Abstract
Introduction
Symptoms and signs in women with Charcot–Marie–Tooth disease type 1X (CMT1X) are often milder from those in men, but the available electrophysiologic evidence regarding CMT1X in women have been characterized in some patients as non-uniform or asymmetric.
Methods
We retrospectively reviewed electrodiagnostic findings from 45 women and 31 men with CMT1X.
Results
Motor nerve conduction parameters in CMT1X women were less abnormal (P <0.05), and a wider range of motor conduction velocities (CVs) were seen in women (P <0.001) compared with men. In women, nerve conduction studies showed only lack of conduction block without temporal dispersion. Motor CVs were more frequently in the normal range in women compared with men. There was no significant relationship to age of presentation and motor CV or motor compound muscle action potential in women.
Conclusion
NCS parameters in CMT1X women did not demonstrate features suggestive of acquired demyelinating neuropathy.
Keywords: CMT1X, EDx, nerve conduction studies, women, X-inactivation
Charcot–Marie–Tooth disease type IX (CMT1X) is an X-linked dominant disorder and the second most common inherited peripheral neuropathy caused by mutations in the gap junction beta 1 (GJB1) gene, which is located on the X chromosome and encodes the gap junction protein connexin32 (Cx32).1 Whereas men with CMT1X consistently present with a uniform progressive phenotype characterized by difficulty walking, distal weakness, and sensory loss, women present with a variable phenotype (a small proportion are asymptomatic, approximately two-thirds have a mild, non-progressive phenotype, and approximately one-third have a relatively severe progressive phenotype).2 Indeed, the CMT1X phenotype is usually milder in women than in men; exceptions include cases of certain mutations, such as one that results in the Phe235Cys substitution and leads to a severe neuropathy.3 The gender difference in most forms of CMTIX has been attributed to X-inactivation in women, who have normal expression of Cx32 from the wild-type X chromosome in a subset of tissues.2
With the advent of a wide variety of genetic testing for diagnosis of Charcot–Marie–Tooth (CMT) disease, choosing the appropriate genetic test will avoid unnecessary costs. Electrodiagnostic (EDx) testing guides genetic testing by categorizing CMT subtypes into axonal, demyelinating, or intermediate based on nerve conduction studies (NCS); however, electrophysiological characterization of CMT1X in women based on NCS is uncertain, because the electrophysiological features of women with CMT1X are different from those in men.4 In a study carried out by Birouk and colleagues, 90% (n =21) of men with CMT1X exhibited slowed motor nerve conduction (intermediate velocities of 30–40 m/s), but only 40% (n =27) of women exhibited slowing.5 Compared with men, women had a wide range of nerve conduction velocities (CVs), from mild slowing to normal velocities.5 Another study likewise reported that a smaller percentage of women featured slowing, but in that case the degree of heterogeneity of motor conduction between nerves (28–50 m/s) was higher.6 Consistent with these observations, other studies in women have shown that conduction slowing is often non-uniform4,7–10 and asymmetric,11 with the median nerve more affected than the ulnar nerve.12 These studies suggest that EDx studies in women with CMT1X have features similar to those seen in an acquired demyelinating neuropathy, such as chronic inflammatory demyelinating polyneuropathy (CIDP). CIDP is an autoimmune disease that targets peripheral nerve sheath myelin. The diagnosis of CIDP is often difficult to make because it is multifocal and has a predilection for proximal nerve segments. EDx criteria of CIDP include electrophysiological changes of slowing of CV, temporal dispersion, conduction block, and prolonged F-wave and distal latencies.13 We hypothesized that, in women, CMT1X may look electrophysiologically like CIDP. We wanted to determine how often this was the case in our experience. To this end, we reviewed the EDx data from a cohort of 45 women and 31 men with CMT1X who had been evaluated at Wayne State University and the University of Iowa between 1994 and 2014.
METHODS
Subjects and Chart Review
This study was approved by the institutional review board. EDx data from 50 women and 33 men previously diagnosed with CMT1X were reviewed retrospectively. The women were evaluated in the CMT clinic at Wayne State University or the University of Iowa by a highly experienced neurologist (M.E.S). Patients had been diagnosed as having CMT1X if they had any of the following: (1) a positive genetic test result (performed by Athena Diagnostics, Worchester, Massachusetts); (2) they were obligate carriers of CMT1X (daughter of an affected man); or (3) they had a first- or second-degree relative who had a positive genetic test and had a CMT phenotype.2 Among the 50 women in the data set, 1 had diabetes, 1 had CMT type 1B, and 3 had not undergone EDx and were therefore excluded from analysis. Thus, a total of 45 women were evaluated. Similarly, 2 men had not undergone EDx. Thus, a total of 31 men without diabetes or other conditions causing a neuropathy were reviewed.
Motor nerve conduction studies (MNCS) and sensory nerve conduction studies (SNCS) (including the left median, ulnar, fibular, and sural nerves) had been performed using conventional methods (Nicolet Viking, Synergy EMG, or Nihon Kohden system). Skin temperature was maintained at >32 °C to ensure accuracy in NCS. Surface electrodes were used in all studies. SNCS were performed using anti-dromic techniques. By convention, median compound muscle action potentials (CMAPs) were recorded from the abductor pollicis brevis (APB), and ulnar CMAPs were recorded from the abductor digiti minimi (ADM). The fibular CMAPs were recorded from the extensor digitorum brevis (EDB). In addition, fibular nerve studies recording from the tibialis anterior (TA) were performed using distal stimulation at the fibular head and proximal stimulation at the popliteal fossa. The following variables were obtained from the chart review: CMAP; SNAP (sensory nerve action potential); CV; distal motor latency (DML); and distal sensory latency (DSL). Normal NCS values were derived from normal values in the electromyography (EMG) labs. F-waves were not evaluated in this study. Partial motor conduction block was defined as follows: a ≥50% amplitude reduction of the proximal negative peak CMAP relative to the distal, if distal negative peak CMAP was ≥ 20% of the lower limit of normal.14
We divide asymmetries into 2 categories within an individual. One between various nerves such as the median and ulnar nerves; we term this “asymmetry between nerves.” The other asymmetry is along the length of an individual nerve evidenced by conduction block and/or temporal dispersion, and we term this “non-uniformity.”
Statistical Analysis
Percentages and means were compared using the Student t-test, and differences were considered significant at P <0.05. MNCS and SNCS were compared among the 4 nerves, with the responses categorized as abnormal or normal. This was done using a generalized linear mixed model for a logit link function. To adjust for the multiple tests, pairwise comparison between the nerves was performed using the Tukey test (Table 3). The estimates of the mean proportion of abnormal MNCS are presented in Table 1. NCS with no response (NR) were recorded as NR; these “NR” values were not used in calculating the mean, but they were used in calculating the percent abnormal. NCS of women and men with CMT1X were compared using a 2-sided t-test. An unpaired Student t-test was used to compare those unaffected to the remaining age groups (Table 5).
Table 3.
Comparison of MNCS in upper and lower limbs of women with CMT1X
| Variable | Nerve | Proportion abnormal (%) | Tukey adjusted P-value
|
||
|---|---|---|---|---|---|
| Vs. median | Vs. ulnar | Vs. fibular EDB | |||
| CMAP | Median (≤4) | 37% | — | — | — |
| Ulnar (≤6) | 42% | 1 | — | — | |
| Fibular EDB (≤3) | 85% | 0.0002‡ | 0.0005‡ | — | |
| Fibular TA (≤5) | 90% | 0.002‡ | 0.005‡ | 0.9 | |
| CV | Median (≤48) | 55% | — | — | — |
| Ulnar (≤49) | 42% | 0.5 | — | — | |
| Fibular EDB (≤41) | 70% | 0.4 | 0.02* | — | |
| Fibular TA (≤44) | 39% | 0.5 | 1.0 | 0.04* | |
| DML | Median (≤4.5) | 33% | — | — | — |
| Ulnar (≤3.5) | 23% | 0.5 | — | — | |
| Fibular EDB (≤5.5) | 42% | 0.7 | 0.1 | — | |
| Fibular TA (≤6.7) | 0% | 0.008‡ | 0.08 | 0.002‡ | |
The fibular CMAPs recorded from EDB and TA were more frequently affected than median and ulnar CMAPs (P <0.01).
P <0.05;
P <0.01;
P<0.001.
Table 1.
MNCS in women and men with CMT1X.
| Nerve | Normal value | CMT1X women | Percent abnormal | CMT1X men | Percent abnormal |
|---|---|---|---|---|---|
| Median nerve | n =41 | n =30 | |||
| MNCV (m/s) Range | >48 | 46 ±1.4‡ | 55%‡ | 35 ±1.5 | 100% |
| Range | 29–59‡ | 21–44 | |||
| CMAP (mV) | >4 | 5 ±0.5* | 37%‡ | 3 ±0.6 | 77% |
| DML (ms) | <4.5 | 4 ±0.3 | 33%‡ | 5 ±0.3 | 73% |
| No response | 3‡ | 7%‡ | 10 | 33% | |
| Ulnar nerve | n =44 | n =29 | |||
| MNCV (m/s) | >49 | 47 ±1.9‡ | 42%‡ | 34 ±1.7 | 100% |
| Range | 25–64‡ | 24–46 | |||
| CMAP (mV) | >6 | 6 ±0.3‡ | 42%‡ | 4 ±0.5 | 79% |
| DML (ms) | <3.5 | 3 ±0.1‡ | 23%‡ | 4 ±0.2 | 86% |
| No response | 0 | 0% | 2 | 7% | |
| Fibular nerve, record EDB | n =40 | n =29 | |||
| MNCV (m/s) | >41 | 38 ±1.8‡ | 70%‡ | 31 ±2.3 | 100% |
| Range | 24–62‡ | 22–39 | |||
| CMAP (mV) | >3 | 1.8 ±2.0 | 85% | 1.7 ±0.8 | 97% |
| DML (ms) | <5.5 | 5.5 ±1.4‡ | 42%‡ | 7.6 ±0.8 | 97% |
| No response | 9‡ | 23%‡ | 21 | 72% | |
| Fibular nerve, record TA | n =23 | n =19 | |||
| MNCV (m/s) | >44 | 45.0 ±2.1‡ | 39%‡ | 32.6 ±4.8 | 89% |
| Range | 24–61 | 21–58 | |||
| CMAP (mV) | >5.0 | 3.4 ±1.7* | 90% | 4.4 ±0.42 | 100% |
| DML (ms) | <6.7 | 3.2 ±0.6‡ | 0%‡ | 1.8 ±0.35 | 58% |
| No response | 0‡ | 0%‡ | 10 | 53% |
MNCS findings in women were less severe than those in men (P<0.05) for almost all parameters.
P <0.05;
P <0.01;
P<0.001.
Table 5.
The age of symptom onset of CMTX in women did not have an effect on motor conduction velocity abnormality or CMAP abnormality
| Symptom onset | Mean median motor CV (m/s) | Mean ulnar motor CV (m/s) | Mean fibular motor CV (m/s) | Mean median CMAP (mV) | Mean ulnar CMAP (mV) | Mean fibular motor CMAP (mV) |
|---|---|---|---|---|---|---|
| 0–10 years | 44 | 51 | 38 | 3.7 | 6.5 | 1.5 |
| 10–19 years | 32 | 47 | 36 | 5.8 | 6.1 | 1.6 |
| 20–30 years | 47 | 48 | 43 | 4.4 | 5.7 | 1.6 |
| 30–60 years | 44 | 48 | 35 | 5.2 | 6.4 | 1.1 |
| Unaffected | 54‡ | 55* | 47* | 5.1 | 6.6 | 3.8‡ |
Those with no symptoms had a faster conduction velocity (P<0.05) and a higher fibular CMAP amplitude (P <0.01).
P <0.05;
P <0.01;
P<0.001.
RESULTS
Subjects
Results for 45 women and 31 men with CMT1X were analyzed. The age range of the women was 7–84 years and the age range of the men was 13–86 years, with mean ages of 44 and 43 years, respectively. In women, symptom onset ranged from childhood (hammer toes, high arches, clumsiness, paresthesias, frequent falls, weak ankles) to 60s (weakness, burning pain in hands/feet, cramps). Five women were asymptomatic and were age 9, 27, 37, 49, and 53 years, respectively, at the time of diagnosis. The average CMT neuropathy score (CMTNS) for women was 9 of 36, with a range of 0–17 (0 =normal, 36 =maximum abnormal score). The average CMTNS for men was 12 of 36, with a range of 1–24.15
Comparisons of EDX Studies in Men and Women with CMT1X
MNCS in women with CMT1X were lesss severe in almost all parameters compared to men with CMT1X (P <0.05) (Table 1). The motor CV ranges were wider in women compared with men (P <0.001), except in the fibular motor response recorded from the tibialis anterior (Table 1). Motor CVs were more frequently in the normal range for women compared with men (Table 2). There was no conduction block noted in any of the NCS, and temporal dispersion was not seen. Furthermore, there was no significant correlation between CMTNS and any of the MNCS parameters in either gender. When excluding for cases of carpal tunnel syndrome, the average of the individual difference between the median and ulnar motor CVs was 5 m/s in women and 4 m/s in men; thus, there was no significant individual difference between median and ulnar motor CVs in men and in women.
Table 2.
Comparison of degree of MNCV slowing between men and women
| Median motor NCV
|
Ulnar motor NCV
|
Fibular (EDB) motor NCV
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Mean age for women (y) | Men | Women | Mean age for women (y) | Men | Women | Mean age for women (y) | |
| Slow (<32 m/s) | 8/20 (40%) | 3/38 (8%) | 51 | 10/27 (37%) | 2/44 (4.5%) | 54 | 4/8 (50%) | 5/30 (16.7%) | 38 |
| Intermediate (33–41 m/s) | 8/20 (40%) | 7/38 (18.4%) | 50 | 13/27 (48%) | 3/44 (6.8%) | 35 | 4/8 (50%) | 10/30 (33.3%) | 46 |
| Normal (>42 m/s) | 4/20 (20%) | 28/38 (73.6%) | 43 | 4/27 (15%) | 39/44 (88.6%) | 44 | 0 | 15/30 (50%) | 37 |
More women had MNCVs in the normal range.
Comparison of MNCS among Women with CMT1X
Among the CMT1X women, the scores for the lower extremity CMAPs (fibular CMAP recording from EDB and TA) were more affected than those of the upper extremity CMAPs (ulnar and median nerves, P <0.01) (Table 3). However, the fibular motor DML measured at the TA was not affected in any of the women (P <0.01) and the fibular motor CV recorded from the TA was less affected than the fibular motor CV recorded from the EDB (P <0.05) (Table 3). Although there was a slight trend for older women to have slow median and ulnar motor nerve CVs, this was not seen for the fibular motor nerve (Table 2).
SNCS in CMT1X
There was a higher frequency of non-response (NR) in the sural than the median and ulnar nerves in women (P <0.001) (Fig. 1). In addition, the sural NR rate in women was seen more frequently than the sural NR rate in men (P <0.001). The men had the same rate of NR between all 3 sensory nerves as opposed to the women who had variable NR rates between the sensory nerves. In addition, the sensory CVs and SNAPs for the median and ulnar nerves in women were all more frequently affected than the equivalent MNCS parameters (P <0.001) (Table 4).
FIGURE 1.
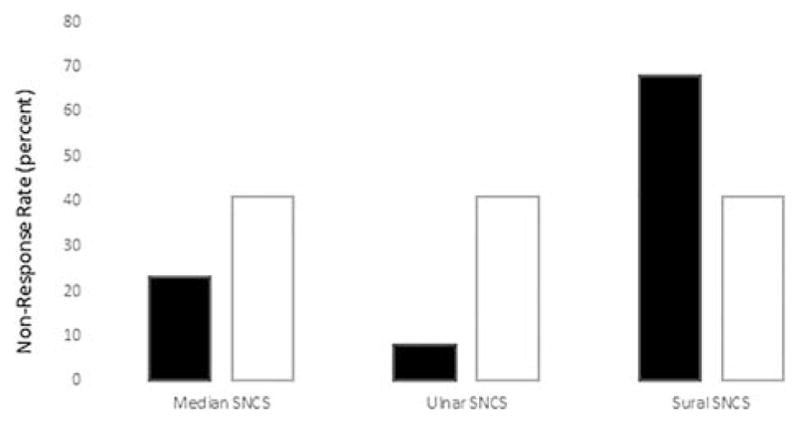
Percentage of no response in sensory nerve conduction studies (SNCS) in median, ulnar, and sural sensory nerves. The sural SNCS has a higher non-response rate than the median and ulnar SNCS in women and than the sural SNCS in men (P <0.0001). Men had the same rate of abnormality for all 3 sensory nerves. Black bars: women; white bars: men.
Table 4.
SNCS frequency of abnormality to equivalent MNCS response parameters
| Variable | Nerve | Proportion abnormal | Tukey adjusted P-value
|
Compared with corresponding motor response | |
|---|---|---|---|---|---|
| Vs. median | Vs. ulnar | ||||
| DSL | Median (<3.5) | 31% | — | — | >0.99 |
| Ulnar (<3.5) | 24% | 0.8 | — | >0.99 | |
| Sural (<4.5) | 8% | 0.08 | 0.2 | — | |
| CV | Median (>56) | 87% | — | — | 0.001‡ |
| Ulnar (>56) | 77% | 0.3 | — | 0.0004‡ | |
| Sural (>43) | 89% | 0.99 | 0.3 | — | |
| SNAP | Median (>25) | 85% | — | — | <0.0001‡ |
| Ulnar (>10) | 76% | 0.5 | — | 0.001‡ | |
| Sural (>6) | 84% | 0.99 | 0.6 | — | |
Median and ulnar CV and SNAP are more frequently abnormal than the corresponding MNCS parameters (P <0.001).
P <0.05;
P <0.01;
P<0.001.
Age of Onset and EDX Abnormality in Women
The age of symptom onset of CMTX in women did not have an effect on motor CV abnormality or CMAP abnormality (Table 5). Also, those with no symptoms had a faster CV (P <0.05) and a higher fibular CMAP amplitude (P <0.01).
Clinical Presentation in Women
Fifty-eight percent (25 of 43) of the women had weak ankles as a child. Nineteen percent (8 of 43) had balance difficulties as a child. Fourteen percent (6 of 43) were asymptomatic and were known to have CMTX by family history, EDx, and genetic testing. Five percent (2 of 43) had weak hands as a child. Five percent (2 of 43) had foot drop in middle age, but either were non-athletic as a child or had a positive family history. The time course was slowly progressive over years.
DISCUSSION
Previous studies have suggested that conduction blocks or temporal dispersion may be characteristic of CMT1X in women, such that some were thought to have CIDP. However, we did not find this in our study (Table 1). There was only mild individual asymmetry, with the distal motor latencies in CMT1X women being less frequently abnormal compared with men. However, this was not suggestive of an acquired demyelinating neuropathy. The median and ulnar SNAPs and CVs were more frequently affected than the corresponding motor values, implying a mild asymmetry between motor and sensory nerves in women with CMT1X (Table 4). However, none of these results suggested a diagnosis of CIDP.
CMT1X may not have uniform conduction slowing as seen in CMT1A. Particularly as women with CMT1X may have late onset, clinicians may mistakenly consider the possibility of a treatable acquired neuropathy such as CIDP. There are certain key features that make presentation less likely to be CIDP, such as: (1) a lack of temporal dispersion or conduction block on NCS; (2) a family history of CMT1X; and (3) the clinical history, as the majority of women with CMT1X present with either weak ankles or balance difficulties in childhood. Furthermore, no patient had more individual variability between the median and ulnar motor nerve conduction velocity than men with CMT1X.
Our results confirm that women with CMT1X have less severe MNCS than men. The findings are consistent with previous reports of less abnormal MNCV in women compared with men (Table 1).11 Although the MNCVs were not consistently markedly abnormal compared with men, the median and ulnar MNCVs in women had a wider range than those of men (P <0.001) (Table 1). The NCS have an underlying length-dependent component in women with CMT1X. Our analysis indicates that the MNCS response parameters for the lower extremities (CMAP and CV) are abnormal more frequently than those for the upper extremity in women (Table 3). The sural SNAP is more frequently absent than that in the median or ulnar sensory nerves in women (Fig. 1). As previously reported, the milder findings in most women can be explained by random X-inactivation. Because only a subset of Schwann cells will express mutant GJB1 in women with CMT1X, they are likely to have milder disease in most situations. This is also reflected in the faster nerve conduction velocity in most women with CMT1X compared with men. However, in some cases, women are affected as significantly as men, which suggests that, on occasion, X-inactivation can be skewed and result in a more severe phenotype.1,2 We hypothesized that similar skewing may occur in focal areas along a nerve, resulting in focal slowing and resembling CIDP. However, we did not find this in our CMT1X cohort, which suggests that such occurrences must be quite rare.
In conclusion, our results do not suggest nonuniformity and segmental abnormalities in CMT1X in women, but they do confirm previous findings of less severe MNCS in women with CMT1X compared to men with CMT1X. Although women also exhibited a range of CVs, they were generally in the normal range when compared with men. It would be rare for a woman with CMT1X to be mistakenly diagnosed with an acquired demyelinating neuropathy such as CIDP.
Acknowledgments
This study was supported by grants from the National Institute of Neurological Disorders and Stroke (to M.E.S.), Office of Rare Diseases (U54NS065712 to M.E.S.), Muscular Dystrophy Association (to M.E.S.), and Charcot–Marie–Tooth Association (to M.E.S.), and also by a Muscular Dystrophy Association clinical research training grant and University of Iowa internal funding initiatives award (to N.U.J.).
Abbreviations
- APB
abductor pollicis brevis
- ADM
abductor digiti minimi
- CIDP
chronic inflammatory demyelinating polyneuropathy
- CMT1X
Charcot–Marie–Tooth disease type 1X
- CMAP
compound muscle action potential
- CMTNS
CMT neuropathy score
- CV
conduction velocity
- Cx32
connexin32
- DML
distal motor latency
- DSL
distal sensory latency
- EDB
extensor digitorum brevis
- EDx
electrodiagnostic testing
- EMG
electromyography
- GJB1
gap junction beta 1 gene
- MNCV
motor nerve conduction velocity
- NCS
nerve conduction studies
- NR
no response
- SNAP
sensory nerve action potential
- SNCV
sensory nerve conduction velocity
- TA
tibialis anterior
References
- 1.Shy ME, Siskind C, Swan ER, Krajewski KM, Doherty T, Fuerst DR, et al. CMT1X phenotypes represent loss of GJB1 gene function. Neurology. 2007;68:849–855. doi: 10.1212/01.wnl.0000256709.08271.4d. [DOI] [PubMed] [Google Scholar]
- 2.Siskind CE, Murphy SM, Ovens R, Polke J, Reilly MM, Shy ME. Phenotype expression in women with CMT1X. J Periph Nerv Syst. 2011;16:102–107. doi: 10.1111/j.1529-8027.2011.00332.x. [DOI] [PubMed] [Google Scholar]
- 3.Liang GS, de Miguel M, Gomez-Hernandez JM, Glass JD, Scherer SS, Mintz M, et al. Severe neuropathy with leaky connexin32 hemichannels. Ann Neurol. 2005;57:749–754. doi: 10.1002/ana.20459. [DOI] [PubMed] [Google Scholar]
- 4.Lewis RA, Sumner AJ, Shy ME. Electrophysiological features of inherited demyelinating neuropathies: a reappraisal in the era of molecular diagnosis. Muscle Nerve. 2000;23:1472–1487. doi: 10.1002/1097-4598(200010)23:10<1472::aid-mus3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Birouk N, LeGuern E, Maisonobe T, Rouger H, Gouider R, Tardieu S, et al. X-linked Charcot-Marie-Tooth disease with connexin 32 mutations: clinical and electrophysiologic study. Neurology. 1998;50:1074–1082. doi: 10.1212/wnl.50.4.1074. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez A, England JD, Sumner AJ, Ferer S, Warner LE, Lupski JR, et al. Unusual electrophysiological findings in X-linked dominant Charcot–Marie–Tooth disease. Muscle Nerve. 2000;23:182–188. doi: 10.1002/(sici)1097-4598(200002)23:2<182::aid-mus6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Hahn AF, Brown WF, Koopman WJ, Feasby TE. X-linked dominant hereditary motor and sensory neuropathy. Brain. 1990;113:1511–1525. doi: 10.1093/brain/113.5.1511. [DOI] [PubMed] [Google Scholar]
- 8.Lewis RA, Shy ME. Electrodiagnostic findings in CMTX: a disorder of the Schwann cell and peripheral nerve myelin. Ann NY Acad Sci. 1999;883:504–507. [PubMed] [Google Scholar]
- 9.Nicholson G, Nash J. Intermediate nerve conduction velocities define X-linked Charcot-Marie-Tooth neuropathy families. Neurology. 1993;43:2558–2564. doi: 10.1212/wnl.43.12.2558. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson GA, Yeung L, Corbett A. Efficient neurophysiologic selection of X-linked Charcot-Marie-Tooth families: ten novel mutations. Neurology. 1998;51:1412–1416. doi: 10.1212/wnl.51.5.1412. [DOI] [PubMed] [Google Scholar]
- 11.Dubourg O, Tardieu S, Birouk N, Gouider R, Leger JM, Maisonobe T, et al. Clinical, electrophysiological and molecular genetic characteristics of 93 patients with X-linked Charcot-Marie-Tooth disease. Brain. 2001;124:1958–1967. doi: 10.1093/brain/124.10.1958. [DOI] [PubMed] [Google Scholar]
- 12.Pareyson D, Scaioli V, Laura M. Clinical and electrophysiological aspects of Charcot-Marie-Tooth disease. Neuromol Med. 2006;8:3–22. doi: 10.1385/nmm:8:1-2:3. [DOI] [PubMed] [Google Scholar]
- 13.Latov N. Diagnosis of CIDP. Neurology. 2002;59(suppl 6):S2–S6. doi: 10.1212/wnl.59.12_suppl_6.s2. [DOI] [PubMed] [Google Scholar]
- 14.Joint Task Force of the European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Periph Nerv Syst. 2010;15:1–9. doi: 10.1111/j.1529-8027.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- 15.Shy ME, Blake J, Krajewski K, Fuerst DR, Laura M, Hahn AF, et al. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology. 2005;64:1209–1214. doi: 10.1212/01.WNL.0000156517.00615.A3. [DOI] [PubMed] [Google Scholar]


