Abstract
Diverse animals ranging from worms and insects to birds and turtles perf orm impressive journeys using the magnetic field of the earth as a cue. Although major cellular and molecular mechanisms for sensing mechanical and chemical cues have been elucidated over the past three decades, the mechanisms that animals use to sense magnetic fields remain largely mysterious. Here we survey progress on the search for magnetosensory neurons and magnetosensitive molecules important for animal behaviors. Emphasis is placed on magnetosensation in insects and birds, as well as on the magnetosensitive neuron pair AFD in the nematode Caenorhabditis elegans. We also review conventional criteria used to define animal magnetoreceptors and suggest how approaches used to identify receptors for other sensory modalities may be adapted for magnetoreceptors. Finally, we discuss prospects for under-utilized and novel approaches to identify the elusive magnetoreceptors in animals.
Keywords: magnetoreception, magnetosensation, orientation, migration, magnetic orientation
INTRODUCTION
Many animals sense the earth’s magnetic field to accomplish spectacular migrations. European robins fly across the Mediterranean Sea to North Africa (Wiltschko & Wiltschko 2005). Monarch butterflies cross wide plains to fly from Canada to Mexico (Reppert et al. 2010). Sea turtles hatched on the East Coast of the United States hobble into the Atlantic to launch a circular trek (Lohmann et al. 2001). Sea mollusks orient to the earth’s magnetic field in phase with the moon (Lohmann & Willows 1987). Although these animals use other cues such as the position of the sun or moon; the flow of continental wind currents; and the location of mountain ranges, seas, and rivers, the earth’s magnetic field provides a valuable additional cue for migration. Emanating from the rotating iron core of the earth, these more reliable magnetic cues allow animals to move onward when visual, thermal, and mechanical cues on the earth’s surface are obscured by clouds, fog, rain, and transient disturbances in wind patterns.
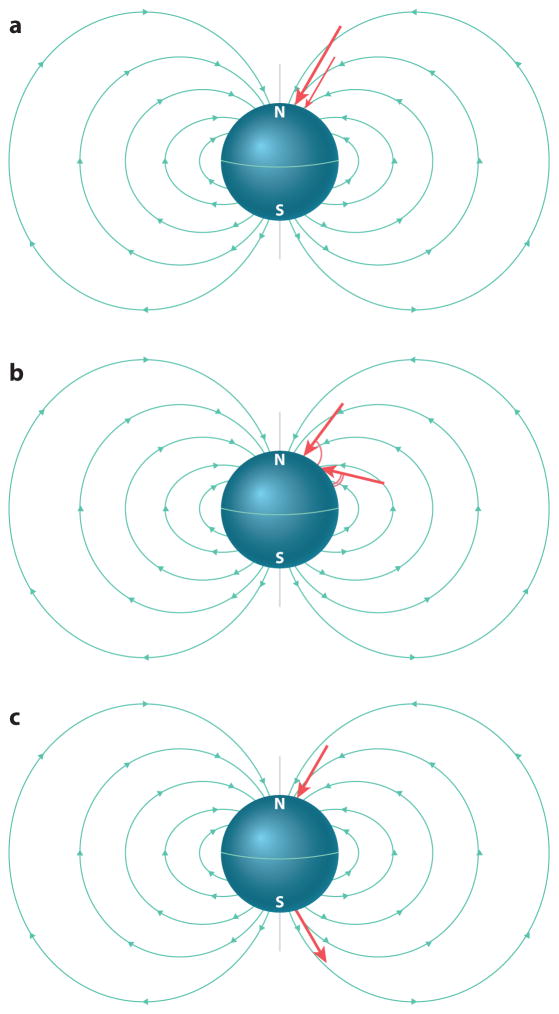
The earth’s magnetic field varies across the globe, and these variations can be used for orientation in a variety of ways (Figure 1). Some animals may use differences in field direction to deduce their heading, akin to a magnetic compass. Other animals seem able to infer their global position by detecting differences in magnetic field strength and inclination angle along their route (e.g., Lohmann et al. 2004). Decades worth of clever behavioral experiments in the field of magnetic orientation have provided evidence for animals using magnetoreception for both compass and position sensing (Lohmann et al. 2001, Walker et al. 2002, Zapka et al. 2009). Although no animal is believed to rely solely on geomagnetic cues for orientation, magnetic sensation is a solution widely used among animals to orient across distances on our planet.
Figure 1.
Different cues derived from the earth’s magnetic field. The magnetic field of the earth originates from the Southern Hemisphere, extends into the atmosphere and space, and then pierces into the Northern Hemisphere. Organisms may gain cues regarding their position and orientation on earth by detecting distinct aspects of the geomagnetic field in order to guide their behaviors. This includes sensing the intensity of the magnetic field (a), the inclination of the magnetic field (b), and the polarity of the magnetic field (c).
Despite the prevalence of magnetic orientation in animals, we still do not understand all the physiological, molecular, and genetic underpinnings of this sensory modality (Edelman et al. 2015). Importantly, although candidates have been proposed, the precise molecular sensor of the earth’s magnetic field and its transduction pathway have not been identified in any animal (Gould 2010). The search for a magnetosensor is far behind the identification of receptors for other sensory cues, primarily because researchers do not even know where to look. The earth’s magnetic field freely pierces the bodies of small and large animals alike. Whereas animals have evolved prominent sensory organs to optimally collect and amplify other sensory cues, such as eyes for light and cone-shaped pinna for sound, no such obvious magnetosensory organ appears to exist in animals. Also unhelpful in this search, humans do not appear to possess an obvious magnetic sense, so we cannot benefit from personal insight. Magnetoreceptor cells might be in subtle, overlooked areas on the body surface or buried deep within the body. The most advanced theories posit that magnetosensory neurons are embedded within organs that transduce other sensory modalities (Figure 2). In fact, specialized neurons that specifically sense aspects of magnetic fields may not exist; many researchers predict that a subset of neurons that sense another sensory modality will also sense the earth’s magnetic field. Candidate sensors that may also share a magnetic sense include mechanosensory neurons involved in proprioception in birds, light-sensing neurons in birds and salamanders, and chemosensory neurons in salmon (Demaine & Semm 1985, Diebel et al. 2000, Mann et al. 1988, Zapka et al. 2009).
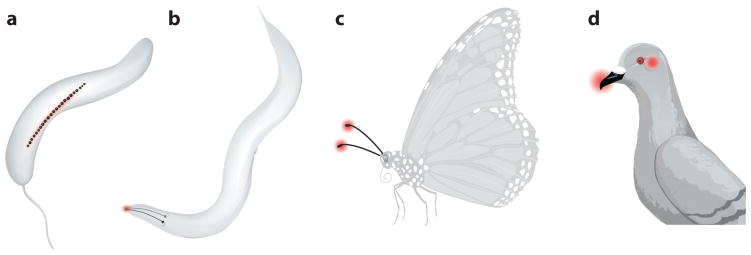
Figure 2.
Magnetosensory loci across organisms. Many organisms have been found to orient to the earth’s magnetic field with different sensory structures. (a) Magnetotactic bacteria use a micron-scale compass needle composed of a string of iron beads. (b) The nematode Caenorhabditis elegans uses the left-right pair of AFD sensory neurons that project sensory structures to the tip of the worm’s head. (c) Insects including butterflies and flies may use a cryptochrome-based chemical magnetic sensor in their antennae. (d) Birds may sense magnetic fields using magnetosensory cells in the inner ear and beak with an iron-based mechanism, and in eyes with a cryptochrome-based mechanism.
Many lucid reviews have summarized important aspects of magnetic orientation and possible mechanisms of magnetoreception (e.g., Gould 2010, Johnsen & Lohmann 2008, Wiltschko & Wiltschko 2005). Here we attempt to bridge the expanding field of magnetosensation across studies on animals traditionally investigated for their magnetic orientation, newer studies that exploit genetics to define transduction mechanisms and cells required for magnetosensation, and approaches on the horizon for this exciting field.
CRITERIA FOR A MAGNETOSENSORY NEURON AND RECEPTOR
In the historic search for magnetosensory neurons, researchers have been aided by thinking about factors that may constrain the form and function of a magnetoreceptor. As with all sensory systems, the magnetoreceptor must function within basic parameters (Block 1992). The detector must sense magnetic cues relevant to the earth’s magnetic field present in the animal’s natural environment, and the presence of the magnetosensory detector would need to contribute a selective advantage to the individual animal.
Expectations of General Sensory Systems
All sensory systems possess a detection stage mediated by a primary transducer. For some sensory systems, this may be a single peptide or molecular complex. To date, definitive evidence for a primary transducer for magnetosensation has proved elusive. However, tantalizing evidence has accumulated for the light-sensitive protein cryptochrome in birds, butterflies, and the fruit fly Drosophila (Gegear et al. 2008, 2010; Ritz et al. 2002). This molecule detects blue light to regulate circadian rhythms in animals and plants (Cashmore et al. 1999). Because its function depends in part on earth-strength magnetic fields, cryptochrome may transduce magnetic field information into a biochemical signal in sensory neurons. Newer yet weaker evidence has been presented for the MagR protein (Qin et al. 2016). This protein has been proposed to act like a compass needle because it binds iron and forms a rod-shaped complex when physically associated with cryptochrome.
The sensitivity of a proposed magnetosensory neuron and its primary magnetotransducer must match the appropriate sensitivity range of recorded behavioral feats. Data from pigeons and honey bees demonstrate sensitivities in the minute range of 1–20 μT (Kirschvink 1989). Physiological recordings of central neurons in the pigeon and of the magnetosensory neuron pair AFD in the nematode Caenorhabditis elegans show that neuronal responses saturate at larger than earth-strength magnetic fields, about 0.65 Gauss (Wu & Dickman 2012, Vidal-Gadea et al. 2015). Thus, the primary magnetotransducer for geomagnetic orientation in animals may not be adapted to respond to strong fields found in natural deposits of rare earth magnetic material and electrical systems devised by humans.
The sensitivity of a magnetosensory neuron must also make sense with any process of amplification. Cryptochrome may take advantage of the built-in amplification processes found in photoreceptor neurons (Ritz et al. 2000). Although the primary magnetotransducer has not yet been identified in C. elegans, a photoreceptor-like, cyclic nucleotide–based amplification system was found to be required for magnetic responses in the worm (Vidal-Gadea et al. 2015). Both magnetic orientation behavior and responses of the magnetosensory neuron pair AFD required cyclic GMP signaling components specifically in the AFD neurons. These include guanylyl cyclases that control intracellular cGMP levels and downstream cGMP-gated cation channel components TAX-4 and TAX-2. Intracellular signaling may serve to amplify tiny biochemical or physical changes imposed by the earth’s magnetic field on primary magnetodetectors in these different systems.
Alternatively, body structures may serve to amplify magnetic signals. Some evidence suggests that neurons within the beak of some birds detect the earth’s magnetic field (Falkenberg et al. 2009, Fleissner et al. 2003). Fleissner & Fleissner (2010) observed magnetic iron structures arranged in multiple linear arrays in the dendrites of upper beak neurons. Interspersed throughout the arrays are membrane-attached magnetite clusters. When these arrays align with the external field, they may serve to amplify the signal, such that an ambient magnetic field of approximately 0.5 Gauss is predicted to induce a field of 10 Gauss inside the dendrite (Solov’yov & Greiner 2007). The membrane-attached magnetite clusters then reorient with respect to the amplified field. This mechanism is predicted to exert a force of 0.2 pN on the membrane and nearby mechanosensors (Solov’yov & Greiner 2007). If true, then the beak may represent a magnetosensory organ that has been hidden in plain sight.
We should also prepare for the possibility that a magnetotransducer may be much more sensitive than appears necessary for natural behavior in an animal’s environment, as in other sensory modalities. For instance, photoreceptors in humans can detect single photons (Tinsley et al. 2016), and hair cells can detect mere nanometers of displacement of their stereocilia (Martin & Hudspeth 1999). The research challenge for these fields is how the signal is distinguished among the noise in these ultrasensitive detectors. Hinting at this potential consideration, the magnetosensory preferences of European robins in a laboratory setting were found to be not only sensitive to earth-strength fields but also disrupted by weaker electromagnetic fields from common radio waves (Engels et al. 2014). This surprising finding may explain anecdotal behavioral observations of birds misguided near electrical poles as well as some of the difficulty of replication in magnetic orientation studies (Kirschvink 2014).
Once magnetosensory information is detected by a primary magnetotransducer, it must then be relayed within the sensory neuron. The first sensory neuron with physiological evidence of responding to earth-strength magnetic stimuli in the lab is the sensory neuron pair AFD in the nematode C. elegans (Figure 2b, Figure 3) (Vidal-Gadea et al. 2015). These neurons likely represent actual magnetosensory neurons because magnetoresponsive activity persisted even after the neurons were synaptically isolated by deletion of genes critical for synaptic transmission. In other words, the AFD sensory neurons are not relaying a signal from an upstream sensory neuron. Although the primary magnetoreceptive transducer has not been identified in AFD, an intracellular signaling network using cyclic GMP was found to be required for both physiological responses of the AFD neurons and magnetic orientation behavior of the worm (Vidal-Gadea et al. 2015). This magnetotransducer is predicted to increase intracellular levels of cGMP upon changes in earth-strength magnetic fields, which in turn activate the cGMP-gated cation channel.
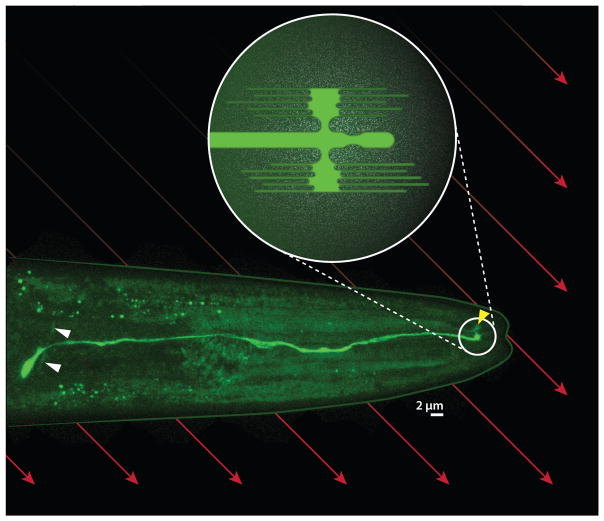
Figure 3.
AFD magnetosensory neuron in Caenorhabditis elegans. Fluorescent micrograph shows the right AFD neuron expressing a green fluorophore. The cell body has a curved axon (white arrowheads) that synapses onto other neurons and a dendrite that extends toward the tip of the worm’s head, where it features a complex sensory structure (circled). Reconstructions by White et al. (1986) and Doroquez et al. (2014) at the level of electron microscopy suggest that this structure includes a single cilium and antenna-shaped arrays of anterioposterior-directed microvilli on the dorsal and ventral sides (schematized in inset) Although most of the bilaterally symmetric left AFD neuron is out of view, its sensory structure is visible (yellow arrowhead). If the microvilli are associated with iron, the earth’s magnetic field (red arrows) may impose a mechanical force on the microvilli that depends on the orientation of the worm.
Information from magnetosensory neurons then passes through downstream circuitry to brain regions that encode informative aspects of the geomagnetic field. Several groups have recorded electrical activity from central neurons in the brains of birds. For instance, Semm & Demaine (1986) found that earth-strength magnetic stimuli activated different brain regions in birds, including the optic tectum, a structure homologous to the superior colliculus, as well as a nucleus within the basal optic root. Notably, neurons in the optic tectum fired with directional selectivity. Most impressive, Wu & Dickman (2012) discovered that single neurons in the brainstem of pigeons displayed firing activity that correlated with the direction, intensity, and/or polarity of imposed magnetic fields. These three factors are critical to calculating compass direction and geographic map location. The next step is to trace back the source of the magnetosensory input, as well as to determine how the neurons compute these geomagnetic qualities from the yet to be identified magnetosensory neurons.
Many sensory systems respond to diverse ranges and types of input by making rapid adjustments with feedback pathways. Although activity of central neurons has been recorded in both birds and mollusks (e.g., Lohmann et al. 1991, Wang et al. 2004, Wu & Dickman 2012), it remains to be tested whether efferent signaling pathways carry magnetosensory information back to sensory organs. This may represent an untapped approach to search for magnetosensory neurons.
Expectations Specific to Magnetoreceptors
When considering a primary transducer for magnetic reception, researchers have advanced evidence for two primary models of how properties of the earth’s magnetic field are converted to electrochemical signals by sensory neurons in animals. We do not discuss a third model, electromagnetic induction, because it allows an animal to sense a magnetic field indirectly by sensing an internal electric field that is generated when an animal moves through a magnetic field (Johnsen & Lohmann 2008).
Iron-based magnetoreception
The iron-based models of magnetoreception propose that magnetic field information is first sensed by an iron-containing molecule. Hypothetical iron sensors are most commonly believed to be composed of biogenic magnetite (Fe3O4) (Kirschvink & Gould 1981). The primary inspiration for magnetite as a biological sensor comes from bacteria that harbor a micron-scale bar of magnetite that resembles a string of black pearls (Figure 2a) (Blakemore 1975). Several recent reviews have been published on the diversity and evolution of magnetoreception in bacteria (Lefèvre & Bazylinski 2013, Lin et al. 2014). Genes required for bacteria to extract iron from the environment and form a magnetite compass have been deduced (Greene & Komeili 2012). Notably, none of these genes appear to have obvious orthologs in animals.
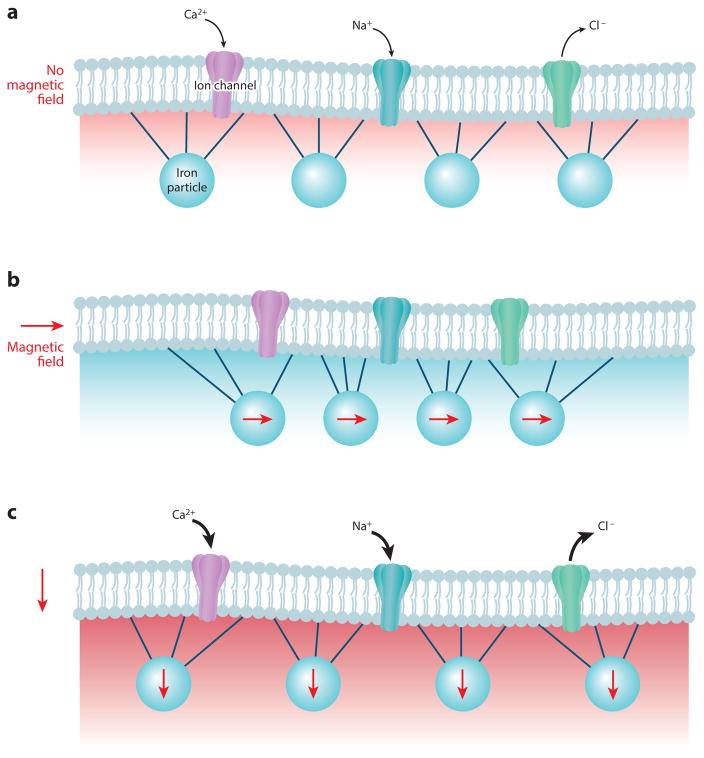
Magnetite may be used in two major ways to transduce magnetic fields. The first model is most intuitive, as it relates to how a piece of iron with a permanent magnetic moment will spin physically in alignment with the earth’s magnetic field, just like a needle rotating in a handheld compass. The iron may be shaped as a bar, akin to a compass needle, or may be more symmetrical but still carry an asymmetrical distribution of charge. A second model proposes the use of superparamagnetic iron molecules to transduce field information. Here, iron molecules with nonpermanent magnetic moments can become transiently magnetized based on surrounding field properties. In both models, specialized cells that carry magnetite particles must transduce this change in magnetization or position of iron molecules into a corresponding change in neuronal activity. To convert the physical movements of the iron complex into an electrochemical signal within the sensory neurons, the magnetite might be positioned within or on the cell, where it could yank or push on an ion channel through direct tethering to the channel or through indirect exertion of tension on the cell membrane (Figure 4). In either case, the magnetically generated force would cause an alteration of channel properties. Winklhofer & Kirschvink (2010) give a detailed account of the biophysical constraints on and expectations for how these mechanisms might operate. A short chain of iron in an earth-strength field of 0.5 Gauss would translate to 1 pN of force applied to an attached mechanoreceptive channel, enough to change the open state probability of an attached channel from 50% to 70% (Corey & Howard 1994). Alternatively, iron may conceivably alter the configuration of an intercellular enzyme whose product alters neuronal activity indirectly.
Figure 4.
Biological magnetic particles associated with ion channels may provide an iron-dependent mechanism for magnetosensation. Iron-containing particles that are physically associated with ion channels may influence the activity of a hypothetical magnetosensory neuron. Iron particles may link directly with the ion channels or with nearby membrane proteins or lipids to alter channel activity. (a) When the magnetic field is in one orientation, the ion channels may conduct a baseline current. (b) When the magnetic field is oriented in a different orientation, the imposed movement on the iron particles may impede channel conductance and change the cell membrane potential. (c) When the magnetic field is in another orientation, the channels may increase conductance. Although progress is being made to engineer analogous magnetosensory ion channels, it remains to be determined whether animals possess similar ion channels associated with magnetic particles for magnetic dependent behaviors. Schematic modified with permission from Johnsen & Lohmann (2008).
Examples are abundant of iron in cells proposed to participate in magnetosensation. Sophisticated approaches have been used to document subcellular formations of iron in cells, including confocal microscopy, magnetic force microscopy, and pulse remagnetization (Diaz-Ricci et al. 1991, Diebel et al. 2000, Wiltschko et al. 2002). An excellent review on these approaches along with their varying strengths and weaknesses has been published recently (Shaw et al. 2015). These putative miniature iron compasses often appear as dark objects within cross sections of cells in scanning or transmission electromicrographs (Fleissner & Fleissner 2010). Unfortunately, doubt has been cast on whether iron in cells from some of these studies represents iron that could sense the earth’s magnetic field (Edelman et al. 2015). Careful analysis found that common techniques used to search for iron particles in cells can be fraught with errors, including contamination by iron and titanium found in laboratories (Edelman et al. 2015). Importantly, although some of these cells can rotate in response to artificial magnetics when dissociated (e.g., Eder et al. 2012), none of the documented iron-containing cells have been tested for physiological responses to magnetic fields yet.
Many researchers appear to be looking within putative magnetosensory neurons for microscopic iron compasses similar to those used in magnetotactic bacteria. However, iron may instead be distributed on or beneath the surface of the complex sensory structures of neurons. The magnetosensory neuron AFD in C. elegans may be a prime example (Figure 3). The morphology of each of the 302 neurons that comprise the worm’s nervous system has been described at the level of electron microscopic reconstruction (White et al. 1986). Most worm sensory neurons have cilia that consist of one or two lobes. The AFD sensory neurons, by contrast, boast a sophisticated structure at the dendritic end that is embedded beneath the cuticle of the anterior tip of the worm. This sensory structure is made up of both a single cilium as well as dorsal and ventral arrays of microvilli that fan in anterior and posterior directions. The AFD sensory neurons were first demonstrated to be required for thermotaxis and sensitive to temperature with calcium imaging and patch-clamp recording (Clark et al. 2006, Kimura et al. 2004, Mori & Ohshima 1995, Ramot et al. 2008). AFD was later also found to be sensitive to carbon dioxide (Bretscher et al. 2011). It has not been obvious why detection of temperature or CO2 would require the unique anterioposterior-directed arrangement of the microvilli. In fact, when isolated in culture, the AFD neurons do not form antenna-shaped microvilli but nevertheless maintain the ability to respond to temperature above a memory of recent temperature, analogous to how AFD appears to function in vivo (Kobayashi et al. 2016). The antenna-shaped sensory structure may instead contribute to magnetosensitivity by acting as a scaffold for an ordered field of magnetized iron distributed over microns. In this model, each microvillus prong in the iron antenna exhibits small rotational changes that combine to produce a larger and possibly synergistic signal throughout the over 50 microvilli by opening mechanoreceptive ion channels. Iron may lie beneath or on the surface of microvilli. Consistent with this theory, dark objects have been reported in and around AFD microvilli (Doroquez et al. 2014). The glial cell that surrounds AFD microvilli, which supports their antennae-shaped distribution, is also required for magnetotaxis in C. elegans (Vidal-Gadea et al. 2015). Future studies need to determine whether iron is associated with AFD microvilli and whether it resides in the AFD cell processes, the surrounding glial cell, or both locations. It is also conceivable that these glia themselves may detect the magnetic field, akin to how Merkel cells have been discovered recently to detect and convey tonic mechanical stimuli through mechanosensory neurons that responded to acute stimuli (Ikeda et al. 2014, Ranade et al. 2014, Woo et al. 2014).
The major criterion for defining an iron-based mechanism for magnetoreception is whether demagnetization with a brief magnetic pulse perturbs magnetic-oriented behavior or physiology. So far, this approach suggests that birds, bees, reptiles, and bats use an iron compass (Holland 2010, Holland & Helm 2013, Holland et al. 2008, Irwin & Lohmann 2005, Kirschvink & Kirschvink 1991, Wiltschko et al. 1998). Because this approach should not affect biochemical-based magnetoreceptive mechanisms, described below, these positive results strongly favor iron-based magnetoreceptive mechanisms.
Biochemical magnetoreception
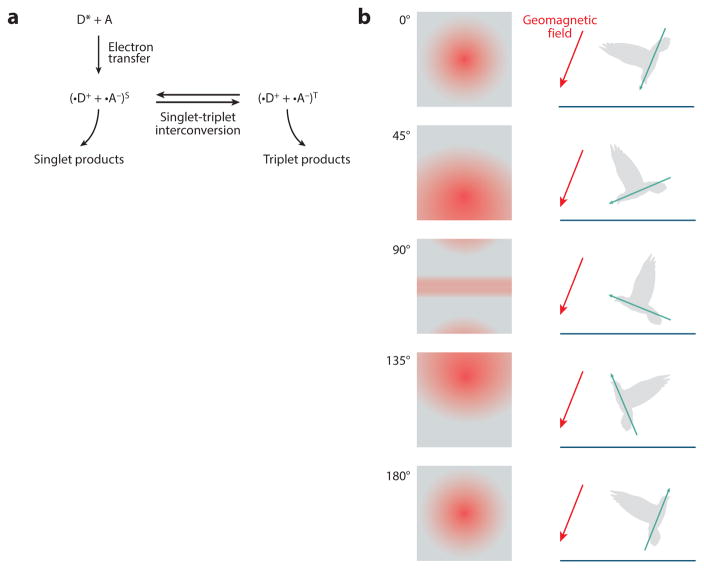
The second, less intuitive major model for magnetoreception is the radical-pair mechanism advanced by Leask (1977) and Schulten et al. (1978). This mechanism involves detecting the earth’s magnetic field via biochemical reactions that are sensitive to magnetic fields (Figure 5a). In this model, an electron exchange occurs between two atoms when the first becomes excited by a stimulus. This electron donation causes a paramagnetic radical pair to form. The newly formed radical pair can then interact with the earth’s magnetic field when in this state. Ritz et al. (2000) provide a more detailed treatment of this process and its biophysical expectations. Note that this mechanism needs an initial excitation step to occur. Light activation of a photosensitive protein is a likely first excitatory step in this transduction mechanism, as evidenced by the light sensitivity of magnetosensation in a variety of organisms (Stapput et al. 2008, 2010; Wiltschko & Wiltschko 1995; Zapka et al. 2009). One candidate in particular, cryptochrome, has accumulated ample evidence for its role in magnetoreception with its light-harvesting cofactor flavin adenine dinucleotide. Detailed reviews have been published recently on how cryptochromes are thought to transform geomagnetic stimuli in birds and insects and how genetic alteration of cryptochromes in Drosophila interferes with magnetic responsive behaviors (Dodson et al. 2013, Ritz et al. 2010). The light-dependent mechanism by which cryptochrome mediates magnetosensation may be more complicated than the initial tryptophan triad-generated radical-pairs model, however, because transgenic versions of Drosophila and monarch cryptochromes lacking this key tryptophan were sufficient to rescue magnetosensitivity (Gegear et al. 2010).
Figure 5.
Cryptochrome provides a chemical-dependent mechanism for magnetosensation. The earth’s magnetic field may be detected by how it influences chemical reactions in sensory neurons. (a) The most promising model suggests that light may alter the propensity of doublet versus triplet reaction products when blue light interacts with the photoenzyme cryptochrome. (b) In this model, cryptochrome-expressing photoreceptors in the eye may visualize aspects of the geomagnetic field superimposed across their field of vision. Note that although this model allows the bird to distinguish its position with respect to the inclination angle of the geomagnetic field, it does not provide accurate information regarding the polarity of the geomagnetic field. Schematic modified with permission from Ritz et al. (2000).
One weakness of the cryptochrome model for magnetic orientation is that cryptochrome reactivity depends on the strength but not the north-south polarity of a magnetic field. Thus, a cryptochrome-based mechanism may be less informative than an iron-based mechanism to detect a compass direction (Figure 5b).
Although various experimental lines have produced solid evidence for cryptochrome as a potential magnetosensor, the precise cells where cryptochrome acts to detect the earth’s magnetic field in animals remain to be determined. In birds, the obvious location is a subset of neurons in the retina that express cryptochromes (Mouritsen et al. 2004). In this model, birds might perceive the earth’s magnetic field as a hazy pattern superimposed over their normal field of vision, analogous to how polarized light appears as horizontal bands when humans view the horizon while wearing sunglasses (Figure 5b). In insects, the locus for cryptochrome-based magnetosensation might be the antennae, which are full of sensory neurons (Reppert et al. 2016). The search for cryptochrome-based magnetosensory neurons might be hampered by the fact that most animals have an expanded array of cryptochrome genes, many of which are expressed widely in neuronal as well as nonneuronal tissue. Future genetic studies on a cryptochrome basis for magnetosensation may also be impeded by the prospect that multiple cryptochromes function redundantly, so that deleting a single cryptochrome gene may produce no obvious deficit in magnetic orientation despite its importance. So far, this is not the case in Drosophila (Gegear et al. 2008).
Although evidence has been gathered for both compass and biochemical reaction models of magnetosensation, healthy debate continues regarding which process may be the most common and whether certain animals use both mechanisms. Strong evidence suggests that some migratory bird species, newts, monarch butterflies, and fruit flies use biochemical magnetoreception (e.g., cryptochrome radical-pair model) because of their light dependency to respond to magnetic cues (Phillips & Borland 1992; Phillips & Sayeed 1993; Wiltschko & Wiltschko 1995, 1996). However, until light-dependent magnetosensory neurons are identified, it will remain difficult to distinguish whether these magnetotransducers require light to function and/or if the sensory neuron or its downstream neural circuitry requires light to function. This is especially questionable for magnetosensitive neurons thought to be in photoreceptive circuits.
Other animals such as mole rats and sea turtles perform magnetic orientation independent of light, suggestive of an iron-based molecular sensor (Wiltschko & Wiltschko 2012). This is also the case for C. elegans, which performs magnetic orientation in the dark (Vidal-Gadea et al. 2015). Different research groups have proposed that birds use both cryptochrome-based and iron-based magnetosensation in different parts of their bodies. Despite the mounting evidence for magnetoreception at many levels of analysis, there is still substantial debate regarding the identity of the primary molecular detector as well as the mechanisms of transducing and encoding magnetic cues. There is such disagreement that some are comfortable ruling out the possibility of radical pair–mediated magnetoreception altogether (Kirschvink et al. 2001).
APPROACHES TO IDENTIFY MAGNETOSENSORY NEURONS AND RECEPTORS
Behavior
Evidence for the presence of magnetosensory neurons and their primary transducers is inferred from the plentiful collection of behavioral studies showing that many animals respond to magnetic fields. The list is too long to categorize here, so we highlight some examples. One classic study found that homing pigeons had their preferred migratory route perturbed predictably when the local geomagnetic field varied (Keeton et al. 1974). Phillips & Sayeed (1993) discovered that they could train male fruit flies to respond to a magnetic field depending on the ambient wavelength of light, providing some of the first evidence for cryptochrome-based magnetosensation. More recently, Lohmann et al. (2004) found that sea turtles artificially displaced on their migrations could compensate in a manner that suggested they had a map sense that was in part based on the local distribution of magnetic field.
Once an experimenter shows that an animal’s behavior correlates with magnetic field cues, a common next step is to build evidence for magnetoreceptivity by altering or overriding magnetic reception with artificial magnets. Often these are placed near the hypothesized structure to interfere selectively with an animal’s sensitivity to the magnetic field. For example, Walcott & Green (1974) found that on overcast days, homing pigeon orientation was perturbed when magnets conflicting with the geomagnetic field were placed on their heads. Others have tried to demagnetize or remagnetize hypothetical iron-based receptors contained in a portion of an animal’s body, an approach first used in magnetotactic bacteria, to ask whether the animal is still capable of magnetic orientation (Kalmijn & Blakemore 1978). For animals believed to sense the field through light-dependent mechanisms, researchers have altered the ability to orient with respect to a magnetic field by altering the spectra of available light (Wiltschko & Wiltschko 1996, Wiltschko et al. 1993). Because all these approaches have distinct advantages and disadvantages, determining the identity of a magnetosensory neuron and its transduction mechanism will require using more than one behavioral approach.
Physiology
After showing that an animal can respond to an aspect of the earth’s magnetic field, the next challenge is to determine which structure is necessary for magnetic orientation. This is most often accomplished by attempting to physically obstruct, pharmacologically block, remove, or damage the proposed structure. Examples include how physical obstruction of the pineal complex (or deeper brain regions such as the hypothalamus) in the eastern red-spotted newt perturbed light-dependent magnetic orientation (Deutschlander et al. 1999). Studies in birds have implicated the beak, retina, and inner ear as possible sites of magnetosensation (Harada et al. 2001, Mora et al. 2004, Ritz et al. 2009, Wu & Dickman 2011). Evidence for the upper beak is provided by Mora et al. (2004), who found that severing the trigeminal nerve in four pigeons stopped their ability to discriminate a magnetic anomaly. Likewise, they found that anesthetic blockade of trigeminal nerve transmission from the upper beak, but not the olfactory nerve, interfered reversibly with the birds’ magnetic discrimination ability. Because European robins display light-dependent magnetic orientation, Möller et al. (2004) suggested that a variety of cryptochromes identified in their retina might play a role in magnetotransduction. Experimental manipulation of specific cryptochromes in bird eyes has not been attempted, however. Evidence for the inner ear acting as a magnetosensory organ comes from intracellular iron observed in otoliths and the cuticular plate inside this organ (Harada et al. 2001, Lauwers et al. 2013). Wu & Dickman (2011) provided indirect functional evidence of the inner ear as a magnetoreceptor by probing immediate-early gene expression in the brains of pigeons exposed to magnetic fields. Not only did they find evidence for activation of brain regions important for directional sense and memory, but many of these regions failed to show magnetic-induced expression when the vestibular lagena was ablated. A study by Zapka et al. (2009) demonstrated that the European robin could no longer orient to a magnetic field when a night-active visual center of the brain called cluster N was lesioned. Interestingly, in contrast to the pigeon, the European robin was still able to orient to a field when its trigeminal nerve was sectioned.
These direct measurements and disruptions of neuronal activity, however, are invasive and require a priori hypotheses about where magnetic field information is processed for behavioral responses. By probing the expression of immediate-early genes, which express upon prolonged neuronal activity, regions across the brain responsive to magnetic fields can be identified in an unbiased and noninvasive way. Studies in the Zambian mole rat using this approach identified magnetic field–induced activity in the superior colliculus (Němec et al. 2001). Similarly, Wu & Dickman (2011) identified sets of neurons in the pigeon vestibular brainstem whose immediate-early gene expression correlated with magnetic field and the presence of intact pigeon lagena receptor organs. Because immediate-early genes appear to function similarly in all animals, this general approach may be used to search for magnetosensory organs and neurons in diverse animals.
Genetics
Genetic approaches to identify magnetotransducers and their neurons have been slow to take off. This is largely due to the fact that most animals in which magnetoreception is well characterized are difficult to manipulate genetically. Nevertheless, recent studies using traditional genetic model animals are proving useful as new approaches to reveal transduction mechanisms and neurons required for magnetosensation. Several studies have found that the fruit fly Drosophila has the ability to detect magnetic fields with innate preference or through associative learning (Phillips & Sayeed 1993). Gegear et al. (2008) provided genetic evidence for a transduction mechanism. They demonstrated that flies were unable to perform an associative learning task involving magnetic field cues when their ultraviolet-A/blue light receptor cryptochrome gene was knocked out. This is some of the most compelling evidence yet that cryptochrome is necessary for a magnetic sense in at least some animals. Now the challenge is to determine which cryptochrome-expressing cells represent the magnetosensory neurons. Cell-specific gene knockdown of cryptochrome may provide an approach to answer this question.
Taking a hint that magnetotactic bacteria use the geomagnetic field to orient their motion roughly up and down along the geomagnetic incline, Vidal-Gadea et al. (2015) recently asked whether the nematode C. elegans was similarly capable of using the geomagnetic field to orient vertically while burrowing. Worms showed no preference for burrowing to either side in agar-filled tubes when they were positioned horizontally but showed a bias to burrow down when tubes were positioned vertically. Evidence that the earth’s magnetic field was used as a cue rather than gravity was provided by several experiments. First, they found that worms would burrow upward if an equal but oppositely directed artificial magnetic field was imposed. Second, they found that Australian worms displayed the opposite burrowing preferences as the common lab strain N2, which was isolated originally in England. Thus, just as magnetotactic bacteria from the Northern and Southern Hemispheres generally migrate vertically in opposite directions when tested in the United States (Blakemore et al. 1980), distinct C. elegans wild strains appear to have a genetic basis for orienting their migrations to the geomagnetic field consistent with their site of isolation.
Vidal-Gadea et al. (2015) next deduced which sensory neurons were required for magnetic orientation by assaying mutants with defects in subsets of sensory neurons. A pattern emerged in which mutation of genes critical for the developmental fate of the sensory neuron pair AFD or its cGMP-dependent signal transduction caused defective magnetic orientation. Further calcium imaging experiments showed that the AFD neurons respond to earth-strength magnetic fields even when isolated synaptically. This response depended on the presence of the cGMP-gated cation channel TAX-4. Owing to the ease of genetic manipulation and behavioral assays in the worm, future studies may quickly test the requirement of hundreds of candidate magnetotransducers and transduction mechanisms.
The genetic approach described above for Drosophila and C. elegans is called reverse genetics because the experimenter must decide which genes to interfere with by mutation. Unfortunately, because the reverse-genetic approach is limited by a priori hypotheses on which molecules to test for their requirement in magnetic responses, it would miss novel molecules involved in magnetosensation. In the future, complementary forward-genetic approaches may be leveraged to screen in an unbiased manner novel or uncharacterized molecules that are required for magnetic responses. Current magnetic responsive assays in fly and worm, although reliable, are unfortunately not robust enough to conduct forward-genetic screens without yielding too many false positives. Until these behavioral assays are optimized for forward-genetic screens, applying reverse genetics to batches of novel and uncharacterized genes expressed in the AFD and cryptochrome-expressing neurons may yield novel insight into magnetoreception and its modulation.
A parallel transgenic approach could be used to test empirically the feasibility of candidate primary magnetotransducers in flies and worms. Here, candidate genes are expressed ectopically in other cells to test whether the encoded molecule is sufficient to confer magnetic responsiveness. By analogy, two of the three receptor-type guanylyl cyclase molecules that appear necessary for magnetoreception in AFD neurons, GCY-18 and GCY-23, were shown recently to confer thermoresponsiveness to other neurons and even muscle cells in C. elegans (Takeishi et al. 2016). The third guanylyl cyclase required for magnetotaxis in worms, GCY-8, was shown recently to be required to maintain the complex antenna-shaped microvilli of the AFD neurons (Singhvi et al. 2016). Thus, it remains to be seen whether GCY-8 has a functional role or only a developmental role in AFD magnetosensation. Soon we will learn whether GCY molecules also convey magnetic stimuli ectopically in ordinary neurons.
The vertebrate genetic model zebrafish may prove to be a useful alternative model to identify magnetosensory neurons. Many fish, including salmon and Mozambique tilapia, perform seasonal migrations, in part by using magnetic cues, but the nonmigratory zebrafish can also be trained to respond to magnetic stimuli (Shcherbakov et al. 2005). The full power of zebrafish genetics has not yet been used to discern magnetosensory neurons and transduction mechanisms. Owing to the compact size of the zebrafish and Drosophila nervous systems, magnetosensory neurons may be identified in these animals by current whole-brain functional imaging techniques (e.g., Chhetri et al. 2015).
Aside from forward and reverse genetics, quantitative genetic techniques may be applied as an orthogonal genetic approach. Here, the genomic loci that contribute to natural variation in behavioral responses across individuals within a species can be identified using quantitative correlative analyses such as quantitative trait locus analyses or genome-wide association studies. C. elegans appears to be an excellent model for this approach because worms isolated from around the world respond differently to magnetic fields (Vidal-Gadea et al. 2015). As mentioned above, a British strain moved in the opposite direction of a strain isolated in Australia, concordant with the pole reversal at their respective sites of origin. Moreover, when assayed in an agar plate with the field in parallel to the substrate, rather than simply moving toward magnetic north or south, strains from England, Hawaii, and Australia migrated at angles with respect to a magnetic field that would optimize their vertical migration at their site of isolation. Lastly, the magnetotaxis performance of 11 wild isolates correlated with the strength of the geomagnetic field at their point of isolation. Together, these results suggest that there is natural standing variation in how wild C. elegans isolates respond to a magnetic field. For the first time, a quantitative genetic approach may be used to identify the underlying architecture of magnetoreception.
In vitro Analyses
In the search for a magnetotransducer, other researchers have started with in vitro analyses of molecules rather than with animals. A recent study by Qin et al. (2016) used a bioinformatic approach to predict which subset of proteins interacts with cryptochrome, binds iron, and is expressed in the head of Drosophila. They focused on one protein, MagR, which, when viewed with electron microscopy, forms rod-shaped structures with cryptochrome. Although it remains to be seen whether mutation of MagR interferes with magnetic responsive behaviors in Drosophila, Long et al. (2015) found that ectopic expression of MagR may confer magnetoresponsive calcium activity in C. elegans touch neurons in vivo and in transfected mammalian cells. Doubt has been cast on the hypothesized magnetotransduction mechanism in these studies, however, because fundamental physics predicts that the amount of iron believed to associate with the MagR/Cry complex would not form a permanent dipole moment that could be influenced by earth-strength or typical artificial magnetic stimuli (Meister 2016). This approach to start with candidate iron-binding molecules rather than identifying magnetosensory neurons, however, represents a promising complementary strategy to reveal potential magnetotransducers in animals and perhaps develop molecular tools for the emerging field of magnetogenetics.
Future Prospects
Novel techniques to study the activity and molecular constituents of neurons are opening new avenues to search for magnetosensory neurons and their magnetotransducers. Here are some directions to look in the near future.
Researchers have tracked the expression of immediate-early genes in the brains of birds as an indicator of which neural pathways may be active in response to magnetic fields (e.g., Wu & Dickman 2011). Future studies may take this powerful approach a step further with new imaging and transgenic techniques in animals traditionally favored in magnetic orientation research. For example, researchers have used viruses to transfect the brains of mice with transgenes that will express only fluorescent reporters driven by an immediate-early gene promoter (Renier et al. 2016, Ye et al. 2016). Mice are then sacrificed just as they perform a behavior and subsequently imaged for whole-brain connectivity using the CLARITY method. With this sophisticated method, the activity of intact whole-brain circuits of many animals can be visualized conveniently for different behavioral responses at the single-cell level. Future studies in magnetosensation will surely benefit from similar approaches.
Another way to identify magnetosensory neurons is to literally sift for them. Progress in maintaining the health of cells while sorting them via fluorescent-activated cell sorting may allow the detection of magnetosensitive neurons dissociated from tissue suspected of containing a hidden sensor. The activity of sorted cells may be observed with antibody labeling of immediate-early genes (Guez-Barber et al. 2011). Neurons that display fluorescence induced by a magnetic field can be sorted and investigated at the levels of their transcriptome with RNA sequencing and proteome with mass spectrometry to determine candidate magnetotransducers from molecules enriched in magnetosensitive neurons.
Serious studies led by Kirschvink are beginning to investigate the human potential to sense magnetic fields (described by Hand 2016). Although we may not consciously perceive the geomagnetic field, we may unknowingly take advantage of it for our own direction sensitivity (Baker 1989), and human cryptochrome is sensitive to the earth’s magnetic field (Foley et al. 2011). To peer into the unconscious brain, Kirschvink and colleagues are testing whether randomly imposed earth-strength magnetic fields alter brain function predictably as revealed by electroencephalogram recordings. In this way, the human subject and experimenter can both be blind to treatment while results are analyzed objectively afterward. These and other physiological investigations may provide evidence for a possible human magnetic sense.
CONCLUDING REMARKS
With the cumulative strengths of protein biochemistry, molecular biology, behavioral genetics, and cellular physiology, sensory neuroscientists have witnessed the discovery of a collection of primary detectors for many of the major sensory modalities. In a general order of discovery, this includes light, olfaction, taste, osmolality, temperature, pain, touch, and hearing. The primary detector for the earth’s magnetic field remains undiscovered. With the identification of the first magnetosensory neurons in C. elegans, compelling evidence for cryptochrome as a potential magnetosensitive molecule in Drosophila and butterflies, and a growing kit of genetic tools to target specific cells and molecules in any animal, the field of magnetic orientation is honing in on answers.
Acknowledgments
We thank Sarah Nordquist for helpful comments on the manuscript and Andres Vidal-Gadea for composing the figure of the AFD neuron pair. Graphics were prepared by Samantha Peters and Jenna Luecke. This work was supported by National Institute of Neurological Disorders and Stroke grant NS075541 to J.T.P.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Baker RR. Human Navigation and Magnetoreception. Manchester, UK/New York: Manchester Univ. Press; 1989. [Google Scholar]
- Blakemore RP. Magnetotactic bacteria. Science. 1975;190(4212):377–79. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- Blakemore RP, Frankel RB, Kalmijn AJ. South-seeking magnetotactic bacteria in the Southern Hemisphere. Nature. 1980;286:384–85. [Google Scholar]
- Block SM. Biophysical principles of sensory transduction. In: Corey DP, Roper SD, editors. Sensory Transduction. New York: Rockefeller Univ. Press; 1992. pp. 1–18. [PubMed] [Google Scholar]
- Bretscher AJ, Kodama-Namba E, Busch KE, Murphy RJ, Soltesz Z, et al. Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron. 2011;69(6):1099–113. doi: 10.1016/j.neuron.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore AR, Jarillo JA, Wu Y-J, Liu D. Cryptochromes: blue light receptors for plants and animals. Science. 1999;284:760–65. doi: 10.1126/science.284.5415.760. [DOI] [PubMed] [Google Scholar]
- Chhetri RK, Amat F, Wan Y, Höckendorf B, Lemon WC, Keller PJ. Whole-animal functional and developmental imaging with isotropic spatial resolution. Nat Methods. 2015;12(12):1171–78. doi: 10.1038/nmeth.3632. [DOI] [PubMed] [Google Scholar]
- Clark DA, Biron D, Sengupta P, Samuel AD. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J Neurosci. 2006;26(28):7444–51. doi: 10.1523/JNEUROSCI.1137-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D, Howard J. Models for ion channel gating with compliant states. Biophys J. 1994;66:1254–57. doi: 10.1016/S0006-3495(94)80909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaine C, Semm P. The avian pineal gland as an independent magnetic sensor. Neurosci Lett. 1985;62(1):119–22. doi: 10.1016/0304-3940(85)90294-0. [DOI] [PubMed] [Google Scholar]
- Diaz-Ricci JC, Woodford BJ, Kirschvink JL, Hoffman MR. Alteration of the magnetic properties of Aquaspirillum magnetotacticum by a pulse magnetization technique. Appl Environ Microbiol. 1991;57:3248–54. doi: 10.1128/aem.57.11.3248-3254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebel CE, Proksch R, Green CR, Nielson P, Walker MM. Magnetite defines a vertebrate magnetoreceptor. Nature. 2000;406:299–302. doi: 10.1038/35018561. [DOI] [PubMed] [Google Scholar]
- Deutschlander ME, Borland SC, Phillips JB. Extraocular magnetic compass in newts. Nature. 1999;400(6742):324–25. doi: 10.1038/22450. [DOI] [PubMed] [Google Scholar]
- Dodson CA, Hore PJ, Wallace MI. A radical sense of direction: signalling and mechanism in cryptochrome magnetoreception. Trends Biochem Sci. 2013;38(9):435–46. doi: 10.1016/j.tibs.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Doroquez DB, Berciu C, Anderson JR, Sengupta P, Nicastro D. A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in Caenorhabditis elegans. eLife. 2014;3:e01948. doi: 10.7554/eLife.01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman NB, Fritz T, Nimpf S, Pichler P, Lauwers M, et al. No evidence for intracellular magnetite in putative vertebrate magnetoreceptors identified by magnetic screening. PNAS. 2015;112(1):262–67. doi: 10.1073/pnas.1407915112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder SH, Cadiou H, Muhamad A, McNaughton PA, Kirschvink JL, Winklhofer M. Magnetic characterization of isolated candidate vertebrate magnetoreceptor cells. PNAS. 2012;109(30):12022–27. doi: 10.1073/pnas.1205653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels S, Schneider NL, Lefeldt N, Hein CM, Zapka M, et al. Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory bird. Nature. 2014;509(7500):353–56. doi: 10.1038/nature13290. [DOI] [PubMed] [Google Scholar]
- Falkenberg G, Fleissner G, Schuchardt K, Kuehbacher M, Thalau P, et al. Avian magnetoreception: elaborate iron mineral containing dendrites in the upper beak seem to be a common feature of birds. PLOS ONE. 2009;5(2):e9231. doi: 10.1371/journal.pone.0009231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner G, Fleissner G. Encyclopedia of Animal Behavior. Vol. 1. Cambridge, MA: Academic; 2010. Magnetoreception; pp. 324–35. [Google Scholar]
- Fleissner G, Holtkamp-Rötzler E, Hanzlik M, Winklhofer M, Fleissner G, et al. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J Comp Neurol. 2003;458:350–60. doi: 10.1002/cne.10579. [DOI] [PubMed] [Google Scholar]
- Foley LE, Gegear RJ, Reppert SM. Human cryptochrome exhibits light-dependent magnetosensitivity. Nat Commun. 2011;2:356. doi: 10.1038/ncomms1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegear RJ, Casselman A, Waddell S, Reppert SM. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature. 2008;454(7207):1014–18. doi: 10.1038/nature07183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegear RJ, Foley LE, Casselman A, Reppert SM. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature. 2010;463(7282):804–7. doi: 10.1038/nature08719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould JL. Magnetoreception. Curr Biol. 2010;20(10):R431–35. doi: 10.1016/j.cub.2010.03.045. [DOI] [PubMed] [Google Scholar]
- Greene SE, Komeili A. Biogenesis and subcellular organization of the magnetosome organelles of magnetotactic bacteria. Curr Opin Cell Biol. 2012;24(4):490–95. doi: 10.1016/j.ceb.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guez-Barber D, Fanous S, Golden SA, Schrama R, Koya E, et al. FACS identifies unique cocaine-induced gene regulation in selectively activated adult striatal neurons. J Neurosci. 2011;31(11):4251–59. doi: 10.1523/JNEUROSCI.6195-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand E. What and where are the body’s magnetometers? Science. 2016;352(6293):1510–11. doi: 10.1126/science.352.6293.1510. [DOI] [PubMed] [Google Scholar]
- Harada Y, Taniguchi M, Namatame H, Iida A. Magnetic materials in otoliths of bird and fish lagena and their function. Acta Otolaryngol. 2001;121(5):590–95. [PubMed] [Google Scholar]
- Holland RA. Differential effects of magnetic pulses on the orientation of naturally migrating birds. J R Soc Interface. 2010;7(52):1617–25. doi: 10.1098/rsif.2010.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland RA, Helm B. A strong magnetic pulse affects the precision of departure direction of naturally migrating adult but not juvenile birds. J R Soc Interface. 2013;10(81):20121047. doi: 10.1098/rsif.2012.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland RA, Kirschvink JL, Doak TG, Wikelski M. Bats use magnetite to detect the earth’s magnetic field. PLOS ONE. 2008;3(2):e1676. doi: 10.1371/journal.pone.0001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell. 2014;157(3):664–75. doi: 10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin WP, Lohmann KJ. Disruption of magnetic orientation in hatchling loggerhead sea turtles by pulsed magnetic fields. J Comp Physiol A. 2005;191(5):475–80. doi: 10.1007/s00359-005-0609-9. [DOI] [PubMed] [Google Scholar]
- Johnsen S, Lohmann KJ. Magnetoreception in animals. Phys Today. 2008;61(3):29–35. [Google Scholar]
- Kalmijn AJ, Blakemore RP. The magnetic behavior of mud bacteria. In: Schmidt-Koenig K, Keeton WT, editors. Animal Migration, Navigation, and Homing. Berlin: Springer; 1978. pp. 354–55. [Google Scholar]
- Keeton WT, Larkin TS, Windsor DM. Normal fluctuations in the earth’s magnetic field influence pigeon orientation. J Comp Physiol. 1974;95:95–103. [Google Scholar]
- Kimura KD, Miyawaki A, Matsumoto K, Mori I. The C. elegans thermosensory neuron AFD responds to warming. Curr Biol. 2004;14(14):1291–95. doi: 10.1016/j.cub.2004.06.060. [DOI] [PubMed] [Google Scholar]
- Kirschvink JL. Magnetite biomineralization and geomagnetic sensitivity in higher animals: an update and recommendations for future study. Bioelectromagnetics. 1989;10(3):239–59. doi: 10.1002/bem.2250100304. [DOI] [PubMed] [Google Scholar]
- Kirschvink JL. Sensory biology: Radio waves zap the biomagnetic compass. Nature. 2014;509:296–97. doi: 10.1038/nature13334. [DOI] [PubMed] [Google Scholar]
- Kirschvink JL, Gould JL. Biogenic magnetite as a basis for magnetic field detection in animals. Biosystems. 1981;13(3):181–201. doi: 10.1016/0303-2647(81)90060-5. [DOI] [PubMed] [Google Scholar]
- Kirschvink JL, Kirschvink AK. Is geomagnetic sensitivity real? Replication of the Walker-Bitterman magnetic conditioning experiment in honey bees. Am Zool. 1991;31:169–85. [Google Scholar]
- Kirschvink JL, Walker MM, Diebel CE. Magnetite-based magnetoreception. Curr Opin Neurobiol. 2001;11(4):462–67. doi: 10.1016/s0959-4388(00)00235-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Nakano S, Amano M, Tsuboi D, Nishioka T, et al. Single-cell memory regulates a neural circuit for sensory behavior. Cell Rep. 2016;14(1):11–21. doi: 10.1016/j.celrep.2015.11.064. [DOI] [PubMed] [Google Scholar]
- Lauwers M, Pichler P, Edelman NB, Resch GP, Ushakova L, et al. An iron-rich organelle in the cuticular plate of avian hair cells. Curr Biol. 2013;23(10):924–29. doi: 10.1016/j.cub.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Leask MJM. A physicochemical mechanism for magnetic field detection by migratory birds and homing pigeons. Nature. 1977;267:144–45. doi: 10.1038/267144a0. [DOI] [PubMed] [Google Scholar]
- Lèfevre CT, Bazylinski DA. Ecology, diversity, and evolution of magnetotactic bacteria. Microbiol Mol Biol Rev. 2013;77(3):497–526. doi: 10.1128/MMBR.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Bazylinski DA, Xiao T, Wu LF, Pan Y. Life with compass: diversity and biogeography of magnetotactic bacteria. Environ Microbiol. 2014;16(9):2646–58. doi: 10.1111/1462-2920.12313. [DOI] [PubMed] [Google Scholar]
- Lohmann KJ, Cain SD, Dodge SA, Lohmann CMF. Regional magnetic fields as navigational markers for sea turtles. Science. 2001;294(5541):364–66. doi: 10.1126/science.1064557. [DOI] [PubMed] [Google Scholar]
- Lohmann KJ, Lohmann CM, Ehrhart LM, Bagley DA, Swing T. Animal behaviour: geomagnetic map used in sea-turtle navigation. Nature. 2004;428(6986):909–10. doi: 10.1038/428909a. [DOI] [PubMed] [Google Scholar]
- Lohmann KJ, Willows AO. Lunar-modulated geomagnetic orientation by a marine mollusk. Science. 1987;235(4786):331–34. doi: 10.1126/science.3798115. [DOI] [PubMed] [Google Scholar]
- Lohmann KJ, Willows AO, Pinter RB. An identifiable molluscan neuron responds to changes in earth-strength magnetic fields. J Exp Biol. 1991;161:1–24. doi: 10.1242/jeb.161.1.1. [DOI] [PubMed] [Google Scholar]
- Long X, Ye J, Zhao D, Zhang SJ. Magnetogenetics: remote non-invasive magnetic activation of neuronal activity with a magnetoreceptor. Sci Bull. 2015;60:2107–19. doi: 10.1007/s11434-015-0902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S, Sparks NH, Walker MM, Kirschvink JL. Ultrastructure, morphology and organization of biogenic magnetite from sockeye salmon, Oncorhynchus nerka: implications for magnetoreception. J Exp Biol. 1988;140:35–49. doi: 10.1242/jeb.140.1.35. [DOI] [PubMed] [Google Scholar]
- Martin P, Hudspeth AJ. Active hair-bundle movements can amplify a hair cell’s response to oscillatory mechanical stimuli. PNAS. 1999;96(25):14306–11. doi: 10.1073/pnas.96.25.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M. Physical limits to magnetogenetics. eLife. 2016;5:e17210. doi: 10.7554/eLife.17210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller A, Sagasser S, Wiltschko W, Schierwater B. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften. 2004;91(12):585–88. doi: 10.1007/s00114-004-0578-9. [DOI] [PubMed] [Google Scholar]
- Mora CV, Davison M, Wild JM, Walker MM. Magnetoreception and its trigeminal mediation in the homing pigeon. Nature. 2004;432(7016):508–11. doi: 10.1038/nature03077. [DOI] [PubMed] [Google Scholar]
- Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376(6538):344–48. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- Mouritsen H, Janssen-Bienhold U, Liedvogel M, Feenders G, Stalleicken J, et al. Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. PNAS. 2004;101:14294–99. doi: 10.1073/pnas.0405968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Němec P, Altmann J, Marhold S, Burda H, Oelschläger HHA. Neuroanatomy of magnetoreception: the superior colliculus involved in magnetic orientation in a mammal. Science. 2001;294(5541):366–68. doi: 10.1126/science.1063351. [DOI] [PubMed] [Google Scholar]
- Phillips JB, Borland C. Behavioural evidence for use of a light-dependent magnetoreception mechanism by a vertebrate. Nature. 1992;359:142–44. [Google Scholar]
- Phillips JB, Sayeed O. Wavelength-dependent effects of light on magnetic compass orientation in Drosophila melanogaster. J Comp Physiol. 1993;172:303–8. doi: 10.1007/BF00216612. [DOI] [PubMed] [Google Scholar]
- Qin S, Yin H, Yang C, Dou Y, Liu Z, et al. A magnetic protein biocompass. Nat Mater. 2016;15(2):217–26. doi: 10.1038/nmat4484. [DOI] [PubMed] [Google Scholar]
- Ramot D, MacInnis BL, Goodman MB. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat Neurosci. 2008;11(8):908–15. doi: 10.1038/nn.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014;516(7529):121–25. doi: 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, et al. Mapping of brain activity by automated volume analysis of immediate early genes. Cell. 2016;165(7):1789–802. doi: 10.1016/j.cell.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Gegear RJ, Merlin C. Navigational mechanisms of migrating monarch butterflies. Trends Neurosci. 2010;33(9):399–406. doi: 10.1016/j.tins.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Guerra PA, Merlin C. Neurobiology of monarch butterfly migration. Annu Rev Entomol. 2016;61:25–42. doi: 10.1146/annurev-ento-010814-020855. [DOI] [PubMed] [Google Scholar]
- Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J. 2000;78(2):707–18. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T, Dommer DH, Phillips JB. Shedding light on vertebrate magnetoreception. Neuron. 2002;34:503–6. doi: 10.1016/s0896-6273(02)00707-9. [DOI] [PubMed] [Google Scholar]
- Ritz T, Wiltschko R, Hore PJ, Rodgers CT, Stapput K, et al. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys J. 2009;96(8):3451–57. doi: 10.1016/j.bpj.2008.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T, Yoshii T, Helfrich-Foerster C, Ahmad M. Cryptochrome: a photoreceptor with the properties of a magnetoreceptor? Commun Integr Biol. 2010;3(1):24–27. doi: 10.4161/cib.3.1.9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulten K, Swenberg CE, Weller A. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z Phys Chem. 1978;111(1):1–5. [Google Scholar]
- Semm P, Demaine C. Neurophysiological properties of magnetic cells in the pigeon’s visual system. J Comp Physiol A. 1986;159(5):619–25. doi: 10.1007/BF00612035. [DOI] [PubMed] [Google Scholar]
- Shaw J, Boyd A, House M, Woodward R, Mathes F, et al. Magnetic particle-mediated magnetoreception. J R Soc Interface. 2015;12(110):0499. doi: 10.1098/rsif.2015.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbakov D, Winklhofer M, Petersen N, Steidle J, Hilbig R, Blum M. Magnetosensation in zebrafish. Curr Biol. 2005;15(5):R161–62. doi: 10.1016/j.cub.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Singhvi A, Liu B, Friedman CJ, Fong J, Lu Y, et al. A glial K/Cl transporter controls neuronal receptive ending shape by chloride inhibition of an rGC. Cell. 2016;165(4):936–48. doi: 10.1016/j.cell.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solov’yov I, Greiner W. Theoretical analysis of an iron mineral-based magnetoreceptor in birds. Biophys J. 2007;93:1493–509. doi: 10.1529/biophysj.107.105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapput K, Güntürkün O, Hoffmann KP, Wiltschko R, Wiltschko W. Magnetoreception of directional information in birds requires nondegraded vision. Curr Biol. 2010;20(14):1259–62. doi: 10.1016/j.cub.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Stapput K, Thalau P, Wiltschko R, Wiltschko W. Orientation of birds in total darkness. Curr Biol. 2008;18(8):602–6. doi: 10.1016/j.cub.2008.03.046. [DOI] [PubMed] [Google Scholar]
- Takeishi A, Yu YV, Hapiak VM, Bell HW, O’Leary T, Sengupta P. Receptor-type guanylyl cyclases confer thermosensory responses in C. elegans. Neuron. 2016;90(2):235–44. doi: 10.1016/j.neuron.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley JN, Molodtsov MI, Prevedel R, Wartmann D, Espigulé-Pons J, et al. Direct detection of a single photon by humans. Nat Commun. 2016;7:12172. doi: 10.1038/ncomms12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gadea A, Ward K, Beron C, Ghorashian N, Gokce S, et al. Magnetosensitive neurons mediate geomagnetic orientation in Caenorhabditis elegans. eLife. 2015;4:e07493. doi: 10.7554/eLife.07493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott C, Green RP. Orientation of homing pigeons altered by a change in the direction of an applied magnet field. Science. 1974;184:180–82. doi: 10.1126/science.184.4133.180. [DOI] [PubMed] [Google Scholar]
- Walker MM, Dennis TE, Kirschvink JL. The magnetic sense and its use in long-distance navigation by animals. Curr Opin Neurobiol. 2002;12:735–44. doi: 10.1016/s0959-4388(02)00389-6. [DOI] [PubMed] [Google Scholar]
- Wang JH, Cain SD, Lohmann KJ. Identifiable neurons inhibited by Earth-strength magnetic stimuli in the mollusc Tritonia diomedea. J Exp Biol. 2004;207(Pt. 6):1043–49. doi: 10.1242/jeb.00864. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc B. 1986;314(1165):1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wiltschko R, Wiltschko W. Magnetoreception. Adv Exp Med Biol. 2012;739:126–41. doi: 10.1007/978-1-4614-1704-0_8. [DOI] [PubMed] [Google Scholar]
- Wiltschko W, Munro U, Ford H, Wiltschko R. Red light disrupts magnetic orientation of migratory birds. Nature. 1993;364:525–27. [Google Scholar]
- Wiltschko W, Munro U, Ford H, Wiltschko R. Effect of a magnetic pulse on the orientation of silvereyes, Zosterops l. lateralis, during spring migration. J Exp Biol. 1998;201(Pt. 23):3257–61. doi: 10.1242/jeb.201.23.3257. [DOI] [PubMed] [Google Scholar]
- Wiltschko W, Munro U, Wiltschko R, Kirschvink J. Magnetite-based magnetoreception in birds: the effect of a biasing field and a pulse on migratory behavior. J Exp Biol. 2002;205:3031–37. doi: 10.1242/jeb.205.19.3031. [DOI] [PubMed] [Google Scholar]
- Wiltschko W, Wiltschko R. Migratory orientation of European robins is affected by the wavelength of light as well as by a magnetic pulse. J Comp Physiol. 1995;177:363–69. [Google Scholar]
- Wiltschko W, Wiltschko R. Magnetic orientation in birds. J Exp Biol. 1996;199(Pt. 1):29–38. doi: 10.1242/jeb.199.1.29. [DOI] [PubMed] [Google Scholar]
- Wiltschko W, Wiltschko R. Magnetic orientation and magnetoreception in birds and other animals. J Comp Physiol A. 2005;191(8):675–93. doi: 10.1007/s00359-005-0627-7. [DOI] [PubMed] [Google Scholar]
- Winklhofer M, Kirschvink JL. A quantitative assessment of torque-transducer models for magnetoreception. J R Soc Interface. 2010;7(Suppl 2):S273–89. doi: 10.1098/rsif.2009.0435.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509(7502):622–26. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LQ, Dickman JD. Magnetoreception in an avian brain in part mediated by inner ear lagena. Curr Biol. 2011;21(5):418–23. doi: 10.1016/j.cub.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LQ, Dickman JD. Neural correlates of a magnetic sense. Science. 2012;336(6084):1054–57. doi: 10.1126/science.1216567. [DOI] [PubMed] [Google Scholar]
- Ye L, Allen WE, Thompson KR, Tian Q, Hsueh B, et al. Wiring and molecular features of prefrontal ensembles representing distinct experiences. Cell. 2016;165(7):1776–88. doi: 10.1016/j.cell.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapka M, Heyers D, Hein CM, Engels S, Schneider N, et al. Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature. 2009;461:1274–77. doi: 10.1038/nature08528. [DOI] [PubMed] [Google Scholar]