and etanercept, on the number of NK and T cells and cancer metastasis was investigated using an experimental lung metastasis mouse model with a mouse colon cancer cell line.
Materials and methods
Laboratory animals
Female Balb/c mice were obtained from Charles River Laboratories Japan, Inc. (Yokohama, Japan). The mice were housed under specific-pathogen-free conditions and were used in experiments at 6 weeks of age. The mean weight of mice was 20.5 g (20.3–20.8 g). In total, 32 mice were used for each experiment (a total of 96 mice were used in the present study). Mice were housed in cages and received standard mouse chow (CRF1; Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum. The environment was maintained between 23 and 24°C with a time-regulated light period between 8 a.m. and 8 p.m. Experiments were conducted in accordance with the institutional Ethics Guidelines of Fukuoka University in Japan (Fukuoka, Japan). The present study was approved by the Fukuoka University Animal Experiment Committee (approval no. 1404735).
Cell line
The mouse rectal colon 26 (C26) cancer cell line, was obtained from the RIKEN BioResource Center (Tsukuba, Japan). C26 cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin (all from Thermo Fisher Scientific, Inc., Waltham, MA, USA). C26 cells were incubated at 37°C in air containing 5% CO2.
Experimental metastasis assay
On day 0, the mice were treated with each agent (tofacitinib, MR16-1 or etanercept) as described subsequently. C26 cells were suspended in sodium bicarbonate-free RPMI-1640 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). A single injection of C26 cells (1.0×104 cells/mouse) was injected into mice via the lateral tail vein on day 3. On day 14, the mice were sacrificed by removal of blood from caudal vena cava under isoflurane anesthesia (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Blood was subsequently collected from the vena cava, and the spleen and lung were resected. The spleen was dispersed in PBS and contaminated red blood cells were lysed with lysing solution (BD Pharm Lyse; BD Biosciences, Franklin Lakes, NJ, USA). The total number of leukocytes in a splenocyte suspension and a whole blood sample were counted using an automatic cell counter (Nihon Kohden Corporation, Tokyo, Japan). Each lung was then weighed and placed in Bouin's solution (Wako Pure Chemical Industries, Ltd.) for ≥24 h, and the number of surface nodules was then counted using a light stereo microscope (magnification, ×10; SW-301; Wraymer Inc., Osaka, Japan).
Treatment of all mice groups
For each experiment with tofacitinib, MR16-1 or etanercept, the mice were divided into the following four groups (n=8 per group): No agent + no cancer cell group; no agent + C26 cell injection group; vehicle/control + C26 cell injection group; and agent + C26 injection group. The vehicle/controls used were poly (ethylene glycol) 300 (PEG300; Wako Pure Chemical Industries, Ltd.), rat immunoglobulin G (IgG) and human IgG (both from MP Biomedicals, LLC, Santa Ana, CA, USA) for tofacitinib, MR16-1 and etanercept, respectively. The dose of each treatment was determined as the effective dose reported on a collagen-induced arthritis model in previous studies (17–19).
Tofacitinib treatment
Tofacitinib (Selleck Chemicals, Houston, TX, USA) was dissolved in a sterile solution of PEG300, as used previously (18). Mice in the tofacitinib and vehicle treatment groups were anesthetized with isoflurane, and their dorsal surface was shaved 1 day prior to pump insertion. On day 0, a subcutaneous pocket was created under anesthesia with isoflurane, and an ALZET Mini-Osmotic Pump (model 2002, release rate 0.5 µl/h; Durect Co., Cupertino, CA, USA) was then inserted to deliver tofacitinib at a dosage of 15 mg/kg/day, or PEG300 as a control, as previously described (18).
MR16-1 treatment
As tocilizumab is an anti-human IL-6R Ab, it does not cross-react with murine IL-6R (20). Therefore, in the present study MR16-1 [obtained from hybridoma, established and gifted by Chugai Pharmaceutical Co., Ltd., Tokyo, Japan (20)], a specific rat anti-mouse IL-6R Ab, was used instead of tocilizumab. An intraperitoneal (i.p.) dose of 10 mg/ml MR16-1 in PBS or rat IgG (cat. no. 55951; MP Biomedicals, LLC) of 8 mg/mouse was injected once a week.
Etanercept treatment
Etanercept is a human TNF receptor-Fc fusion protein that inhibits TNF-α function of humans and mice (17). Etanercept was purchased from Pfizer, Inc. (Tokyo, Japan). Etanercept or human IgG (cat. no. 55908; MP Biomedicals, LCC) (1 mg/mouse, 3 times a week) was injected i.p. in mice.
Flow cytometric analysis
Splenocyte suspension was incubated with the Fc-receptor-blocking antibodies anti-CD16 and anti-CD32 (BD Biosciences) and then stained for 30 min with fluorescent antibodies (Table I) at 4°C. Blood sample was incubated with the Fc-receptor-blocking antibodies anti-CD16 and anti-CD32 (BD Biosciences) and stained with fluorescent antibodies (Table I) for 30 min at room temperature. Red blood cells were then lysed with lysing solution (BD Pharm Lyse; BD Biosciences). Following antibodies (all from BD Biosciences): Fluorescein isothiocyanate (FITC)-conjugated anti-CD3, phycoerythrin (PE)-conjugated anti-natural killer cell p46-related protein (NKp46), allophycocyanin (APC)-cyanine (Cy)7-conjugated anti-CD11b, APC-conjugated anti-CD27, PE-Cy7-conjugated anti-granulocyte-differentiation antigen-1 (Gr1)/Ly6 G and 6c for analysis of NK cell populations; and FITC-conjugated anti-CD3, APC-conjugated anti-CD4, PE-conjugated anti-CD8 and PE-Cy7-conjugated anti-CD19 were used for analysis of lymphocyte populations. Manufacturer-recommended isotype controls were used for each antibody. Antibodies used for FACS in the present study are summarized in Table I. The frequency of labeled cells was visualized using FACSCanto™II (BD Bioscience). In flow cytometric analysis of splenocyte and blood, T cells were gated as the CD3+ cells, and NK cells were gated as the CD3− NKp46+ Gr1− cells.
Table I.
Antibodies used.
| A, Antibodies used for FACS | ||
|---|---|---|
| Fluorescent antibody for FACS | Cat. no. | Volume, µl |
| FITC anti-mouse CD3 | 561798 | 1 |
| PE anti-mouse CD335 (NKp46) | 560757 | 1 |
| APC-Cy7 rat anti-mouse CD11b | 557657 | 1 |
| APC hamster anti-mouse CD27 | 560691 | 1 |
| PE-Cy7 rat anti-mouse Ly6g and | 552985 | 1 |
| Ly6c (Gr1) | ||
| APC rat anti-mouse CD4 | 553051 | 1 |
| PE anti-mouse CD8a | 553032 | 1 |
| PE-Cy7 rat anti-mouse CD19 | 552854 | 1 |
| B, Isotype controls | ||
| Isotype control | Cat. no. | Volume, µl |
| FITC rat IgG2bκ | 556923 | 1 |
| PE rat IgG2aκ | 553930 | 1 |
| APC-Cy7 rat IgG2bκ | 552773 | 1 |
| APC hamster IgG1κ | 553974 | 1 |
| PE-Cy7 rat IgG2bκ | 552849 | 1 |
| APC rat IgG2aκ | 553932 | 1 |
| PE rat IgG2aκ | 353930 | 1 |
| PE-Cy7 rat IgG2aκ | 552784 | 1 |
All antibodies were purchased from BD Biosciences. FACS, fluorescence-activated cell sorting; FITC, fluorescein isothiocyanate; CD, cluster of differentiation; APC, allophycocyanin; PE, phycoerythrin; NK, natural killer; Cy, cyanine; IgG, immunoglobulin G.
Statistical analysis
All data are presented as the mean ± standard error of the mean. Statistical analysis was performed using one-way analysis of variance with Dunnett's test as a post hoc comparison. P<0.05 was considered to indicate a statistically significant difference. All data were analyzed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA).
Results
Tofacitinib treatment
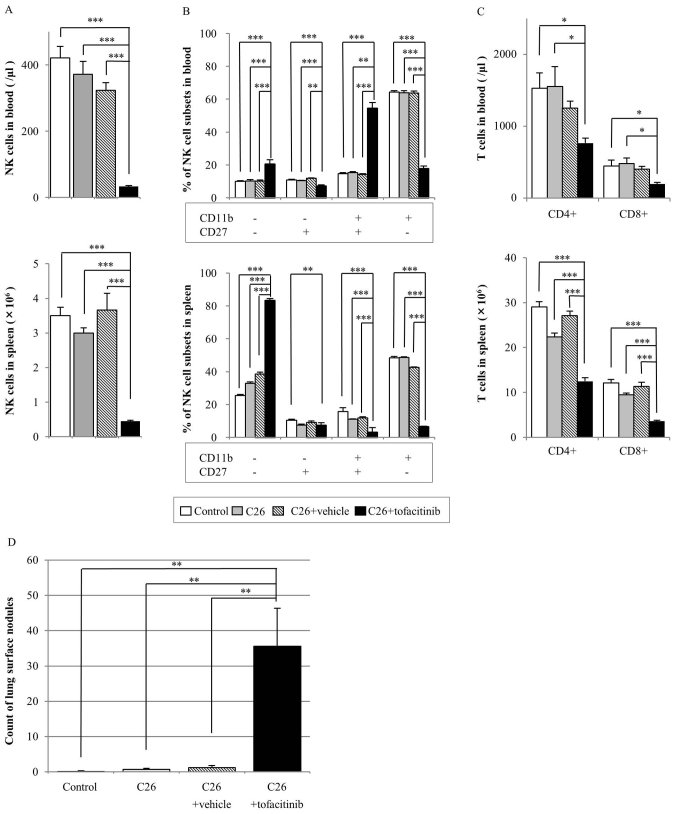
The tofacitinib-treated group had significantly reduced numbers of NK cells in the blood and spleen compared with those in all other groups (P<0.001; Fig. 1A). Compared with those in the vehicle-treated group, the number of NK cells in blood and spleen samples in the tofacitinib-treated group was decreased by 90 and 88%, respectively (Fig. 1A).
Figure 1.
Effect of tofacitinib treatment on NK cells, T cells and metastatic nodules in a mouse tumor model. Balb/c mice were injected with or without C26 colon cancer cells, and with or without tofacitinib or a vehicle [poly (ethylene glycol) 300] control as indicated. The following parameters were assayed after 14 days: (A) NK cell count in the blood (top) and spleen (bottom); (B) percentage of NK cell subsets, which were defined based on CD27 and CD11b expression, in the blood (top) and spleen (bottom); (C) CD3+CD4+ and CD3+CD8+ cell counts in the blood (top) and spleen (bottom); and (D) number of metastatic lung surface nodules. Data are presented as the mean ± standard error of the mean (control group n=7, C26 group n=7, C26+vehicle n=5, C26+tofacitinib n=7). *P<0.05, **P<0.01, ***P<0.001. CD, cluster of differentiation; NK, natural killer.
In addition, the effect of tofacitinib treatment on the percentage of NK cell subsets defined by CD11b and CD27 surface expression was assayed to analyze NK cell activity (Fig. 1B). The percentage of CD11b+CD27− NK cells in the blood and spleen samples of the tofacitinib-treated group was significantly decreased compared with that in the other three groups. By contrast, the percentage of CD11b−CD27− NK cell subsets was significantly increased in the tofacitinib-treated group compared with that in the other groups for blood and spleen analyses.
The number of CD4+ and CD8+ T cells in the blood samples of the tofacitinib-treated group was significantly decreased compared with that in the control and C26 cell-injected groups (Fig. 1C). No significant differences were identified in the number of CD4+ (P=0.381) or CD8+ (P=0.189) T cells in the blood samples between the tofacitinib-treated and vehicle-treated groups.
In the spleen of the tofacitinib-treated group, the number of CD4+ and CD8+ T cells was significantly decreased compared with that in all the other groups (Fig. 1C). The number of CD4+ and CD8+ T cells in the spleens of the tofacitinib-treated group was 39 and 51% lower, respectively compared with that in the vehicle-treated group.
In the experimental lung metastasis assay, no significant difference was observed in the lung weight among all groups (data not shown). The number of lung surface nodules was significantly increased in the tofacitinib-treated mice compared with that in the other three groups (vehicle-treated, 1.20±0.58; tofacitinib-treated, 35.6±10.81; all P<0.01; Fig. 1D).
The following mice were excluded from this analysis: One mouse in the vehicle-treated group died prior to being injected with C26 cells due to trouble at surgery; two mice in the vehicle-treated group failed to receive the C26 injection due to mistake of tail vein injection; and one mouse in the tofacitinib group had problem at drug administration (failure of skin anastomosis).
MR16-1 treatment
The blood NK cell numbers in the MR16-1-treated group were significantly decreased compared with those in the control group (Fig. 2A). In the spleen, no significant differences were identified between groups. The percentage of CD11b+CD27− NK cells in the blood and spleen was highest in all NK cell subsets in all groups, and the percentages of this subset in the MR16-1-treated group were not different among the other groups (Fig. 2B).
Figure 2.
Effect of MR16-1 treatment on NK cells, T cells and metastatic nodules in a mouse tumor model. Balb/c mice were injected with or without C26 colon cancer cells, and with or without MR16-1 or a rat IgG control as indicated. The following parameters were assayed following 14 days: (A) NK cell count in the blood (top) and spleen (bottom); (B) percentage of NK cell subsets defined based on CD27 and CD11b expression in the blood (top) and spleen (bottom); (C) CD3+CD4+ and CD3+CD8+ cell counts in the blood (top) and spleen (bottom); and (D) number of metastatic lung surface nodules. Data are presented as the mean ± standard error of the mean (control group n=8, C26 group n=8, C26+IgG group n=7, C26+MR16-1 n=8). *P<0.05, **P<0.01, ***P<0.001. CD, cluster of differentiation; NK, natural killer; IgG, Immunoglobulin G.
The CD4+ and CD8+ T cell numbers in the blood of the MR16-1-treated group were not different from those of any other groups (Fig. 2C). The CD4+ and CD8+ T cell numbers in blood exhibited similar results, although the CD4+ T cell number was decreased in the MR16-1-treated group compared with that in the control group (Fig. 2C). In the splenocyte of the MR16-1-treated group, the CD4+ and CD8+ T cell number was significantly decreased compared with that in the rat IgG-treated group, but not with that in the control or C26-injected groups (Fig. 2C).
In the experimental lung metastasis model, no significant difference was observed in lung weight (data not shown) or in the number of lung surface nodules between the MR16-1-treated group and any other groups (Fig. 2D). For one mouse in the rat IgG group, the spleen cells could not be analyzed due to technical failure (missing the sample).
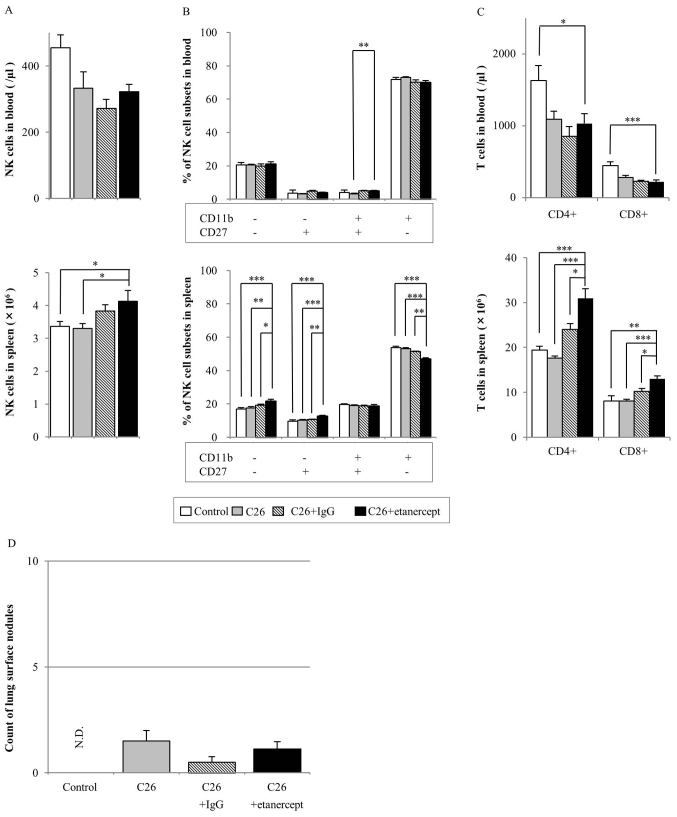
Etanercept treatment
The NK cell numbers in the blood of the etanercept-treated group did not differ from those of any of the other groups. The NK cell number in the spleens of the etanercept-treated group was not different from that in the human IgG-treated group, but was significantly increased compared with that in the control and C26-injected group (Fig. 3A). The percentage of CD11b+CD27− NK cells in blood and spleen was the highest of all the NK cell subsets in all the groups (Fig. 3B). However, in the spleen, the percentage of CD11b+CD27− NK cells of the etanercept-treated group was significantly decreased compared with that of the other groups.
Figure 3.
Effect of etanercept treatment on NK cells, T cells and metastatic nodules in a mouse tumor model. Balb/c mice were injected with or without C26 colon cancer cells, and with or without etanercept or human IgG control as indicated. The following parameters were assayed following 14 days: (A) NK cell count in the blood (top) and spleen (bottom); (B) percentage of NK cell subsets defined based on CD27 and CD11b expression in the blood (top) and spleen (bottom); (C) CD3+CD4+ and CD3+CD8+ cell counts in the blood (top) and spleen (bottom); and (D) number of metastatic lung surface nodules. Data are presented as the mean ± standard error of the mean (n=8 animals). *P<0.05, **P<0.01, ***P<0.001. CD, cluster of differentiation; NK, natural killer; IgG, Immunoglobulin G.
CD4+ and CD8+ T cell numbers in the blood of the etanercept-treated group were significantly decreased compared with those of the C26 only-injected group (Fig. 3C). However, the CD4+ and CD8+ T cell numbers in the spleen were significantly increased in the etanercept-treated group compared with those in all other groups.
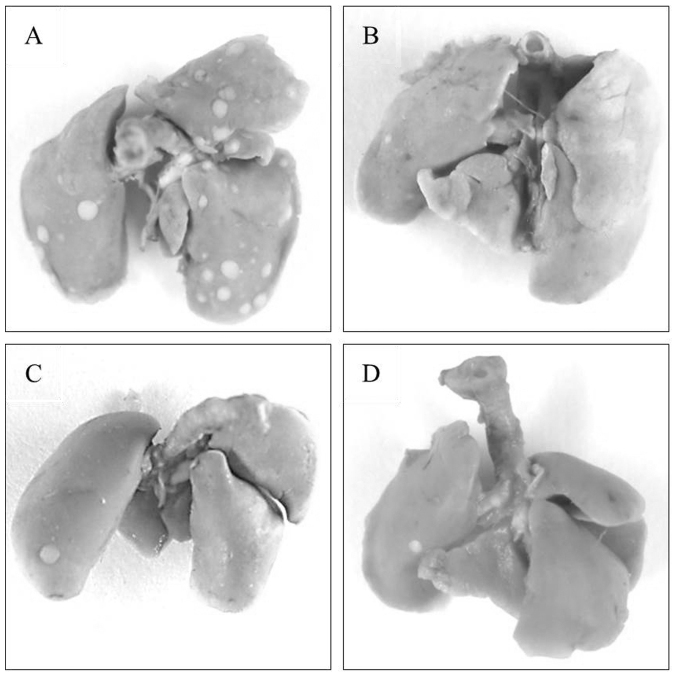
In the experimental lung metastasis assay, no significant difference was identified in the number of lung surface nodules between the etanercept-treated group and any other groups (Fig. 3D). Representative images of the lungs of mice treated with tofacitinib, MR16-1 and etanercept are shown in Fig. 4.
Figure 4.
Representative excised lungs from mice injected with C26 colon cancer cells and treated with (A) tofacitinib, (B) MR16-1, (C) etanercept, and (D) no treatment.
Discussion
In the present study, the effect of three cytokine signal inhibitors, tofacitinib, MR16-1 and etanercept, on NK cells, T cells and cancer metastasis was investigated. Only tofacitinib significantly enhanced cancer metastasis as determined by the number of lung surface nodules, with a significant decrease in NK cells in the mouse model.
Several previous reports have suggested that tofacitinib reduces NK cell counts in vivo (21,22). Clinically, tofacitinib does not significantly decrease NK cell counts in patients with RA (23). However, the Food and Drug Administration has reported that NK numbers exhibit a dose-dependent decrease following tofacitinib treatment (24). It was therefore suggested that tofacitinib reduces NK cells depending on the status of the patient. Additionally, it was reported that infiltration of CD8+ T cells into the tumor was associated with an improved prognosis, and that the depletion of CD8+ T cells reduces anti-tumor immunity and enhances growth and metastasis in a mouse lung metastasis model (10,25,26). It is therefore assumed that NK and CD8+ T cell reduction following tofacitinib treatment can promote cancer metastasis. Tofacitinib is a JAK inhibitor that suppresses inflammatory signaling downstream of γc-chain cytokines, IL-2, −4, −7 and −15 (22). IL-15 has an important role in the life and death of NK and CD8+ T cells (27,28). It is considered that IL-15 inhibition following tofacitinib treatment is the main mechanism underlying the significant reduction observed in NK and CD8+ T cell numbers.
Regarding the effect of tofacitinib on NK cell numbers and NK subsets in the present study, the results suggest that tofacitinib reduces total NK cell numbers and the percentage of the CD11b+CD27− NK cell subset. It has been proposed that CD11b−CD27−, CD11b−CD27+, CD11b+CD27+ and CD11b+CD27− NK subsets are present in proportion to maturation of murine and human NK cells (29,30). CD11b+CD27− NK cells are considered to be effector cells, expressing a high level of CD107a and producing interferon (IFN)-γ and cytotoxic granules, including granzyme B and perforin (31). It was suggested that perforin and IFN-γ in particular, produced by NK cells, have an important role in tumor surveillance (32,33). Therefore, it is considered that the CD11b+CD27− subset has the most important role for immunosurveillance of cancer. Thus, in the current study, it was considered that the reduction of CD8+ and NK cell counts, and the inhibition of NK cell maturation following tofacitinib treatment promotes lung metastasis due to the activities described above.
Cancer metastasis and NK cell count was not significantly affected by MR16-1 treatment in the present study. IL-6 is an inflammatory cytokine that serves multiple roles, including developmental differentiation, proliferation, survival and anti-apoptosis of various cells (34). These same signaling pathways serve to maintain cell progression towards neoplastic growth, protecting cells from apoptotic death (35). With regards to NK cell activity, a previous study reported that human NK cells exposed to IL-6 exhibited reduced perforin and granzyme-B expression, which was recovered in the presence of the anti-human IL-6R Ab tocilizumab (36). In that study, no significant differences in NK cell expression of CD69 or CD107a were observed between IL-6 transgenic, and wild-type mice. However, perforin and granzyme expression in NK cells was reduced in IL-6 transgenic mice compared with that in wild-type mice (36). Therefore, it may be assumed that NK cell activity is inhibited by IL-6; however, in the present study, the IL-6R Ab did not affect NK cell numbers or maturation, and did not promote cancer metastasis in the lung metastasis mouse model.
Etanercept is a recombinant human TNF receptor-Fragment crystallizable (R-Fc) fusion protein that inhibits TNF-α activity (37). Due to the immunosuppressive properties of this TNF-α inhibitor, it has been suggested that TNF-α inhibitor therapy may increase the risk of malignancy (38,39). However, a consensus has not been reached on whether this TNF-α inhibitor enhances carcinogenesis, tumor growth and metastasis in patients with cancer. The present study revealed no enhancement of lung metastasis in etanercept-treated mice. Etanercept has been reported to reduce the number and size of tumors in a spontaneous colon cancer mouse model associated with chronic colitis (40). Furthermore, blockade of TNF-α has been reported to inhibit lung metastasis in a mouse model (41,42). Concerning the effect of etanercept on NK cells, etanercept was reported to inhibit the production of transforming growth factor (TGF)-β1, which subsequently led to the inhibition of NK cells and cytotoxic activity (42). In an experimental lung metastasis mouse model, etanercept inhibited TGF-β1 production, which induced IL-13, restored CD8+ cell cytolytic activity and reduced lung metastasis (42). In the present study, there was a significant decrease in the percentage of CD11b+CD27− NK cells in the spleen following treatment with etanercept compared with that in other groups. Accompanied by the decrease in the CD11b+CD27− ratio, the ratio of CD11b−CD27− and CD11b−CD27+ was increased; however, the ratio of CD11b+CD27+ to total NK cells was unchanged. However, the total NK cell count in the etanercept-treated group was significantly increased compared with that in the untreated control and C26-treated groups. Furthermore, no statistically significant differences were identified in the total count of CD11b+CD27− NK cells in the spleen compared with those in other groups. The effect of etanercept may depend on the TNF-α status of the experimental model; for example, whether the model exhibits enhanced TNF-α expression or not. It was assumed that lung metastasis was not significantly enhanced following etanercept treatment in the present study, as etanercept exhibited little effect on NK cells. This finding does not conflict with previous studies reporting that TNF blockade inhibits carcinogenesis and cancer metastasis (41,42).
The present study has certain limitations. Firstly, the study used an experimental mouse model. Thus, the dose or administration method of each drug was referred from other previous experimental animal reports, and the clinical use of these drugs in humans may differ from the lung metastasis model used. In particular, tofacitinib is orally administered in humans, and therefore, it is unclear whether an increase in cancer metastasis would occur in patients with cancer following tofacitinib treatment as it did in the mice. Therefore, validation of the results of the current study in patients is warranted. Secondly, the present study used a normal mouse-bearing cancer cell line but not a RA mouse model. Thus, further studies are required to address these limitations.
Out of the three cytokine signal inhibitors evaluated in the present study, only tofacitinib significantly enhanced lung metastasis with inhibition of the proliferation and differentiation of NK cells in the lung metastasis mouse model. These data suggest that agents that reduce NK cell numbers have the potential to promote cancer metastasis. Monitoring of the NK cell number in patients with RA treated with cytokine signal inhibitors may be important in reducing the risk of cancer.
Acknowledgements
English language editing of the manuscript was received from Elsevier Language Editing Services (Elsevier, San Diego, CA, USA). The research grant and the anti-mouse IL-6R Ab were provided by Chugai Pharmaceutical Co., Ltd. (grant awarded to Dr Shinsuke Takeno; Department of Surgery, Miyazaki University Faculty of Medicine, Miyazaki, Japan).
Glossary
Abbreviations
- C26
colon 26
- IL-6R Ab
IL-6 receptor antibody
- NK
natural killer
- RA
rheumatoid arthritis
References
- 1.Whiteside TL, Herberman RB. The role of natural killer cells in immune surveillance of cancer. Curr Opin Immunol. 1995;7:704–710. doi: 10.1016/0952-7915(95)80080-8. [DOI] [PubMed] [Google Scholar]
- 2.Hanna N, Burton RC. Definitive evidence that natural killer (NK) cells inhibit experimental tumor metastases in vivo. J Immunol. 1981;127:1754–1758. [PubMed] [Google Scholar]
- 3.Kelly SA, Gschmeissner S, East N, Balkwill FR. Enhancement of metastatic potential by gamma-interferon. Cancer Res. 1991;51:4020–4027. [PubMed] [Google Scholar]
- 4.Mailloux AW, Clark AM, Young MR. NK depletion results in increased CCL22 secretion and Treg levels in Lewis lung carcinoma via the accumulation of CCL22-secreting CD11b+CD11c+ cells. Int J Cancer. 2010;127:2598–2611. doi: 10.1002/ijc.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yano S, Nishioka Y, Izumi K, Tsuruo T, Tanaka T, Miyasaka M, Sone S. Novel metastasis model of human lung cancer in SCID mice depleted of NK cells. Int J Cancer. 1996;67:211–217. doi: 10.1002/(SICI)1097-0215(19960717)67:2<211::AID-IJC11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(SICI)1097-0142(19970615)79:12<2320::AID-CNCR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. doi: 10.1002/(SICI)1097-0142(20000201)88:3<577::AID-CNCR13>3.3.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 8.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schild HJ, Kyewski B, von Hoegen P, Schirrmacher V. CD4+ helper T cells are required for resistance to a highly metastatic murine tumor. Eur J Immunol. 1987;17:1863–1866. doi: 10.1002/eji.1830171231. [DOI] [PubMed] [Google Scholar]
- 10.Funada Y, Noguchi T, Kikuchi R, Takeno S, Uchida Y, Gabbert HE. Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep. 2003;10:309–313. [PubMed] [Google Scholar]
- 11.Waldburger JM, Firestein GS. Garden of therapeutic delights: New targets in rheumatic diseases. Arthritis Res Ther. 2009;11:206. doi: 10.1186/ar2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siebert S, Tsoukas A, Robertson J, McInnes I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol Rev. 2015;67:280–309. doi: 10.1124/pr.114.009639. [DOI] [PubMed] [Google Scholar]
- 13.Emery P, Breedveld F, van der Heijde D, Ferraccioli G, Dougados M, Robertson D, Pedersen R, Koenig AS, Freundlich B, Combination of Methotrexate and Etanercept in Early Rheumatoid Arthritis Trial Group Two-year clinical and radiographic results with combination etanercept-methotrexate therapy versus monotherapy in early rheumatoid arthritis: A two-year, double-blind, randomized study. Arthritis Rheum. 2010;62:674–682. doi: 10.1002/art.27268. [DOI] [PubMed] [Google Scholar]
- 14.Genovese MC, Rubbert-Roth A, Smolen JS, Kremer J, Khraishi M, Gómez-Reino J, Sebba A, Pilson R, Williams S, Van Vollenhoven R. Longterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: A cumulative analysis of up to 4.6 years of exposure. J Rheumatol. 2013;40:768–780. doi: 10.3899/jrheum.120687. [DOI] [PubMed] [Google Scholar]
- 15.Lundquist LM, Cole SW, Sikes ML. Efficacy and safety of tofacitinib for treatment of rheumatoid arthritis. World J Orthop. 2014;5:504–511. doi: 10.5312/wjo.v5.i4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, Forejtova S, Zwillich SH, Gruben D, Koncz T, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto M, Serada S, Mihara M, Uchiyama Y, Yoshida H, Koike N, Ohsugi Y, Nishikawa T, Ripley B, Kimura A, et al. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum. 2008;58:3710–3719. doi: 10.1002/art.24126. [DOI] [PubMed] [Google Scholar]
- 18.Milici AJ, Kudlacz EM, Audoly L, Zwillich S, Changelian P. Cartilage preservation by inhibition of Janus kinase 3 in two rodent models of rheumatoid arthritis. Arthritis Res Ther. 2008;10:R14. doi: 10.1186/ar2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takagi N, Mihara M, Moriya Y, Nishimoto N, Yoshizaki K, Kishimoto T, Takeda Y, Ohsugi Y. Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 1998;41:2117–2121. doi: 10.1002/1529-0131(199812)41:12<2117::AID-ART6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki M, Yamada Y, Nishimoto N, Yoshizaki K, Mihara M. Characterization of anti-mouse interleukin-6 receptor antibody. Immunol Lett. 2002;84:231–240. doi: 10.1016/S0165-2478(02)00202-X. [DOI] [PubMed] [Google Scholar]
- 21.Conklyn M, Andresen C, Changelian P, Kudlacz E. The JAK3 inhibitor CP-690550 selectively reduces NK and CD8+ cell numbers in cynomolgus monkey blood following chronic oral dosing. J Leukoc Biol. 2004;76:1248–1255. doi: 10.1189/jlb.0504282. [DOI] [PubMed] [Google Scholar]
- 22.Kudlacz E, Perry B, Sawyer P, Conklyn M, McCurdy S, Brissette W, Flanagan And M, Changelian P. The novel JAK-3 inhibitor CP-690550 is a potent immunosuppressive agent in various murine models. Am J Transplant. 2004;4:51–57. doi: 10.1046/j.1600-6143.2003.00281.x. [DOI] [PubMed] [Google Scholar]
- 23.Sonomoto K, Yamaoka K, Kubo S, Hirata S, Fukuyo S, Maeshima K, Suzuki K, Saito K, Tanaka Y. Effects of tofacitinib on lymphocytes in rheumatoid arthritis: Relation to efficacy and infectious adverse events. Rheumatology (Oxford) 2014;53:914–918. doi: 10.1093/rheumatology/ket466. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Food and Drug Administration, corp-author. Advisory Committee meeting. Tofacitinib for treatment of rheumatoid arthritis (NDA 203214) Pfizer Inc.; 2012. [Google Scholar]
- 25.Ando T, Ito H, Arioka Y, Ogiso H, Seishima M. Combination therapy with α-galactosylceramide and a Toll-like receptor agonist exerts an augmented suppressive effect on lung tumor metastasis in a mouse model. Oncol Rep. 2015;33:826–832. doi: 10.3892/or.2014.3634. [DOI] [PubMed] [Google Scholar]
- 26.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 27.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/S1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 28.Waldmann TA. The biology of IL-15: Implications for cancer therapy and the treatment of autoimmune disorders; J Investig Dermatol Symp Proc; 2013; pp. S28–S30. [DOI] [PubMed] [Google Scholar]
- 29.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 30.Fu B, Wang F, Sun R, Ling B, Tian Z, Wei H. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology. 2011;133:350–359. doi: 10.1111/j.1365-2567.2011.03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinthorne JF, Beli E, Duriancik DM, Gardner EM. NK cell maturation and function in C57BL/6 mice are altered by caloric restriction. J Immunol. 2013;190:712–722. doi: 10.4049/jimmunol.1201837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, Scalzo AA. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- 33.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.V97.1.192. [DOI] [PubMed] [Google Scholar]
- 34.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 35.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Cifaldi L, Prencipe G, Caiello I, Bracaglia C, Locatelli F, De Benedetti F, Strippoli R. Inhibition of natural killer cell cytotoxicity by interleukin-6: Implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 2015;67:3037–3046. doi: 10.1002/art.39295. [DOI] [PubMed] [Google Scholar]
- 37.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 38.Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: Twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151–3158. doi: 10.1002/art.10679. [DOI] [PubMed] [Google Scholar]
- 39.Diak P, Siegel J, La Grenade L, Choi L, Lemery S, McMahon A. Tumor necrosis factor alpha blockers and malignancy in children: Forty-eight cases reported to the Food and drug administration. Arthritis Rheum. 2010;62:2517–2524. doi: 10.1002/art.27511. [DOI] [PubMed] [Google Scholar]
- 40.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choo MK, Sakurai H, Koizumi K, Saiki I. TAK1-mediated stress signaling pathways are essential for TNF-alpha-promoted pulmonary metastasis of murine colon cancer cells. Int J Cancer. 2006;118:2758–2764. doi: 10.1002/ijc.21734. [DOI] [PubMed] [Google Scholar]
- 42.Fichtner-Feigl S, Terabe M, Kitani A, Young CA, Fuss I, Geissler EK, Schlitt HJ, Berzofsky JA, Strober W. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res. 2008;68:3467–3475. doi: 10.1158/0008-5472.CAN-07-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]