Abstract
Mutant mouse models with specific visual pathway defects offer an advantage to comprehensively investigate the role of specific pathways/neurons involved in refractive development. In this review, we will focus on recent studies using mouse models that have provided insight into retinal pathways and neurotransmitters controlling refractive development. Specifically, we will examine the contributions of rod and cone photoreceptors and the ON and OFF retinal pathways to visually driven eye growth with emphasis on dopaminergic mechanisms.
1. Retina
The retina is a complex ocular structure that converts wavelengths of light into neuronal signals that become perceived visual images. The mammalian retina is composed of approximately 55 morphologically distinct cell types, each with a different function [1]. From outer to inner retina, photoreceptors and horizontal cells, bipolar cells, amacrine cells and ganglion cells constitute the major neuronal populations in the mammalian eye [2, 3] (Figure 1). While describing how the retinal neurons are interconnected and the integrated eloquence by which a visual signal is created is beyond the scope of this review, several excellent reviews are available [1, 2, 4].
Figure 1.
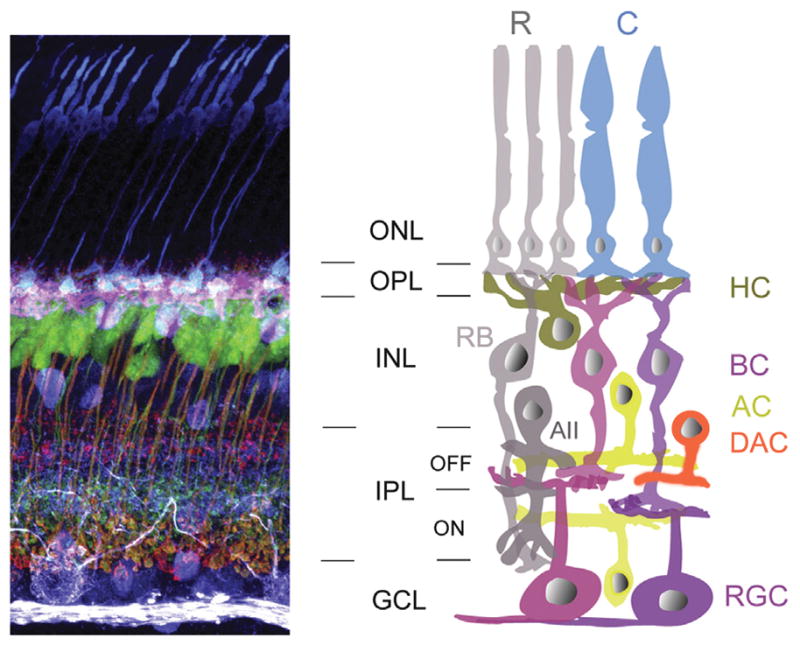
Retinal anatomy and circuitry. During visual processing, the output from the rod and cone photoreceptors in the outer nuclear layer (ONL) are decomposed into a number of different parallel information channels by synapsing in the outer plexiform layer (OPL) to different cells in the inner nuclear layer (INL) (bipolar, amacrine, and horizontal cells). The output from these inner retinal cells are sampled by different retinal ganglion cells in the inner plexiform layer (IPL). Finally, depending on the type of information, specific ganglion cells in the ganglion cell layer (GCL) transmit the signal to higher visual structures in the brain. R, rod; C, cone; RB, rod bipolar cell; CB, cone bipolar cell; H, horizontal cell; A, amacrine cell; AII, AII amacrine cell; DAC, dopaminergic amacrine cell, As, astrocyte; G, ganglion cell; M, microglia. Image modified from http://webvision.med.utah.edu/book/partvi-development-of-cell-types-and-synaptic-connections-in-the-retina and http://webvision.med.utah.edu/book/part-xii-cell-biology-of-retinal-degenerations with permission, made available through Creative Commons.
The retina is required for regulating visually-driven eye growth, yet our knowledge of what visual signals and retinal pathways control eye growth is lacking. In this review, we will focus on a few key retinal cells and neurotransmitters that have been implicated in myopia, and show how investigations to explore complex signaling pathways and retinal circuits using the mouse model have provided some remarkable insights into the retinal mechanisms of refractive development.
2. Retinal input essential for ocular growth
As previously discussed [5–17], a wide range of animal studies have shown that the visual environment influences refractive development of the eye. It has been established that eye growth regulation occurs at the retinal level in response to both diffusers and defocus lenses. Previous studies have shown that severing the optic nerve in young chicks does not prevent the development of myopia in response to both negative lenses [18, 19] and diffusers [20]. Furthermore, in both chicks [21] [7] and primates [22], if partial diffusers are imposed on only half of the visual field, only that corresponding half of the eye elongates and becomes myopic. Similarly, chick eyes compensate for both negative [21, 23] and positive [23] lenses imposed on local retinal areas using hemi-field spectacle lenses with ocular growth restricted to the defocussed parts of the visual field. These studies demonstrate retinal, and not cortical processing, is sufficient to regulate refractive eye growth.
However, there is some evidence that cortical processing may also influence eye growth. While higher order visual processing was not required for compensation to hyperopic defocus in chickens, optic nerve [20] or optic nerve and ciliary nerve sections [24] induced hyperopia in chickens with normal visual input [20, 24]. Together these findings suggest that higher order processing within the visual system may influence ocular growth, but is not a requirement for visually-driven ocular growth. Given that the visual mechanisms regulating refractive development localize principally to the retina, any defect in visual transmission through the retina could potentially influence ocular growth, and may lead to development of refractive errors.
Several studies have suggested the role of various retinal cell types/pathways and neurotransmitters in normal refractive development of the eye. In chickens, physiological and morphological changes in photoreceptors are associated with experimentally induced myopia [25]. In addition, differential eye growth under both normal and FD conditions in response to neurotoxins blocking responses from the photoreceptors [26], ON and OFF pathways [27, 28] and the inner retina [29] have been shown. Several retinal neurotransmitters such as dopamine (DA) [30, 31], glucagon [32], acetylcholine [33], nitric oxide [34, 35] and retinoic acid [36, 37] have also been implicated in defocus induced ocular growth in animals. Abnormalities in visual transmission through the retina may result from mutations in retinal neurons/pathways, changes in various retinal neurotransmitters associated with the mutation, or a combination of both factors. Whist these experiments demonstrate the influence of the retina in normal ocular development, these experimental approaches do not ensure complete and selective blockage of a single pathway or neuronal type. Mutant mouse models with specific visual pathway defects offer an advantage to comprehensively investigate the role of specific pathways/neurons involved in refractive development. In this review, we will focus on recent studies using mouse models that have provided insight into retinal pathways and neurotransmitters controlling refractive development.
3. Mouse – a novel animal model to explore retinal mechanism of refractive development
In the recent years, there has been a growing interest in using mouse models for investigating complex signaling pathways and retinal circuitries, and their influence on ocular refractive development [38–49]. Mouse models offer the advantage of altering both genes and environment in the same animal by using various knockout models that are generated by manipulating the mouse genome, combined with altered visual input with lenses or form deprivation. Additionally, close resemblance of the mouse retinal structure to humans, short gestational period and large litter sizes make them an excellent experimental model for refractive development research. However, small eye size, absence of fovea, nocturnal behavior, poor visual acuity [50] and large depth of field [51] are some limitations of using murine models for refractive development studies (please see review [52]). Despite these limitations, the mouse eye responds to visual form-deprivation with temporal properties and magnitude comparable with other mammalian models (see review, [52]).
Studying the effects of visual manipulations in various mouse mutants provides a unique opportunity to examine the role of gene/environment interactions in refractive development. A number of previous studies have examined the effects of a specific gene defect using various mutant mice under normal and form-deprived visual conditions [41, 42, 47, 48, 53–58]. The mouse model provides the opportunity to investigate how the gene defect and/or the associated changes in the levels of retinal neurotransmitters alter refractive development with normal visual input, as well as the influence of the gene defect on myopia susceptibility. In view of these points, probing genetic and environmental interactions is the most promising aspect of using the mouse to provide important insights into the mechanisms regulating eye growth.
4. Retinal neurotransmitters and refractive development
Before describing studies of mouse models with retinal neuron defects, it is important to acknowledge the rich diversity of neurotransmitters present in the retina that have been associated with experimentally-induced refractive errors in animal models. In order for the rate of ocular growth to change, visual stimulation of the retina must activate signaling pathways that modulate scleral growth. Evidence from pharmacological and genetic studies suggests that several signaling pathways control refractive eye growth [59–61]. For instance, the expression level of ZENK in chickens or the mammalian homologue, Egr-1, has been shown to increase or decrease with hyperopic or myopic eye growth, respectively [62, 63].
A large body of previous studies has examined muscarinic acetylcholine receptor mechanisms in refractive development of the eye. Both non-selective (such as atropine) and partially selective (such as pirenzepine) muscarinic antagonists have been shown to have inhibitory effects on experimental myopia in chickens and mammals (see review, [60, 64]). Clinically, atropine [65–67] and pirenzepine [68–70] has been used to slow myopia progression in children. In both laboratory animals and children, anti-myopia effect of atropine has been found to be independent of the drug’s action on accommodation [64]. Recent results using a mutant mouse suggest that loss of the muscarinic cholinergic receptor gene, M2, or pharmacological blocking M2 will provide resistance to myopia by altering the scleral collagen composition [58].
Another possible signaling pathway candidate is adenosine which is known to be regulated by light and alters collagen synthesis [56]. All adenosine receptor subtypes are expressed in the retina, choroid and sclera of the mammalian eye [71], and have been suggested to play an important role in the regulation of eye growth in both mammalian [71, 72] and human eyes [73]. The adenosine A2A receptor KO mice have relative myopia compared to WT littermates and altered scleral ultrastructure [56].
Nitric oxide, a gaseous neurotransmitter synthesized by both the retina and the choroid [74] is thought to be part of the signal cascade mediating ocular growth inhibition in response to myopic defocus [75]. Studies on chickens have shown pharmacological inhibition of nitric oxide to prevent the increase in choroidal thickness normally induced by myopic defocus, resulting in myopic eye growth [34, 35, 76]. However, some earlier chicken studies have reported inconsistent results compared to these newer studies on the effects of nitric oxide in ocular growth regulation of chickens [77, 78]. The differences in results could be due differences in drug concentrations used by these studies. Furthermore, a transient suppression of retinal nitric oxide synthase (NOS, enzyme catalyzing the production of nitric oxide) activity was observed in guinea pigs with acute form-deprivation, whereas chronic form-deprivation (about 14–21 days) was associated with significant upregulation of NOS levels in the posterior eye [79, 80]. Other potential candidates that either inhibit (such as glucagon [32]), or promote (such as retinoic acid [36, 37]) ocular growth in response to defocus have been suggested to be part of this signaling pathway.
4.1 Role of dopamine in refractive development and myopia susceptibility
In the retina, DA is synthesized and released by a subset of amacrine-interplexiform cells, known as the dopaminergic amacrine cells [81, 82]. Dopaminergic neurons convert tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA) via tyrosine hydroxylase and L-DOPA to DA via aromatic L-amino acid decarboxylase [82]. DA is released by the neuron and metabolized to DOPAC, which is the main DA metabolite in the rodent retina [83].
DA has been implicated as a stop signal in refractive eye growth [61]. In both chicken and mammalian models, form deprivation decreases DA biosynthesis [30, 84, 85], whereas DA mimetic treatment (L-DOPA or receptor agonists) prevents form deprivation myopia (see [61] for review; [30, 31, 86]). Furthermore, particular DA receptor activation may be important for refractive development signaling, as D2R have been shown to be important for the inhibitory effects of form deprivation [87, 88], lens defocus [89] and bright lighting [90, 91] in chickens. In the mammalian guinea pig model of spontaneous myopia, D1-like receptors inhibit and D2-like receptors promote myopic growth [92]. However, the loss of D2R in mice prevents the form deprivation response [55]. It should be noted that some recent reports indicate unaltered retinal dopamine activity in mice with form-deprivation [93]. Together, these results indicate that more studies are needed to elucidate how dopamine modulates refractive development and myopia. Section 3.6 will review dopaminergic modulation of myopia susceptibility in retinal mouse mutants (discussed below).
5. Retinal neurons/pathways and refractive development in mutant mice
5.1 Photoreceptor input to myopia
Since the photoreceptors form the first layer of photo-sensory neurons in the retina, it is plausible that photoreceptors are involved in mechanisms sensing defocus and/or communicating that error signal across the retina to the RPE and the choroid. In fact, studies have suggested that, in emmetropia, the focal plane is located at the photoreceptor inner segments, and both the alignment and directionality of photoreceptors are important components for retinal blur detection [25]. Over the years, morphological changes of photoreceptors (elongation of rod outer segments) [94], reduction in photoreceptor cell density [95], changes in the outer segment shedding under various lighting conditions [96, 97] and changes in electroretinogram [98] associated with experimentally induced myopia have all pointed towards the possible role of photoreceptors in refractive development of the eye.
Whist a number of studies have suggested that cone pathways are likely to dictate the signaling needed for proper eye development, there is also some evidence for the involvement of rods in regulating ocular growth. The requirement of a high acuity retinal image (largely attributed to cone mediated signaling) for emmetropization [99]; development of myopia under dim lighting conditions (when cones are less sensitive) in chickens [100]; increased and decreased susceptibility to form-deprivation myopia in cone and rod dominated animal models, respectively [101, 102]; and reduced response to experimental myopia in chickens treated with formoguanamine, a photoreceptor neurotoxin that causes significant damage to cone outer segments, all suggest the importance of cone activity in emmetropization [26]. However, normal response to form-deprivation myopia in monkeys treated with laser ablation at the cone-rich fovea [103], and similar myopic responses in monkeys with form-deprivation imposed on the rod-dominated peripheral regions or the entire visual field [104] suggest that cone pathways may not completely dominate the signaling for mammalian eye growth, a finding further supported by the following mouse studies of experimental myopia.
5.1.1 rd1−/− and rd10−/− mice – photoreceptor degeneration models
In a recent study, Park et al [47] found that under normal visual conditions, retinal degeneration causes significantly hyperopic refractive errors and shorter axial lengths in rd1−/− and rd10−/− mice compared to wild-type (WT) mice. Pde6brd1/rd1 (rd1−/−, [105–107]) and Pde6brd10/rd10 (rd10−/−, [108, 109]) mice with a mutation in the Pde6b gene are two frequently used mouse models of photoreceptor degeneration. Pde6b mutation disrupts encoding of the β-subunit of cyclic nucleotide phosophodiesterase-6 [110, 111], a mutation also seen in patients with RP [112, 113].
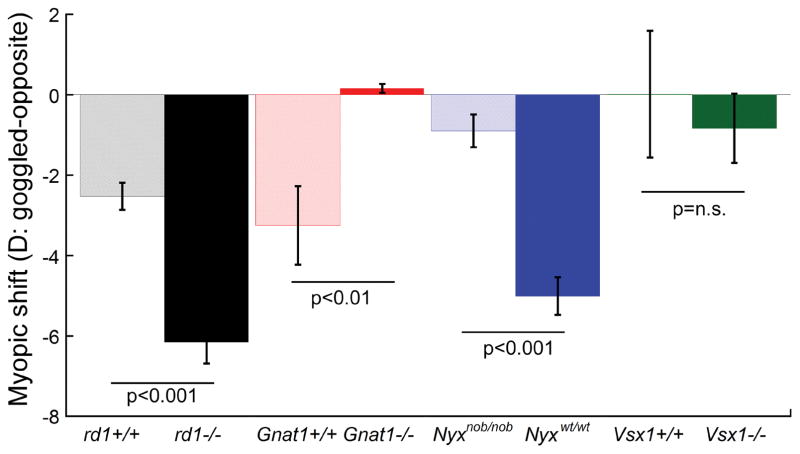
Interestingly, with form-deprivation, both degeneration strains show faster and greater susceptibility to form-deprivation myopia than WT mice (~ 6–7 D in 2 weeks in rd1−/− and rd10−/− mice vs ~ 3 D in 4–5 weeks in rd1+/+ and rd10+/+; Figure 2) [47]. In agreement with previous studies [114, 115], dopamine (DA) levels were altered. Levels of the DA metabolite, 3,4 dihydroxyphenylacetic acid (DOPAC) and the DOPAC/DA ratios (a measure of DA turnover) were significantly lower in rd1−/− and rd10−/− mice compared to the WT mice during normal visual experience, indicating a lower DA metabolism in degenerated mouse retinas [47]. Furthermore, both degenerations models exhibited a high correlation between lower basal levels of retinal DOPAC and greater susceptibility to form-deprivation myopia (Figure 3). These results indicate that retinal degeneration by itself may not cause myopia, but may reduce DA metabolism in the retina, which may lead to an increased susceptibility to myopia under myopigenic visual environments.
Figure 2.
Myopic shift (goggled-opposite eye) across several mutant mouse strains with photoreceptor or ON/OFF retinal pathway defects after 2 weeks of form deprivation. Note that the mutant mice are on different background strains and thus, the WT controls are shown for each strain. Rd1−/− mice and Nyxnob/nob mice show significantly greater myopic shift than their WT controls. In contrast, Gnat1−/− mice with non-functional rods did not show a shift with form deprivation myopia. Vsx1+/+ mice are on a 129SV background and do not respond to form deprivation. The loss of Vsx1 did not change the susceptibility to form deprivation myopia.
Figure 3.
Myopic shift after 2 weeks of form deprivation in several different mutant strains with retinal defects plotted against the DOPAC/DA ratio at 4 weeks of age when the form deprivation was initiated. These results suggest that DA turnover at the time of goggling may influence the susceptibility to form deprivation myopia.
5.1.2 Gnat1−/− mice – non-functional rod model
The importance of rod pathways in visual processing under different light conditions, and their potential role in refractive error development is demonstrated by the presence of myopia in human patients with congenital stationary night blindness due to abnormal visual transmission between rods and ON bipolar cells [116].
Recently, Park et al [53] reported abnormal refractive development in Gnat1−/− mice with non-functional rod photoreceptors (genetic mutation in rhodopsin-associated G protein, transducin α 1, [117]). Unlike normal refractive development in WT mice, Gnat1−/− mice do not show a relative increase in hyperopia with age, instead the refractive curve in Gnat1−/− mice remains stable throughout the developmental period measured from 4 to 12 weeks. Additionally, Gnat1−/− mice are unresponsive to form-deprivation and do not develop a myopic shift (Figure 2). In this study, the authors examined retinal dopamine and DOPAC levels across age, and found significantly lower (and stable) retinal DOPAC levels in Gnat1−/− mice compared to WT mice throughout the period of development. Furthermore, Gnat1−/− retinas exhibit significantly greater dopamine turnover (measured from DOPAC/DA ratio) at early ages of refractive development, which rapidly decline from the second week of development [53]. These results suggest that functional rods are critical to normal refractive development and form-deprivation response in mice, and that dopamine metabolism and tonic levels of dopamine during ocular development are important predictors of susceptibility to form-deprivation myopia in murine eyes. Future studies using Gnat2−/− mutants with non-functional cones [118] may increase our understanding of how different photoreceptors might regulate normal refractive under different ambient lighting conditions.
5.2 ON and OFF pathway contributions to myopia
Effects of ON and OFF pathways on eye development have been examined using various neurotoxins that specifically block the ON and OFF responses to light [27, 28]. In chickens, elimination of the OFF pathway using intravitreal injections of the D isomer of a Müller cell gliotoxin α amino adipic acid (DαAAA) resulted in an enhanced rate of axial elongation under normal visual conditions, but a slower ocular growth rate with form-deprivation [27]. Conversely, inhibition of the ON channel with the L isomer (LαAAA) caused a reduction in axial eye growth of normal eyes, but increased eye growth in form-deprived animals. Similar to chickens, blocking of the ON pathway with D,L-2-amino-4-phosphonobutyric (a selective ON pathway inhibitor) has been shown to cause a significant reduction in axial eye growth of cats [119]. Genetic mutations of neurons or receptor in the ON and OFF pathways in mutant mouse models represent a novel approach to investigating the role of the ON and OFF signaling in refractive development.
5.2.1 Nyxnob/nob mice – ON pathway defect model
Pardue et al [48] examined the refractive development and dopamine levels of the Nyxnob/nob mouse [120], which carries a null mutation in Nyx [121], resulting in a loss of function of the ON pathway [120]. Nyx encodes the protein nyctalopin, which is located on the post-synaptic side of the photoreceptor to ON bipolar cell synapse [122]. ON and OFF channels of the visual system are imperative for processing contrast sensitivity information [123, 124], an important prerequisite for a high resolution retinal image.
Under normal unmanipulated visual conditions, the loss of Nyx causes only slightly more hyperopic refractions in Nyxnob/nob mice compared to WT mice [48]. However, imposing form-deprivation results in a significantly rapid myopic shift in Nyxnob/nob mice compared to Nyxwt/wt mice (Figure 2). Additionally, during normal visual development, dopamine and DOPAC levels were significantly lower in the Nyxnob/nob mice in comparison with the Nyxwt/wtmice. These results indicate that low endogenous dopamine levels or blurred visual input secondary to the ON pathway defect may increase the susceptibility to myopia development in the mouse eye.
5.2.2 Vsx1−/− mice – OFF pathway defect model
Chakraborty et al (2014) examined the role of OFF pathway signaling in refractive development of the eye using the Vsx1−/− mice on a 129S1/Sv background [125], which carry a null mutation in the visual system homeobox 1 gene, Vsx1 [126]. The detection of Vsx1 in the mouse retina at postnatal day 5 in the developing bipolar cell region [126], and a reduction in immunolabeling at the axonal termini of various OFF cone bipolar cells (and a few ON bipolar cells) in adult Vsx1−/− retinas [125, 127, 128] suggest that Vsx1 is essential for late terminal differentiation and functioning of OFF cone bipolar cells. However, Chakraborty et al found that a selective impairment of the retinal OFF visual pathway caused by the Vsx1 mutation does not significantly alter the normal refractive development in Vsx1−/− mice compared to the Vsx1+/+ mice, potentially due to normal visual transmission through other Vsx1 independent ON and OFF bipolar cells in the retina [54]. Interestingly, both Vsx1+/+ and Vsx1−/− mice do not respond to imposed form-deprivation (Figure 2). Furthermore, at 4 weeks of age, 129S1/Sv mice (Vsx1+/+) exhibit a significantly elevated retinal dopamine turnover compared to the commonly used C57BL/6J mice, which may prevent against form-deprivation myopia in both 129S1/Sv Vsx1−/− and Vsx1+/+ mice [54]. Although, these results indicate that OFF pathway signaling may not be critically important for normal refractive development in mice, future studies with mouse mutants that express complete loss of the OFF pathway are required to investigate this further.
5.3 Amacrine and ganglion cell contributions to refractive development
There is also some evidence that the inner retina (especially the amacrine cells and the retinal ganglion cells, RGC) may play some role in refractive development of the eye. While blocking amacrine cell function using cell-specific neurotoxins, such as 6-hydroxy-dopamine (dopaminergic amacrine cells, [129]) and ethylcholine mustard aziridiniumion ion (cholinergic amacrine cells, [130]), does not alter refractive compensation to imposed defocus in chickens, other neurotoxins, like kainic acid [29, 131] causing a non-specific damage to the inner retina (including amacrine cells) at higher doses, lead to increased ocular growth (mostly in the posterior chamber) under normal conditions, and a reduction in myopic eye growth under form-deprived conditions. These findings warrant further investigation using amacrine cell knock out mutants [132]. Finally, blockade of retinal ganglion cell function does not prevent form-deprivation myopia in both chickens [133] and tree shrews [134], suggesting that the outer retina perhaps plays a major role in defocus detection and signaling for ocular development [25].
5.4 Dopamine modulation of myopia susceptibility in retinal mouse mutants
In addition to acute changes in dopamine with abnormal visual input, results from mutant mice also suggest that basal levels of DA turnover (as indicated by DOPAC/DA ratio) may influence susceptibility to form deprivation myopia. For example, mouse models of retinal degeneration (rd1−/− and rd10−/−) have decreased DOPAC and DOPAC/DA levels throughout life and increased susceptibility to form deprivation myopia [47]. Since DA is released via ON pathway stimulation [135], mutations in the ON pathway would decrease retinal DA levels. Such is the case in Nyxwt/wt mice which have reduced DA and DOPAC levels and increased susceptibility to myopia [48]. Alternatively, mice with high levels of retinal DA and/or DOPAC have reduced susceptibility to myopia, such as Gnat1−/− [53] and 129SV Vsx1+/+ mice [54]. Figure 3 shows the relationship between DOPAC/DA levels at 4 weeks of age and the susceptibility to subsequent form deprivation myopia. These results suggest that DA turnover during early development may “preset” the susceptibility to myopigenic stimulation. Further research is needed to determine how DA alters refractive eye growth so that therapeutic approaches can be developed.
6. Conclusions
In conclusion, the retina plays an important role in regulating visually-driven ocular growth in mammals. The mouse is an extremely useful animal model to examine retinal mechanisms controlling eye growth. Using genetic mouse mutants, genes controlling specific retinal receptors, neurotransmitters and cell types can be selectively probed to examine their role in normal refractive development, as well as under altered visual conditions.
Mutations in different retinal neurons/signaling pathways have differential effects on normal and visually-deprived refractive development of the eye. The refractive phenotypes observed in different retinal mutations may result from the mutation itself, changes in various retinal neurotransmitters associated with the mutation (such as changes in dopamine levels), or a combination of both factors. In mice, although photoreceptors are important for normal refractive development, rod pathways in particular (both functional rods and ON pathway) appear to be extremely critical for both normal refractive development as well as response to visual form deprivation. While cone pathways have previously been implicated in normal ocular refractive development, it requires further investigation using mouse mutants (with mutations in functional cones or OFF cone pathways) to determine if their role is essential.
In rodents, alterations in endogenous retinal dopamine (or DOPAC levels) associated with various mutations are important determinants of susceptibility to form deprivation myopia. It should be noted that this chapter specifically reviewed the changes in retinal dopamine levels, and their potential implications in refractive error development in mice. However, the mammalian retina is a hub for many other neurotransmitters (such as nitric oxide, glucagon, retinoic acid, vasoactive intestinal peptide, etc). Changes in other neurotransmitters with experimental myopia, and their interaction with retinal dopamine during refractive error development are yet to be explored, and beyond the scope of this chapter. Further understanding of how abnormal visual signals from the retina are transmitted downstream through the RPE and the choroid, causing long-term changes in the sclera are important for designing therapeutic interventions for myopia control.
References
- 1.Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol. 2001;11(4):431–6. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 2.Masland RH. The neuronal organization of the retina. Neuron. 2012;76(2):266–80. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18(21):8936–46. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4(9):877–86. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 5.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201(4362):1249–51. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- 7.Wallman J, et al. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35(1):37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 8.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28(5):639–57. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 9.Howlett MH, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus) Vision Res. 2006;46(1–2):267–83. doi: 10.1016/j.visres.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 10.Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49(2):219–27. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Smith EL, 3rd, Hung LF. Form-deprivation myopia in monkeys is a graded phenomenon. Vision Res. 2000;40(4):371–81. doi: 10.1016/s0042-6989(99)00184-4. [DOI] [PubMed] [Google Scholar]
- 12.Smith EL, 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39(8):1415–35. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 13.Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33(10):1311–24. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- 14.Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008;8(3):11–12. doi: 10.1167/8.3.1. [DOI] [PubMed] [Google Scholar]
- 15.Van Sluyters RC. Recovery from monocular stimulus deprivation amblyopia in the kitten. Ophthalmology. 1978;85(5):478–88. doi: 10.1016/s0161-6420(78)35649-9. [DOI] [PubMed] [Google Scholar]
- 16.Gao Q, et al. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch Clin Exp Ophthalmol. 2006;244(10):1329–35. doi: 10.1007/s00417-006-0254-1. [DOI] [PubMed] [Google Scholar]
- 17.Shen W, Vijayan M, Sivak JG. Inducing form-deprivation myopia in fish. Invest Ophthalmol Vis Sci. 2005;46(5):1797–803. doi: 10.1167/iovs.04-1318. [DOI] [PubMed] [Google Scholar]
- 18.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35(9):1175–94. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 19.Wildsoet CF. Neural pathways subserving negative lens-induced emmetropization in chicks-insights from selective lesions of the optic nerve and ciliary nerve. Current eye research. 2003;27(6):371–385. doi: 10.1076/ceyr.27.6.371.18188. [DOI] [PubMed] [Google Scholar]
- 20.Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res. 1987;6(8):993–9. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- 21.Wallman J, et al. Local retinal regions control local eye growth and myopia. Science. 1987;237(4810):73–7. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- 22.Smith EL, 3rd, et al. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009;50(11):5057–69. doi: 10.1167/iovs.08-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997;37(6):659–68. doi: 10.1016/s0042-6989(96)00224-6. [DOI] [PubMed] [Google Scholar]
- 24.Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks--insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res. 2003;27(6):371–85. doi: 10.1076/ceyr.27.6.371.18188. [DOI] [PubMed] [Google Scholar]
- 25.Crewther DP. The role of photoreceptors in the control of refractive state. Prog Retin Eye Res. 2000;19(4):421–57. doi: 10.1016/s1350-9462(00)00004-5. [DOI] [PubMed] [Google Scholar]
- 26.Westbrook AM, et al. Formoguanamine-induced inhibition of deprivation myopia in chick is accompanied by choroidal thinning while retinal function is retained. Vision Res. 1995;35(14):2075–88. doi: 10.1016/0042-6989(94)00282-q. [DOI] [PubMed] [Google Scholar]
- 27.Crewther DP, Crewther SG. Pharmacological modification of eye growth in normally reared and visually deprived chicks. Curr Eye Res. 1990;9(8):733–40. doi: 10.3109/02713689008999568. [DOI] [PubMed] [Google Scholar]
- 28.Crewther DP, Crewther SG, Xie RZ. Changes in eye growth produced by drugs which affect retinal ON or OFF responses to light. J Ocul Pharmacol Ther. 1996;12(2):193–208. doi: 10.1089/jop.1996.12.193. [DOI] [PubMed] [Google Scholar]
- 29.Ehrlich D, et al. Effects of selective neurotoxins on eye growth in the young chick. Ciba Found Symp. 1990;155:63–84. doi: 10.1002/9780470514023.ch5. discussion 84–8. [DOI] [PubMed] [Google Scholar]
- 30.Stone RA, et al. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A. 1989;86(2):704–6. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iuvone PM, et al. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991;32(5):1674–7. [PubMed] [Google Scholar]
- 32.Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis Neurosci. 2002;19(6):755–66. doi: 10.1017/s0952523802196064. [DOI] [PubMed] [Google Scholar]
- 33.Stone RA, Lin T, Laties AM. Muscarinic antagonist effects on experimental chick myopia. Exp Eye Res. 1991;52(6):755–8. doi: 10.1016/0014-4835(91)90027-c. [DOI] [PubMed] [Google Scholar]
- 34.Nickla DL, et al. Inhibiting the transient choroidal thickening response using the nitric oxide synthase inhibitor l-NAME prevents the ameliorative effects of visual experience on ocular growth in two different visual paradigms. Exp Eye Res. 2006;83(2):456–64. doi: 10.1016/j.exer.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 35.Nickla DL, Wildsoet CF. The effect of the nonspecific nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on the choroidal compensatory response to myopic defocus in chickens. Optom Vis Sci. 2004;81(2):111–8. doi: 10.1097/00006324-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 36.McFadden SA, et al. Acute effects of dietary retinoic acid on ocular components in the growing chick. Exp Eye Res. 2006;83(4):949–61. doi: 10.1016/j.exer.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 37.McFadden SA, Howlett MH, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44(7):643–53. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Tejedor J, de la Villa P. Refractive changes induced by form deprivation in the mouse eye. Invest Ophthalmol Vis Sci. 2003;44(1):32–6. doi: 10.1167/iovs.01-1171. [DOI] [PubMed] [Google Scholar]
- 39.Qian YS, et al. Sonic hedgehog expression and its role in form-deprivation myopia in mice. Curr Eye Res. 2009;34(8):623–35. doi: 10.1080/02713680903003492. [DOI] [PubMed] [Google Scholar]
- 40.Schmucker C, Schaeffel F. In vivo biometry in the mouse eye with low coherence interferometry. Vision Res. 2004;44(21):2445–56. doi: 10.1016/j.visres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Schippert R, et al. Relative axial myopia in Egr-1 (ZENK) knockout mice. Invest Ophthalmol Vis Sci. 2007;48(1):11–7. doi: 10.1167/iovs.06-0851. [DOI] [PubMed] [Google Scholar]
- 42.Wisard J, et al. Exaggerated eye growth in IRBP-deficient mice in early development. Invest Ophthalmol Vis Sci. 2011;52(8):5804–11. doi: 10.1167/iovs.10-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barathi VA, et al. Two models of experimental myopia in the mouse. Vision Res. 2008;48(7):904–16. doi: 10.1016/j.visres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, et al. The development of the refractive status and ocular growth in C57BL/6 mice. Invest Ophthalmol Vis Sci. 2008;49(12):5208–14. doi: 10.1167/iovs.07-1545. [DOI] [PubMed] [Google Scholar]
- 45.Jiang M, et al. Single-shot dimension measurements of the mouse eye using SD-OCT. Ophthalmic Surg Lasers Imaging. 2012;43(3):252–6. doi: 10.3928/15428877-20120308-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tkatchenko TV, Shen Y, Tkatchenko AV. Analysis of postnatal eye development in the mouse with high-resolution small animal magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2010;51(1):21–7. doi: 10.1167/iovs.08-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park H, et al. Retinal degeneration increases susceptibility to myopia in mice. Mol Vis. 2013;19:2068–79. [PMC free article] [PubMed] [Google Scholar]
- 48.Pardue MT, et al. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008;49(2):706–12. doi: 10.1167/iovs.07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chou TH, et al. Postnatal elongation of eye size in DBA/2J mice compared with C57BL/6J mice: in vivo analysis with whole-eye OCT. Invest Ophthalmol Vis Sci. 2011;52(6):3604–12. doi: 10.1167/iovs.10-6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prusky GT, et al. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45(12):4611–6. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 51.Remtulla S, Hallett PE. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 1985;25(1):21–31. doi: 10.1016/0042-6989(85)90076-8. [DOI] [PubMed] [Google Scholar]
- 52.Pardue MT, Stone RA, Iuvone PM. Investigating mechanisms of myopia in mice. Exp Eye Res. 2013;114:96–105. doi: 10.1016/j.exer.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park HN, et al. Visually-driven ocular growth in mice requires functional rod photoreceptors. Invest Ophthalmol Vis Sci. 2014;55(10):6272–9. doi: 10.1167/iovs.14-14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chakraborty R, et al. Comparison of refractive development and retinal dopamine in OFF pathway mutant and C57BL/6J wild-type mice. Mol Vis. 2014;20:1318–27. [PMC free article] [PubMed] [Google Scholar]
- 55.Huang F, et al. Activation of dopamine D2 receptor is critical for the development of form-deprivation myopia in the C57BL/6 mouse. Invest Ophthalmol Vis Sci. 2014;55(9):5537–44. doi: 10.1167/iovs.13-13211. [DOI] [PubMed] [Google Scholar]
- 56.Zhou X, et al. Genetic deletion of the adenosine A2A receptor confers postnatal development of relative myopia in mice. Invest Ophthalmol Vis Sci. 2010;51(9):4362–70. doi: 10.1167/iovs.09-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou G, et al. Modulation of retinal cell populations and eye size in retinoic acid receptor knockout mice. Mol Vis. 2001;7:253–60. [PubMed] [Google Scholar]
- 58.Barathi VA, et al. Muscarinic cholinergic receptor (M2) plays a crucial role in the development of myopia in mice. Dis Model Mech. 2013;6(5):1146–58. doi: 10.1242/dmm.010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stone RA, Khurana TS. Gene profiling in experimental models of eye growth: clues to myopia pathogenesis. Vision Res. 2010;50(23):2322–33. doi: 10.1016/j.visres.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ganesan P, Wildsoet CF. Pharmaceutical intervention for myopia control. Expert Rev Ophthalmol. 2010;5(6):759–787. doi: 10.1586/eop.10.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013;114:106–19. doi: 10.1016/j.exer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Ashby R, et al. Alterations in ZENK and glucagon RNA transcript expression during increased ocular growth in chickens. Mol Vis. 2010;16:639–49. [PMC free article] [PubMed] [Google Scholar]
- 63.Ashby RS, et al. Egr-1 mRNA expression is a marker for the direction of mammalian ocular growth. Invest Ophthalmol Vis Sci. 2014;55(9):5911–21. doi: 10.1167/iovs.13-11708. [DOI] [PubMed] [Google Scholar]
- 64.Stone RA, et al. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47. doi: 10.1016/j.exer.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walline JJ, et al. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2011;(12):CD004916. doi: 10.1002/14651858.CD004916.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chua WH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285–91. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 67.Li SM, et al. Atropine slows myopia progression more in Asian than white children by meta-analysis. Optom Vis Sci. 2014;91(3):342–50. doi: 10.1097/OPX.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 68.Siatkowski RM, et al. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Arch Ophthalmol. 2004;122(11):1667–74. doi: 10.1001/archopht.122.11.1667. [DOI] [PubMed] [Google Scholar]
- 69.Siatkowski RM, et al. Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS. 2008;12(4):332–9. doi: 10.1016/j.jaapos.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 70.Tan DT, et al. One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology. 2005;112(1):84–91. doi: 10.1016/j.ophtha.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 71.Cui D, et al. Adenosine receptor protein changes in guinea pigs with form deprivation myopia. Acta Ophthalmol. 2010;88(7):759–65. doi: 10.1111/j.1755-3768.2009.01559.x. [DOI] [PubMed] [Google Scholar]
- 72.Cui D, et al. Effects of 7-methylxanthine on the sclera in form deprivation myopia in guinea pigs. Acta Ophthalmol. 2011;89(4):328–34. doi: 10.1111/j.1755-3768.2009.01688.x. [DOI] [PubMed] [Google Scholar]
- 73.Chen X, et al. Assessment of exonic single nucleotide polymorphisms in the adenosine A2A receptor gene to high myopia susceptibility in Chinese subjects. Mol Vis. 2011;17:486–91. [PMC free article] [PubMed] [Google Scholar]
- 74.Fischer AJ, Stell WK. Nitric oxide synthase-containing cells in the retina, pigmented epithelium, choroid, and sclera of the chick eye. J Comp Neurol. 1999;405(1):1–14. doi: 10.1002/(sici)1096-9861(19990301)405:1<1::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 75.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–68. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nickla DL, Damyanova P, Lytle G. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor L-NAME. Exp Eye Res. 2009;88(6):1092–9. doi: 10.1016/j.exer.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujikado T, et al. The effect of nitric oxide synthase inhibitor on form-deprivation myopia. Curr Eye Res. 1997;16(10):992–6. doi: 10.1076/ceyr.16.10.992.9021. [DOI] [PubMed] [Google Scholar]
- 78.Fujikado T, et al. Effect of a nitric oxide synthase inhibitor on lens-induced myopia. Ophthalmic Res. 2001;33(2):75–9. doi: 10.1159/000055647. [DOI] [PubMed] [Google Scholar]
- 79.Wu J, et al. Time-course of changes to nitric oxide signaling pathways in form-deprivation myopia in guinea pigs. Brain Res. 2007;1186:155–63. doi: 10.1016/j.brainres.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 80.Wu J, et al. Changes of nitric oxide synthase and cyclic guanosine mono-phosphate in form deprivation myopia in guinea pigs. Chin Med J (Engl) 2007;120(24):2238–44. [PubMed] [Google Scholar]
- 81.Dowling JE, Ehinger B. The interplexiform cell system. I. Synapses of the dopaminergic neurons of the goldfish retina. Proc R Soc Lond B Biol Sci. 1978;201(1142):7–26. doi: 10.1098/rspb.1978.0030. [DOI] [PubMed] [Google Scholar]
- 82.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108(1):17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 83.Nir I, Haque R, Iuvone PM. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 2000;870(1–2):118–25. doi: 10.1016/s0006-8993(00)02409-4. [DOI] [PubMed] [Google Scholar]
- 84.Pendrak K, et al. Retinal dopamine in the recovery from experimental myopia. Curr Eye Res. 1997;16(2):152–7. doi: 10.1076/ceyr.16.2.152.5090. [DOI] [PubMed] [Google Scholar]
- 85.Rohrer B, Iuvone PM, Stell WK. Stimulation of dopaminergic amacrine cells by stroboscopic illumination or fibroblast growth factor (bFGF, FGF-2) injections: possible roles in prevention of form-deprivation myopia in the chick. Brain Res. 1995;686(2):169–81. doi: 10.1016/0006-8993(95)00370-6. [DOI] [PubMed] [Google Scholar]
- 86.Iuvone PM, et al. Dopamine synthesis and metabolism in rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Vis Neurosci. 1989;2(5):465–71. doi: 10.1017/s0952523800012360. [DOI] [PubMed] [Google Scholar]
- 87.Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993;10(3):447–53. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- 88.McCarthy CS, et al. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res. 2007;84(1):100–7. doi: 10.1016/j.exer.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 89.Nickla DL, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res. 2011;93(5):782–5. doi: 10.1016/j.exer.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen Y, et al. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012;103:33–40. doi: 10.1016/j.exer.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 91.Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51(10):5247–53. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- 92.Jiang L, et al. Effects of dopaminergic agents on progression of naturally occurring myopia in albino guinea pigs (Cavia porcellus) Invest Ophthalmol Vis Sci. 2014;55(11):7508–19. doi: 10.1167/iovs.14-14294. [DOI] [PubMed] [Google Scholar]
- 93.Wu XH, et al. Unaltered retinal dopamine levels in a C57BL/6 mouse model of form-deprivation myopia. Invest Ophthalmol Vis Sci. 2015;56(2):967–77. doi: 10.1167/iovs.13-13362. [DOI] [PubMed] [Google Scholar]
- 94.Liang H, et al. A role for photoreceptor outer segments in the induction of deprivation myopia. Vision Res. 1995;35(9):1217–25. doi: 10.1016/0042-6989(94)00241-d. [DOI] [PubMed] [Google Scholar]
- 95.Beresford JA, Crewther SG, Crewther DP. Anatomical correlates of experimentally induced myopia. Aust N Z J Ophthalmol. 1998;26(Suppl 1):S84–7. doi: 10.1111/j.1442-9071.1998.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 96.Basinger S, Hoffman R, Matthes M. Photoreceptor shedding is initiated by light in the frog retina. Science. 1976;194(4269):1074–6. doi: 10.1126/science.1086510. [DOI] [PubMed] [Google Scholar]
- 97.Currie JR, Hollyfield JG, Rayborn ME. Rod outer segments elongate in constant light: darkness is required for normal shedding. Vision Res. 1978;18(8):995–1003. doi: 10.1016/0042-6989(78)90027-5. [DOI] [PubMed] [Google Scholar]
- 98.Fujikado T, et al. Retinal function with lens-induced myopia compared with form-deprivation myopia in chicks. Graefes Arch Clin Exp Ophthalmol. 1997;235(5):320–4. doi: 10.1007/BF01739642. [DOI] [PubMed] [Google Scholar]
- 99.Nevin ST, Schmid KL, Wildsoet CF. Sharp vision: a prerequisite for compensation to myopic defocus in the chick? Curr Eye Res. 1998;17(3):322–31. doi: 10.1076/ceyr.17.3.322.5220. [DOI] [PubMed] [Google Scholar]
- 100.Lauber JK, Kinnear A. Eye enlargement in birds induced by dim light. Can J Ophthalmol. 1979;14(4):265–9. [PubMed] [Google Scholar]
- 101.McBrien NA, et al. Experimental myopia in a diurnal mammal (Sciurus carolinensis) with no accommodative ability. J Physiol. 1993;469:427–41. doi: 10.1113/jphysiol.1993.sp019821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nathan J, et al. Effects of retinal image degradation on ocular growth in cats. Invest Ophthalmol Vis Sci. 1984;25(11):1300–6. [PubMed] [Google Scholar]
- 103.Smith EL, 3rd, et al. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007;48(9):3914–22. doi: 10.1167/iovs.06-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith EL, 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49(19):2386–92. doi: 10.1016/j.visres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pittler SJ, et al. PCR analysis of DNA from 70-year-old sections of rodless retina demonstrates identity with the mouse rd defect. Proc Natl Acad Sci U S A. 1993;90(20):9616–9. doi: 10.1073/pnas.90.20.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carter-Dawson LD, LaVail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978;17(6):489–98. [PubMed] [Google Scholar]
- 107.LaVail MM, et al. Variability in rate of cone degeneration in the retinal degeneration (rd/rd) mouse. Exp Eye Res. 1997;65(1):45–50. doi: 10.1006/exer.1997.0308. [DOI] [PubMed] [Google Scholar]
- 108.Chang B, et al. Retinal degeneration mutants in the mouse. Vision Res. 2002;42(4):517–25. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 109.Gargini C, et al. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007;500(2):222–38. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pittler SJ, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci U S A. 1991;88(19):8322–6. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang B, et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007;47(5):624–33. doi: 10.1016/j.visres.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McLaughlin ME, et al. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci U S A. 1995;92(8):3249–53. doi: 10.1073/pnas.92.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McLaughlin ME, et al. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993;4(2):130–4. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- 114.Nir I, Iuvone PM. Alterations in light-evoked dopamine metabolism in dystrophic retinas of mutant rds mice. Brain Res. 1994;649(1–2):85–94. doi: 10.1016/0006-8993(94)91051-0. [DOI] [PubMed] [Google Scholar]
- 115.Hankins M, Ikeda H. Early abnormalities of retinal dopamine pathways in rats with hereditary retinal dystrophy. Doc Ophthalmol. 1994;86(3):325–34. doi: 10.1007/BF01203555. [DOI] [PubMed] [Google Scholar]
- 116.Miyake Y, et al. Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol. 1986;104(7):1013–20. doi: 10.1001/archopht.1986.01050190071042. [DOI] [PubMed] [Google Scholar]
- 117.Calvert PD, et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc Natl Acad Sci U S A. 2000;97(25):13913–8. doi: 10.1073/pnas.250478897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chang B, et al. Cone photoreceptor function loss-3, a novel mouse model of achromatopsia due to a mutation in Gnat2. Invest Ophthalmol Vis Sci. 2006;47(11):5017–21. doi: 10.1167/iovs.05-1468. [DOI] [PubMed] [Google Scholar]
- 119.Smith EL, 3rd, Fox DA, Duncan GC. Refractive-error changes in kitten eyes produced by chronic on-channel blockade. Vision Res. 1991;31(5):833–44. doi: 10.1016/0042-6989(91)90150-4. [DOI] [PubMed] [Google Scholar]
- 120.Pardue MT, et al. A naturally occurring mouse model of X-linked congenital stationary night blindness. Invest Ophthalmol Vis Sci. 1998;39(12):2443–9. [PubMed] [Google Scholar]
- 121.Gregg RG, et al. Identification of the gene and the mutation responsible for the mouse nob phenotype. Invest Ophthalmol Vis Sci. 2003;44(1):378–84. doi: 10.1167/iovs.02-0501. [DOI] [PubMed] [Google Scholar]
- 122.Morgans CW, Ren G, Akileswaran L. Localization of nyctalopin in the mammalian retina. Eur J Neurosci. 2006;23(5):1163–71. doi: 10.1111/j.1460-9568.2006.04647.x. [DOI] [PubMed] [Google Scholar]
- 123.Schiller PH. The ON and OFF channels of the visual system. Trends Neurosci. 1992;15(3):86–92. doi: 10.1016/0166-2236(92)90017-3. [DOI] [PubMed] [Google Scholar]
- 124.Schiller PH, Sandell JH, Maunsell JH. Functions of the ON and OFF channels of the visual system. Nature. 1986;322(6082):824–5. doi: 10.1038/322824a0. [DOI] [PubMed] [Google Scholar]
- 125.Chow RL, et al. Control of late off-center cone bipolar cell differentiation and visual signaling by the homeobox gene Vsx1. Proc Natl Acad Sci U S A. 2004;101(6):1754–9. doi: 10.1073/pnas.0306520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chow RL, et al. Vsx1, a rapidly evolving paired-like homeobox gene expressed in cone bipolar cells. Mech Dev. 2001;109(2):315–22. doi: 10.1016/s0925-4773(01)00585-8. [DOI] [PubMed] [Google Scholar]
- 127.Ohtoshi A, et al. Regulation of retinal cone bipolar cell differentiation and photopic vision by the CVC homeobox gene Vsx1. Curr Biol. 2004;14(6):530–6. doi: 10.1016/j.cub.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 128.Cheng CW, et al. The Iroquois homeobox gene, Irx5, is required for retinal cone bipolar cell development. Dev Biol. 2005;287(1):48–60. doi: 10.1016/j.ydbio.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 129.Schwahn HN, Schaeffel F. Chick eyes under cycloplegia compensate for spectacle lenses despite six-hydroxy dopamine treatment. Invest Ophthalmol Vis Sci. 1994;35(9):3516–24. [PubMed] [Google Scholar]
- 130.Fischer AJ, et al. Cholinergic amacrine cells are not required for the progression and atropine-mediated suppression of form-deprivation myopia. Brain Res. 1998;794(1):48–60. doi: 10.1016/s0006-8993(98)00188-7. [DOI] [PubMed] [Google Scholar]
- 131.Wildsoet CF, Pettigrew JD. Kainic acid-induced eye enlargement in chickens: differential effects on anterior and posterior segments. Invest Ophthalmol Vis Sci. 1988;29(2):311–9. [PubMed] [Google Scholar]
- 132.Smith ML, et al. Acetylcholine receptors in the retinas of the alpha7 nicotinic acetylcholine receptor knockout mouse. Mol Vis. 2014;20:1328–56. [PMC free article] [PubMed] [Google Scholar]
- 133.McBrien NA, et al. The effects of blockade of retinal cell action potentials on ocular growth, emmetropization and form deprivation myopia in young chicks. Vision Res. 1995;35(9):1141–52. doi: 10.1016/0042-6989(94)00237-g. [DOI] [PubMed] [Google Scholar]
- 134.Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis Neurosci. 1994;11(1):143–53. doi: 10.1017/s0952523800011184. [DOI] [PubMed] [Google Scholar]
- 135.Boatright JH, Gordon JR, Iuvone PM. Inhibition of endogenous dopamine release in amphibian retina by L-2-amino-4-phosphonobutyric acid (L-AP4) and trans-2-aminocyclopentane-1,3-dicarboxylate (ACPD) Brain Res. 1994;649(1–2):339–42. doi: 10.1016/0006-8993(94)91084-7. [DOI] [PubMed] [Google Scholar]