Abstract
Rheumatoid arthritis (RA) is a negative risk factor for the development of Alzheimer’s disease (AD). While it has been commonly assumed that RA patients’ usage of non-steroidal anti-inflammatory drugs (NSAIDs) helped prevent onset and progression of AD, NSAID clinical trials have proven unsuccessful in AD patients. To determine whether intrinsic factors within RA pathogenesis itself may underlie RA’s protective effect, we investigated the activity of colony-stimulating factors, upregulated in RA, on the pathology and behavior of transgenic AD mice. 5 µg bolus injections of macrophage, granulocyte, and granulocyte-macrophage colony-stimulating factors (M-CSF, G-CSF, or GM-CSF) were administered unilaterally into the hippocampus of aged cognitively-impaired AD mice and the resulting amyloid load reductions determined one week later, using the artificial cerebrospinal fluid-injected contralateral sides as controls. G-CSF and more significantly, GM-CSF reduced amyloidosis throughout the treated brain hemisphere one week following bolus administration to AD mice. 20 daily subcutaneous injections of 5 µg of GM-CSF (the most amyloid-reducing CSF in the bolus experiment) were administered to balanced cohorts of AD mice after assessment in a battery of cognitive tests. Reductions in amyloid load and improvements in cognitive function were assessed. Subcutaneous GM-CSF administration significantly reduced brain amyloidosis and completely reversed the cognitive impairment, while increasing hippocampal synaptic area and microglial density. These findings, along with two decades of accrued safety data using Leukine, recombinant human GM-CSF, in elderly leukopenic patients, suggest that Leukine should be tested as a treatment to reverse cerebral amyloid pathology and cognitive impairment in AD.
Keywords: Alzheimer’s disease, amyloid-β, cognitive interference task, granulocyte-macrophage colony-stimulating factor, intrahippocampal, radial arm water maze, rheumatoid arthritis, subcutaneous, transgenic mice
INTRODUCTION
Although numerous studies have reported that rheumatoid arthritis (RA) reduces the risk of Alzheimer’s disease (AD), the mechanisms for RA’s protective effect are still unknown [1]. It was proposed, and commonly assumed, that RA patients’ usage of non-steroidal anti-inflammatory drugs (NSAIDs) may help prevent the onset and progression of AD [1]. However, the largest NSAID clinical trials have not demonstrated efficacy in reducing the incidence of dementia, and recently Naproxen was reported to be detrimental, with increased risk of cardiovascular and cerebrovascular events [2]. These results suggested to us that intrinsic, probably immunological, factors within RA pathogenesis itself may underlie the protective effect of RA against AD. We surmised that upregulated local cellular populations in RA would have the highest potential to enter into the brain and inhibit the development of AD pathology and/or neuronal dysfunction.
AD is an age-related, progressive neurodegenerative disorder that presents as increasing decline in cognitive and executive function. Alzheimer dementia is associated with cerebrovascular dysfunction [3], extracellular accumulation of amyloid-β (Aβ) peptides in the brain parenchyma and vasculature walls [4,5] (predominantly Aβ1−42 and Aβ1−40), and intraneuronal accumulation of neurofibrillary tangles consisting of hyperphosphorylated tau proteins [6]. Associated neuroinflammation may contribute to AD pathogenesis [7], as the inflammatory proteins apolipoprotein E (ApoE) and α1 antichymotrypsin (ACT) catalyze the polymerization of Aβ peptides into amyloid filaments in vivo and in vitro [8–11], and ACT has been shown to induce the phosphorylation of tau [12]. Conversely, it has also been shown that amyloid plaques form rapidly and then become decorated by microglia [13,14], both resident and bone marrow-derived, suggesting an ability and intention to remove amyloid [15–17]. Thus it is unclear whether neuroinflammation is deleterious or beneficial in the AD brain, and indeed the role of microglia in AD is complex and may involve different states of activation with different activities.
RA is an autoimmune disease in which inflamed synovial tissue and highly vascularized pannus form, irreparably damaging the cartilage and bone. In this inflammatory pannus, leukocyte populations are greatly expanded, perhaps as an endogenous, but ineffective attempt to remove the inflammatory insult. As a result, many proinflammatory factors are produced that work together in feedforward mechanisms, further increasing leukocytosis, cytokine/chemokine release, osteoclastogenesis, angiogenesis, and autoantibody production (rheumatoid factors and anticitrullinated protein antibodies) [18,19]. Additionally, the adaptive immune system presents a Th17 phenotype within CD4+ lymphocytes, with ultimate production of interleukin 17 (IL-17), which is then responsible for inducing much of the proinflammatory effects [20,21]. Further enhancements of leukocyte populations come from increased expression of structurally-unrelated colony-stimulating factors: MCSF (macrophage), GCSF (granulocyte), and GM-CSF (granulocyte-macrophage) [22–25].
Although upregulated leukocytes in response to RA could potentially enter into the brain and inhibit development of AD pathology and/or neuronal dysfunction, lymphocytic infiltrates into AD patient brains have not been reported. The lack of infiltration suggests that RA-induced proliferation and activation of the innate immune system by the CSFs described above might be responsible for preventing AD pathology in RA patients. Evidence supporting the innate immune system’s role in AD pathogenesis show that complement proteins are upregulated in AD brain, and that inhibition of C3 convertase significantly increases amyloid pathology in AD mice [26]. Bone marrow-derived microglia also play a critical role in restricting amyloid deposition, and indeed, microglia activation and many associated receptors and enzymes, such as CD36, scavenger receptor A, and receptor for advanced glycation end products, neprilysin, insulysin, and matrix metalloproteinases, decline with age as risk of AD pathology increases [17,27,28].
To investigate the interplay of the innate immune system and AD, we studied the effects on AD pathology of the three colony-stimulating factors (M-CSF, G-CSF, and GM-CSF), which are upregulated during RA pathogenesis [22–25]. These CSFs enhance the survival and function of their respective leukocytes and drive their proliferation and differentiation from myeloid lineage precursors. GM-CSF induces dendritic cells, macrophages, and granulocytes (neutrophils, basophils, and eosinophils), while MCSF and GCSF, respectively, induce the macrophage and granulocyte subsets of the innate immune system. These innate cells have the ability to diapedese from the circulatory system and to differentiate further into various specialized immune cells within organs (microglia, Langerhan’s cells, etc.). GM-CSF and G-CSF are also known to be involved in erythropoiesis, and GM-CSF and erythropoietin act synergistically in the maturation and proliferation of the burst-forming and colony-forming erythroid units to the normoblast stage of erythropoiesis [29,30]. Circulating Aβ binds to complement opsonin C3b in an antibody-independent fashion, and C3-opsonized particles bind to the complement receptor, CR1, on erythrocytes and to CR1g on liver-resident kupffer macrophages [31,32]. Thus GM-CSF could function in both the peripheral clearance of Aβ and in bone marrow-derived microglial activity, since it is involved in the proliferation, differentiation, and maintenance of most innate leukocytes.
Here, we report on experiments that investigated the effect of CSF administration on amyloid plaque deposition, microglial activation, synaptic function, and associated cognitive decline in a mouse model of AD. Our results, particularly with GM-CSF, provide a compelling explanation for RA’s inverse relationship with AD. Moreover, the reduction of amyloidosis and enhancement of cognition by GM-CSF warrant clinical investigation of Leukine for the treatment of mild cognitive impairment (MCI) and AD patients, especially with Leukine’s long-standing safety history in leukopenic patients.
MATERIALS AND METHODS
All procedures involving experimentation on animals were performed in accordance with the guidelines set forth by the University of South Florida Animal Care and Use Committee. Transgene detections were performed using QPCR (Bio-Rad iCycler, Hercules, CA).
Transgenic mouse studies involving intracerebral administration of CSFs
PS/amyloid-β protein precursor (AβPP) mice in this study, which begin accumulating robust amyloid plaques at 6–8 months, were generated by crossing heterozygous PDGF-hAβPP (V717F) mice with PDGF-hPS1 (M146L) on both Swiss Webster and C57BL/6 backgrounds.
Bilateral intracerebroventricular infusion of M-CSF
M-CSF was bilaterally infused directly into the lateral ventricles (5 µg/day) for 14 days using a novel intracranial catheter infusion system (patent pending: PCT/US08/73974) [33]. This completely subcutaneously-contained system allows bilateral intracerebral infusion of test substances ipsilaterally and vehicle contralaterally, and overcomes the problem of amyloidosis variance between animals (Supplementary Fig. 1; available online: http://www.j-alz.com/issues/21/vol21-2.html#supplementarydata03), effectively making each animal its own control. Briefly, animals (PS/AβPP, all 8.8–9.6 months, numbered sequentially according to date of birth, 25–35 g, both genders) were anesthetized with 1–2% isoflurane, shaved and scrubbed with 10% Betadine solution at the site of incision, and placed into a stereotaxic frame (Kopf Instruments, Tujunga, Ca.). A small (3 cm) incision was made, exposing the skull, and curved Strabysmus surgical scissors were used to form a subcutaneous pocket along the animal’s back into which 2 osmotic minipumps (Alzet model 1004, average flow rate of 0.12 µL/h, Durect Corp., Cupertino, CA) were inserted. Two holes were drilled into the skull (from Bregma ± 0.1 mm anterior-posterior, ± 0.9 mm medial-lateral), and 30 gauge catheters were inserted at a depth of 3.0 mm, corresponding to the lateral ventricles. Leading from the Alzet pump was a proprietary catheter system with the delivery tips fashioned to the contours of the skull rather than the commercially-available pedestal cannula. The cannulae are affixed to the skull using Locktite 454 adhesive (Plastics One, Roanoke, VA) and secured with 1 cm diameter nitrile, followed by silk sutures to close the scalp.
After 2 weeks of M-CSF infusions, mice were perfused, brain tissues were fixed in 10% neutral buffered formalin, and cryosectioned at 14 µm.
Intrahippocampal injections of CSFs
All three CSFs were stereotaxically-injected (5 µg/injection) into the (ipsilateral) hippocampus, with artificial cerebrospinal fluid vehicle injected contralaterally into four PS/AβPP mice each (all 10–12 months old, 25–35 g, both genders). Two holes were drilled into the skull (from bregma ± 2.5 mm anterior-posterior, ± 2.5 mm medial-lateral, and the 30 gauge needle inserted to a depth of 2.5 mm). Mice were perfused with 0.9% cold saline 7 days later and their brains placed in 10% neutral buffered formalin. Recombinant mouse GM-CSF (rmGM-CSF), recombinant murine G-CSF (rmG-CSF), and recombinant mouse M-CSF (rmM-CSF) (R&D Systems, Minneapolis, MN) will be referred to as GM-CSF, G-CSF, and M-CSF throughout this publication.
Immunohistochemistry and image analysis of intrahippocampal-injected mice
Formalin-fixed brains were either coronally cryosectioned at 14 µm, or paraffin-embedded and sectioned at 5 µm, with standard deparaffination and antigen retrieval steps (boiled in 10 mM sodium citrate buffer for 20 min) performed before immunohistochemical staining. To significantly reduce cost of reagents and antibodies with paraffin-embedded slides, a novel magnetic immunohistochemical staining device was developed (patent pending, Tech ID# 09A015). Standard fluorescent immunohistochemical techniques used primary anti-Aβ antibodies 6E10 (Covance, Emeryville, CA, 1:1000), and MabTech’s 3740-5 (MabTech, Cincinnati, OH, 1:5000) to immunolabel amyloid deposition coupled with Alexa fluorophore-labeled secondary antibodies (Molecular Probes, Eugene, OR, 1:1000, 1:4000), and Hoechst (Sigma) nuclear staining. Immunofluorescence was detected and all pictures per section were taken at the same exposure on a Zeiss Imager Z1 microscope (Oberkochen, Germany) using Axiovision 4.7 software. Digital images were quantified using ImageJ (method described in Supplementary Fig. 6). Briefly, each analyzed picture per coronal section was thresholded equally to the same standard deviation from the histogram mean, and analyzed for area, perimeter, feret diameter, and integrated density parameters of each plaque. Area and Perimeter data were calculated from the total and average number of plaque values in each hemisphere per section, and Feret Diameter and Integrated Density were calculated from the average values of the plaques measured in each hemisphere per section. For GM-CSF-injected mice, each section quantified contained analysis of 15–25 individual 10× pictures of each hemisphere, with fewer pictures quantified in the anterior brain and more in posterior brain. For G-CSF- and M-CSF-injected mice, each section quantified contained analysis of 7–9 individual 5× pictures of each hemisphere Statistical significance was obtained from comparing parameter values of ipsilateral CSF-injected hemispheres versus contralateral artificial cerebrospinal fluid-injected hemispheres. Significance was determined by paired student’s ttest with p values < 0.5 considered significant.
Behavioral transgenic mouse study involving GM-CSF treatment
Mice in this study were derived from the Florida Alzheimer’s Disease Research Center mouse colony, wherein heterozygous mice carrying the mutant AβPPK670N, M671L gene (AβPPsw) are routinely crossed with heterozygous PS1 (line 6.2) mice to obtain AβPPsw/PS1, AβPPsw, PS1, and non-transgenic (NT) genotype offspring with a mixed C57/B6/SW/SJL background. Eleven AβPPsw (Tg) and 17 NT mice, all 12-months old, were selected and evaluated for 8 days in the radial arm water maze (RAWM) task of working memory (as previously described [34] (Supplementary Fig. 7). Briefly, an aluminum insert was placed into a 100 cm circular pool to create 6 radially distributed swim arms emanating from a central circular swim area. The number of errors prior to locating which one of the 6 swim arms contained a submerged escape platform (9 cm diameter) was determined for 5 trials per day. The platform location was changed daily to a different arm, with different start arms for each of the 5 trials semi-randomly selected from the remaining 5 swim arms. The numbers of errors during trials 4 and 5 are both considered indices of working memory and are temporally similar to the standard registration/recall testing of specific items used clinically in evaluating AD patients. Following 8 days of pretreatment RAWM testing, the 11 Tg mice were divided into two groups balanced in RAWM performance. The 17 NT mice were also divided into two groups balanced in RAWM performance.
Two weeks following pretreatment testing, one group of Tg mice (n = 5) and one group of NT mice (n = 9) were started on a 10-day treatment protocol with GM-CSF (5 µg/day given subcutaneously), while animals in the control Tg group (n = 6) and control NT group (n = 8) concurrently received daily vehicle (saline) treatment subcutaneously. On the 11th day of injections, all mice began four days of RAWM evaluation, were given 2 days of rest, then evaluated for 4 days in Cognitive Interference task testing as previously described [35,36]. We designed this task measure-for-measure from a Cognitive Interference task used to discriminate normal aged, MCI, and AD patients from one another [36]. Our analogous interference task for mice RAWM was set-up in two different rooms, each with different sets of visual cues. The task requires animals to remember a set of visual cues, so that following interference with a different set of cues, the initial set of cues can be recalled to successfully solve the RAWM task. A set of four behavioral measures was examined. Behavioral measures were: “A1–A3” (Composite three-trial recall score from first 3 trials performed in RAWM “A”), “B” (proactive interference measure attained from a single trial in RAWM “B”), “A4” (retroactive interference measure attained during a single trial in RAWM “A”), and “A5” (delayed-recall measure attained from a single trial in RAWM “A” following a 20 minute delay between “A4” and “A5”). As a distracter between trials, animals are placed in a Y-maze and allowed to explore for 60 s between successive trials of the three-trial recall task, as well as during the proactive interference task. As with the standard RAWM task, this interference task involves the platform location being changed daily to a different arm for both of the RAWM set-ups utilized, and different start arms for each day of testing for both RAWM set-ups. For A1 and B trials, the animal was initially allowed 1 min to find the platform on their own before they were guided to the platform. Then the actual trial was performed in each case. As with the standard RAWM task, animals were given 60 s to find the escape platform for each trial, with the number of errors recorded for each trial. Animals were tested for cognitive interference performance on four successive days, with statistical analysis performed for the two resultant 2-day blocks. For both RAWM (combined T4 and T5 overall) and cognitive interference testing (each of the four measures overall), swim speed was analyzed by dividing error numbers by latency and statistical significance was determined by one-way ANOVA followed by post hoc Fisher’s LSD (least significant difference) test to determine significant group differences at p < 0.05.
Daily GM-CSF and saline injections were continued throughout the behavioral testing period. After completion of behavioral testing at 20 days into treatment, all mice were euthanatized, brains fixed as described above, and paraffin-embedded. Careful visual examination of all tissues upon necropsy revealed no morphological abnormalities, and the mice tolerated daily subcutaneous injections well. Each analysis was done by a single examiner blinded to sample identities, and statistical analyses were performed by a single examiner blinded to treatment group identities. The code was not broken until analyses were completed.
Immunohistochemistry and image analysis of subcutaneous GM-CSF-treated mice
Five 5 µm sections (150 µm apart) were made of formalin-fixed, paraffin-embedded sections throughout the hippocampus of each mouse and immunoreactivity was developed using the Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA) coupled with the diaminobenzidine reaction, according to the manufacturer’s protocol. Immunostaining used biotinylated anti-Aβ clone 4G8 (Covance, Emeryville, CA, 1:200), synaptophysin (DAKO, Carpinteria, CA, undiluted), and Iba1 (Wako, Richmond, VA, 1:1000) as primary antibodies. Since the 4G8 antibody was obtained with biotin label, the secondary step of the ABC protocol was omitted. However, treatment with 70% formic acid prior to the pre-blocking step was necessary. For 4G8 immunohistochemistry, phosphate-buffered saline (0.1 mM, pH 7.4) was used instead of primary antibody or ABC reagent as a negative control. For Iba 1 and synaptophysin immunohistochemistry, normal rabbit serum was used instead of primary antibody or ABC reagent as a negative control. Images were acquired using an Olympus BX60 microscope and digital images were quantified using SimplePCI software (Compix Inc., Imaging Systems, Cranberry Township, PA), according to previous methods [37]. Briefly, a threshold optical density was obtained that discriminated staining from background, and each region of interest was manually edited to eliminate artifacts. Data are reported as percentage of immunolabeled area captured (positive pixels) relative to the full area captured (total pixels). To evaluate synaptophysin immunoreactivity, after the mode of all images was converted to gray scale, the average intensity of positive signals from each image was quantified in the CA1 and CA3 regions of hippocampus as a relative number from zero (white) to 255 (black).
Statistical significance between GM-CSF-treated versus saline-treated groups was determined by two-tailed homoscedastic Student’s t-test with a p value of < 0.05 considered significant. Each analysis was done by a single investigator blinded to sample identities and genotype.
RESULTS
Intracerebral administration of CSFs
Following bilateral intracerebroventricular infusion of M-CSF for two weeks into PS/AβPP mice, immunohistochemical analysis of both experimental and control mice showed considerable variances of amyloid deposition between mice of similar age (Supplementary Fig. 1), significantly compromising our ability to determine M-CSF’s effect in a limited mouse cohort. While improving our drug delivery system by developing novel bilateral brain infusion catheters [33], we found that parenchymally-infused recombinant peptides remained localized to the infused hemisphere. These findings led us to administer the CSFs as a unilateral intrahippocampal bolus with a contralateral injection of vehicle as control, thus obviating the need for large numbers of transgenic mice and age-matched littermate controls to obtain statistical significance. Each CSF was stereotaxically injected into the hippocampus of 4 mice, with artificial cerebrospinal fluid vehicle injected contralaterally. The mice were sacrificed 7 days post-injection.
M-CSF intrahippocampal injections into mice resulted in swelling of the entire hemisphere as compared to the control side, and in one mouse, an apparent hyperplasia had formed at the injection site (Supplementary Fig. 2). Quantification of amyloid plaque loads from anterior to posterior of each mouse showed similar deposition in the M-CSF-injected hemispheres as compared to the control sides (data not shown). In contrast to M-CSF, G-CSF intrahippocampal injections did not induce swelling and showed some modest reductions of amyloid deposition (Supplementary Fig. 3). Since the analyses were conducted on low magnification (5×) photomicrographs, only the area and integrated density values achieved significance (p < 0.05). Reduction in amyloidosis was subsequently corroborated by independent observations from fellow investigators following peripheral G-CSF administration [37].
GM-CSF-injections, however, demonstrated pronounced decreases in amyloid deposition, as compared to control hemispheres, in visual observations of coronal tissue sections (Fig. 1a; Supplementary Fig. 4). High magnification (10×) quantification of amyloid plaques anterior to posterior revealed significant reductions within individual mice and significant overall reductions for all plaque parameters measured (Fig. 1b and Supplementary Fig. 5).
Fig. 1.
Intrahippocampal injection of GM-CSF (left) and artificial cerebrospinal fluid (aCSF) (right). a) Representative coronal tissue cryo-sectioned at 14 µ m and stained with MabTech α-Aβ/Alexa 546. Image is a montage of about 145 pictures taken at 10×. White spots indicate amyloid plaque immunolabeling (see Supplementary Fig. 4 online for representative montaged sections of all 4 mice). b) Significant overall plaque reductions seen in all 4 plaque parameters measured from 5 quantified sections per mouse (n = 4 mice). Error bars are ± Standard Error of the Mean. (Area: p < 1.11E-07; Perimeter: p < 1.41E-06; Feret Diameter: p < 2.36E-09; Integrated Density: p < 1.11E-07).
Daily subcutaneous injection of GM-CSF
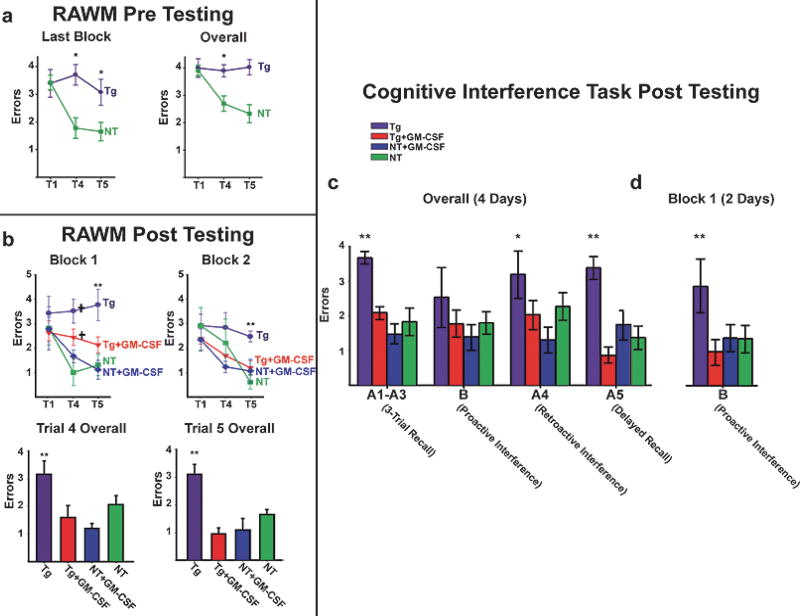
Based upon the positive results from intrahippocampal injections, we investigated the effect of subcutaneous GM-CSF injection on AD pathology and cognitive function. Prior to GM-CSF treatment, AβPPsw mice were first confirmed by RAWM testing to be cognitively-impaired for working memory (Fig. 2a). Both the NT control mice and the Tg mice were then sub-divided into two cognitively-balanced groups, for either GM-CSF or saline treatment. RAWM testing post-injection re-confirmed that Tg control mice were substantially impaired compared to NT control mice. This impairment was not only evident in individual blocks of testing, but also over all 4 days of testing (Fig. 2b). In sharp contrast, GM-CSF-treated Tg mice performed equally well or better than NT control mice during individual blocks and overall. GM-CSF-treated NT mice performed as well as or slightly better than NT controls (Fig. 2b).
Fig. 2.
Behavioral analysis following daily subcutaneous GM-CSF injections. a) Standard RAWM errors prior to treatment. The final block and overall performance of the Tg and NT mice during 8 days of consecutive daily pretreatment testing in the RAWM maze. The data was analyzed in four 2-day blocks and overall (Blocks 1–4). (*p < 0.02 or higher significance). b) Standard RAWM errors after treatment. Tg control mice (n = 6) show substantial impairment on working memory trials T4 and T5 compared to NT control mice (n = 8) in individual blocks of testing (upper), and over all 4 days of testing (lower). GM-CSF-treated Tg mice (n = 5) performed as well as or better than NT control mice on working memory trials T4 and T5 during individual blocks and over all. GM-CSF-treated NT mice (n = 9) performed similarly to or slightly better than NT controls (Note significantly better performance of NT+GM-CSF group versus NT group for T4 of Block 1), although this effect was not significant overall. (**p < 0.02 or higher significance versus all other groups; †p < 0.02 or higher significance versus Tg+GM-CSF and NT+GM-CSF). c) Cognitive Interference Task. Overall (4 Days) Tg control mice are impaired compared to NT mice on all four cognitive measures assessed. GM-CSF-treated Tg mice exhibited significantly better 3-trial recall (A1–A3) and delayed recall (A5) compared to Tg controls and performed similarly to NT mice in all four cognitive measures. GM-CSF treatment of NT mice did not result in significantly better performance compared to NT controls, although trends for a beneficial GM-CSF effect in NT mice were evident overall. (*Tg significantly different from NT+GM-CSF, **Tg significantly different from all other groups). d) Cognitive Interference Task. Proactive Interference testing (First 2 days). GM-CSF-treated Tg mice performed significantly better than Tg controls and equally to NT and GM-CSF-treated NT mice.
Before evaluation in the Cognitive Interference Task, the mice rested two days. This task mimics human interference testing, which discriminates between normal aged, MCI, and AD patients [36]. In three of four cognitive interference measures assessed over 4 days of testing (Fig. 2c), Tg control mice were clearly impaired compared to NT mice, and Tg mice treated with GM-CSF exhibited significantly better three-trial recall and delayed recall compared to Tg controls. Indeed, for all four cognitive measures, GM-CSF-treated transgenic AD mice performed similarly to NT mice. A particularly strong effect of GM-CSF treatment in Tg mice was evident for the proactive interference measure during the first half of testing (Fig. 2d), wherein GM-CSF-treated Tg mice performed substantially better than Tg controls and identically to both groups of NT mice. Including this strong effect on proactive interference testing, GM-CSF treatment resulted in significantly better performance of Tg mice for all four measures of cognitive interference testing. Proactive interference susceptibility has been reported to be a more sensitive marker for differentiating MCI and AD patients from aged controls than traditional measures of delayed recall and rate of forgetting [36]. Parenthetically, even the GM-CSF-treated NT mice showed a trend towards improved cognition in behavioral studies, albeit not statistically significant. Analysis of swim speed for both the RAWM and cognitive interference tasks revealed that Tg control mice were significantly faster than the other three groups in the RAWM task and for two of four measures in the Cognitive Interference task (3-trial recall and delayed recall). However, since error numbers were utilized for statistical analysis of both tasks, this difference in swim speed was negated since it is important only if latency measures had been used.
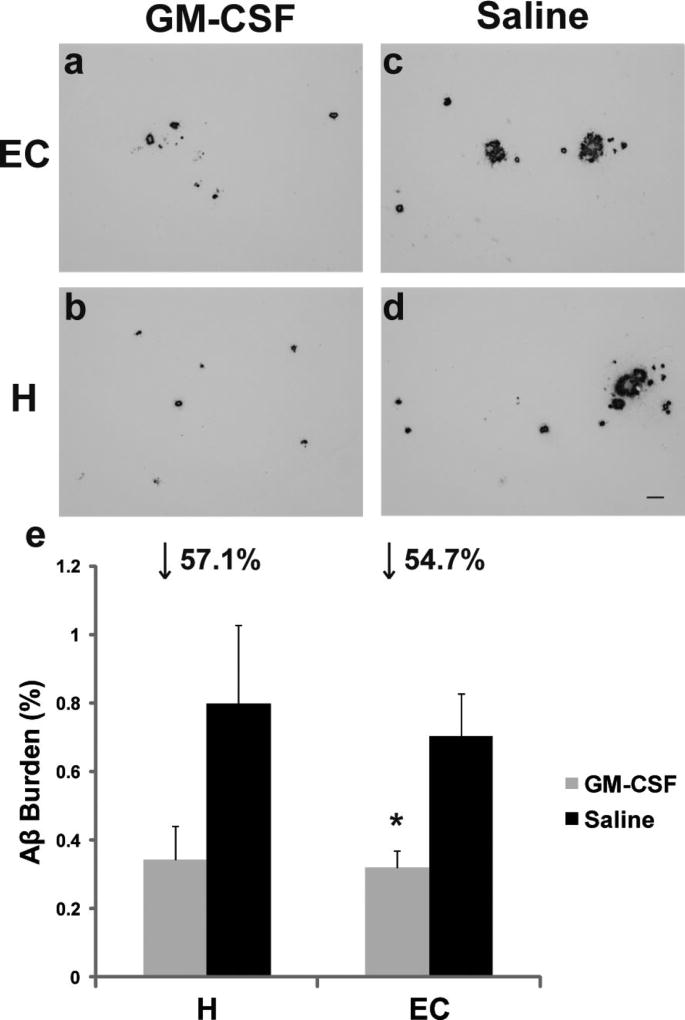
Following completion of all behavioral evaluations, subsequent analysis of brains from Tg mice of this study revealed that GM-CSF treatment induced large reductions in amyloid burdens within entorhinal cortex (↓55%) and hippocampal (↓57%) compared to control Tg mice (Fig. 3). The improved cognitive function and reduced cortical amyloidosis of GM-CSF-treated Tg mice were paralleled by increased microglial density as compared to saline-treated Tg mice (Fig. 4), implying an augmented ability to bind and remove amyloid deposition [27,28]. The GM-CSF-treated Tg mice similarly demonstrated increased synaptophysin immunoreactivity in both CA1 and CA3 regions (Fig. 5), indicating increased synaptic area in these hippocampal regions. Prior work has shown that adult neural stem cells in hippocampal dentate gyrus (DG) express GM-CSF receptors, and GM-CSF increases neuronal differentiation of these cells in a dose-dependent fashion [38]. Thus, one mechanism for the observed GM-CSF-induced cognitive improvement is enhanced removal of deposited Aβ in hippocampus, with ensuing neuronal growth/synaptic differentiation of DG mossy fiber innervation to CA3, resulting in increased innervation/synaptogenesis of Schaffer collaterals into CA1. Removal of deposited Aβ from entorhinal cortex may also increase perforant pathway viability to hippocampal projection fields in DG and CA1.
Fig. 3.
Amyloid deposition in subcutaneous GM-CSF-injected mice. a–d) Photomicrographs of coronal 5 µm paraffin-embedded sections immunolabelled with anti-Aβ antibody (clone 4G8) in entorhinal cortex (EC) and hippocampus (H). Pictures are representative of amyloid load closest to the mean of the GM-CSF- or saline-treated Tg groups. Scale bar = 50 µm. e) Percent of amyloid burden from the average of five 5 µm sections (150 µm apart) through both anatomic regions of interest (hippocampus and entorhinal cortex) per mouse of GM-CSF-treated (n = 5) versus saline-treated (n = 6). Entorhinal cortex (*p < 0.026), and hippocampus (p = 0.12).
Fig. 4.
Microglial immunostaining in subcutaneous GM-CSF-injected mice. a–d) Photomicrographs of coronal 5 µm paraffin-embedded sections immunolabeled with Iba-1 antibody in entorhinal cortex (EC) and hippocampus (H). Pictures are representative of Iba-1 immunolabeling closest to the mean of the GM-CSF- or saline control-treated groups. Scale bar = 50 µm. e) Percent of Iba1 burden from the average of five 5 µm sections (150 µm apart) through both anatomic regions of interest per mouse of GM-CSF-treated (n = 5) versus saline-treated (n = 6). Hippocampus (p < 0.02), entorhinal cortex (p < 0.05).
Fig. 5.
Synaptophysin immunostaining in subcutaneous GM-CSF-injected mice. a–d) Photomicrographs of coronal 5 µm paraffin-embedded sections immunolabeled with antisynaptophysin antibody. Pictures are representative of synaptophysin immunolabeling closest to the mean of the GM-CSF- or saline control-treated groups. Scale bar = 50 µm. e) Percent of synaptophysin immunoreactivity from the average of 5 sections per mouse of GM-CSF-treated (n = 5) versus saline control-treated (n = 6). CA1 (p < 0.0013), CA3 (p < 0.0023).
Thus GM-CSF-induced reduction of amyloidosis and enhancement of hippocampal/entorhinal cortex circuitry, critical for working (short-term) memory, may underlie GM-CSF’s reversal of working memory impairment in Alzheimer’s Tg mice.
DISCUSSION
Since peripheral leukocyte populations are increased in RA and possess the ability to infiltrate into the brain, we initially investigated M-CSF, G-CSF, and GM-CSF to determine which CSF might affect amyloidosis after direct injection into the brain. In the vasculature, all three CSFs work to drive the proliferation, differentiation, and survival of their respective innate leukocytes from monocytic precursors and would be expected to have similar effects on microglial precursors.
In our study, we found different functional effects for each intrahippocampal-injected CSF. In the MCSF injected mice, there was no effect on amyloidosis, but pathological changes were noticed, such as swelling and hyperplasia (Supplementary Fig. 2). Parenchymal overexpression of MCSF in any organ is probably not advisable as overexpression of MCSF and/or its receptor within mammary glands has similarly resulted in tumor formation and hyperplasia [39]. In a study by Boissonneault et al. (2009), the authors published that chronic intraperitoneal injection of MCSF prevents and reverses amyloid deposition and cognitive impairment and induces a large accumulation of bone marrow-derived microglia in the brain [40]. The authors also confirmed previous research [17] that bone marrow-derived microglia efficiently phagocytose and internalize Aβ. Although the authors did not relate their findings to RA’s inverse relationship with AD, their data provide evidence that upregulated MCSF in RA pathogenesis and systemic administration of M-CSF in AD patients may impart protection against AD onset or progression. However, M-CSF’s activation of mature osteoclasts, macrophages, and other innate leukocytes, induced thrombocytopenia, and autocrine signaling in some tumors may limit its usage in the clinical setting.
Peripheral administration of G-CSF has also been found to ameliorate amyloid pathology and reduce cognitive defects in AD models [37,41], which corroborates our observations of modest amyloid reduction by intrahippocampal injection. In the study by Sanchez-Ramos et al. [37], the authors also show a large increase in bone marrow-derived microglia with corresponding amyloid reductions, increased synaptic area, and partial reversal of cognitive impairment.
Although the M-CSF and G-CSF intrahippocampal findings are encouraging, our GM-CSF intrahippocampal injections into an AD mouse model demonstrated a much more pronounced reduction of amyloidosis. These data led us to further examine GM-CSF on the behavior of aged cognitively-impaired AD mice. Subcutaneous administration of GM-CSF resulted in almost complete reversal of cognitive impairment, resulting in function similar to that of wild-type mice, with a corresponding average of about 50% reduction of amyloidosis in entorhinal cortex and hippocampus. In contrast, M-CSF and G-CSF have been shown to only partially reverse cognitive impairment in aged AD mice, although the preventative chronic study of M-CSF administration into young AD mice showed equal cognitive function over time compared to wild-type controls. This cognitive advantage of peripherally-administered GM-CSF into aged AD mice could result from a combinatorial effect of increased macrophage and granulocyte populations from bone marrow-derived monocytic precursors in the periphery, as well as induction of other innate leukocyte subsets in both periphery and brain. Indeed, GM-CSF has been shown to pass the blood brain barrier [42], and a recent study showed that GM-CSF injected into the brains of normal mice activates microglia [43]. Further explanations for the very robust cognitive benefits of GM-CSF in our experiments are multiple, including the aforementioned augmentation of peripheral erythropoietic amyloid-clearance mechanisms [31,32,44], as well as increased neurogenesis [38], increased cerebral angiogenesis [45], neuroprotection from apoptosis [46], reduction in amyloidosis (Figs 1,3, and Supplementary Fig. 5), and increased neuronal plasticity (Fig. 5).
Our results mirror those reported for G-CSF [37], and supports the current practice of interchangeable prescription of either recombinant human GM-CSF (Leukine) or GCSF (Granocyte, Neupogen, and Neulasta) into patients with depressed bone marrow function. G-CSF primarily treats neutropenia while GM-CSF treats all leukopenia, and both have long records of safety data from two decades of FDA-approved usage. Rare adverse events are usually mild febrile incidents that quickly subside upon cessation of administration. G-CSF is currently in clinical trial for stroke and was recently approved for an AD Phase II clinical trial. However, GM-CSF/Leukine is more effective in the AD mouse model and while neutrophils are short-lived leukocytes, the fact that GM-CSF induces the upregulation of all innate cells means that it could potentially impart prolonged protective effects against AD.
The failure of NSAID clinical trials in AD and the multiple studies that show that a defective innate immune system propagates AD pathology encouraged us to develop and test our hypothesis that intrinsic pathogenic properties of RA are protective against AD. Indeed the beneficial effects of all three RA-upregulated CSFs, especially GM-CSF, in mouse models of AD point to a potential new approach to AD therapy and indicate that age-linked depressed hematopoiesis may be etiological for AD pathogenesis.
Supplementary Material
Acknowledgments
Support for this work was provided for by the USF Health Byrd Alzheimer’s Center and Research Institute, the Eric Pfeiffer Chair for research on Alzheimer’s disease, the Florida Alzheimer’s Disease Research Center (P50AG25711), and the James H. and Martha M. Porter Alzheimer’s Fund.
Footnotes
Supplementary data available online at http://www.j-alz.com/issues/21/vol21-2.html#supplementarydata03
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=380).
References
- 1.McGeer PL, Rogers J, McGeer EG. Inflammation, anti-inflammatory agents and Alzheimer disease: the last 12 years. J Alzheimers Dis. 2006;9:271–276. doi: 10.3233/jad-2006-9s330. [DOI] [PubMed] [Google Scholar]
- 2.Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okazaki H, Reagan TJ, Campbell RJ. Clinicopathologic studies of primary cerebral amyloid angiopathy. Mayo Clin Proc. 1979;54:22–31. [PubMed] [Google Scholar]
- 4.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 5.Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 6.Lee VM. Regulation of tau phosphorylation in Alzheimer’s disease. Ann N Y Acad Sci. 1996;777:107–113. doi: 10.1111/j.1749-6632.1996.tb34408.x. [DOI] [PubMed] [Google Scholar]
- 7.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wisniewski T, Castano EM, Golabek A, Vogel T, Frangione B. Acceleration of Alzheimer’s fibril formation by apolipoprotein E in vitro. Am J Pathol. 1994;145:1030–1035. [PMC free article] [PubMed] [Google Scholar]
- 9.Ma J, Yee A, Brewer HB, Das S, Potter H. The Alzheimer amyloid-associated proteins al-antichymotrypsin and apolipoprotein E promote the assembly of the Alzheimer β-protein into filaments. Nature. 1994;372:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 10.Potter H, Wefes IM, Nilsson LN. The inflammation-induced pathological chaperones ACT and apo-E are necessary catalysts of Alzheimer amyloid formation. Neurobiol Aging. 2001;22:923–930. doi: 10.1016/s0197-4580(01)00308-6. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson LN, Arendash GW, Leighty RE, Costa DA, Low MA, Garcia MF, Cracciolo JR, Rojiani A, Wu X, Bales KR, Paul SM, Potter H. Cognitive impairment in PDAPP mice depends on ApoE and ACT-catalyzed amyloid formation. Neurobiol Aging. 2004;25:1153–1167. doi: 10.1016/j.neurobiolaging.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Padmanabhan J, Levy M, Dickson DW, Potter H. Alpha1-antichymotrypsin, an inflammatory protein overexpressed in Alzheimer’s disease brain, induces tau phosphorylation in neurons. Brain. 2006;129:3020–3034. doi: 10.1093/brain/awl255. [DOI] [PubMed] [Google Scholar]
- 13.Koenigsknecht-Talboo J, Meyer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn MB, Hyman BT, Bacskai BJ, Holtzman DM. Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice. J Neurosci. 2008;28:14156–14164. doi: 10.1523/JNEUROSCI.4147-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- 17.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 19.Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Parsonage G, Filer A, Bik M, Hardie D, Lax S, Howlett K, Church LD, Raza K, Wong SH, Trebilcock E, Scheel-Toellner D, Salmon M, Lord JM, Buckley CD. Prolonged, granulocyte-macrophage colony-stimulating factor-dependent, neutrophil survival following rheumatoid synovial fibroblast activation by IL-17 and TNFalpha. Arthritis Res Ther. 2008;10:R47. doi: 10.1186/ar2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox CA, Shi G, Yin H, Vistica BP, Wawrousek EF, Chan CC, Gery I. Both Th1 and Th17 are immunopathogenic but differ in other key biological activities. J Immunol. 2008;180:7414–7422. doi: 10.4049/jimmunol.180.11.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu WD, Firestein GS, Taetle R, Kaushansky K, Zvaifler NJ. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989;83:876–882. doi: 10.1172/JCI113971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura H, Ueki Y, Sakito S, Matsumoto K, Yano M, Miyake S, Tominaga T, Tominaga M, Eguchi K. High serum and synovial fluid granulocyte colony stimulating factor (G-CSF) concentrations in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2000;18:713–718. [PubMed] [Google Scholar]
- 24.Olszewski WL, Pazdur J, Kubasiewicz E, Zaleska M, Cooke CJ, Miller NE. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 2001;44:541–549. doi: 10.1002/1529-0131(200103)44:3<541::AID-ANR102>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Kawaji H, Yokomura K, Kikuchi K, Somoto Y, Shirai Y. [Macrophage colony-stimulating factor in patients with rheumatoid arthritis] Nippon Ika Daigaku Zasshi. 1995;62:260–270. doi: 10.1272/jnms1923.62.260. [DOI] [PubMed] [Google Scholar]
- 26.Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc Natl Acad Sci U S A. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 29.Emerson SG, Sieff CA, Wang EA, Wong GG, Clark SC, Nathan DG. Purification of fetal hematopoietic progenitors and demonstration of recombinant multipotential colony-stimulating activity. J Clin Invest. 1985;76:1286–1290. doi: 10.1172/JCI112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 31.Rogers J, Li R, Mastroeni D, Grover A, Leonard B, Ahern G, Cao P, Kolody H, Vedders L, Kolb WP, Sabbagh M. Peripheral clearance of amyloid beta peptide by complement C3-dependent adherence to erythrocytes. Neurobiol Aging. 2006;27:1733–1739. doi: 10.1016/j.neurobiolaging.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 32.Helmy KY, Katschke KJ, Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 33.Bennett SP, Boyd TD, Norden M, Padmanabhan J, Neame P, Wefes I, Potter H. A novel technique for simultaneous bilateral brain infusions in a mouse model of neurodegenerative disease. J Neurosci Methods. 2009;184:320–326. doi: 10.1016/j.jneumeth.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 35.Echeverria V, Burgess S, Gamble-George J, Zeitlin R, Lin X, Cao C, Arendash GW. Sorafenib inhibits nuclear factor kappa B, decreases inducible nitric oxide synthase and cyclooxygenase-2 expression, and restores working memory in APPswe mice. Neuroscience. 2009;162:1220–1231. doi: 10.1016/j.neuroscience.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 36.Loewenstein DA, Acevedo A, Luis C, Crum T, Barker WW, Duara R. Semantic interference deficits and the detection of mild Alzheimer’s disease and mild cognitive impairment without dementia. J Int Neuropsychol Soc. 2004;10:91–100. doi: 10.1017/S1355617704101112. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Ramos J, Song S, Sava V, Catlow B, Lin X, Mori T, Cao C, Arendash GW. Granulocyte colony stimulating factor (G-CSF) decreases brain amyloid burden and reverses cognitive impairment in Alzheimer’s mice. Neuroscience. 2009;163:55–72. doi: 10.1016/j.neuroscience.2009.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kruger C, Laage R, Pitzer C, Schabitz WR, Schneider A. The hematopoietic factor GM-CSF (granulocyte-macrophage colony-stimulating factor) promotes neuronal differentiation of adult neural stem cells in vitro. BMC Neurosci. 2007;8:88. doi: 10.1186/1471-2202-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirma N, Luthra R, Jones J, Liu YG, Nair HB, Mandava U, Tekmal RR. Overexpression of the colony-stimulating factor (CSF-1) and/or its receptor c-fms in mammary glands of transgenic mice results in hyperplasia and tumor formation. Cancer Res. 2004;64:4162–4170. doi: 10.1158/0008-5472.CAN-03-2971. [DOI] [PubMed] [Google Scholar]
- 40.Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain. 2009;132:1078–1092. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- 41.Tsai KJ, Tsai YC, Shen CK. G-CSF rescues the memory impairment of animal models of Alzheimer’s disease. J Exp Med. 2007;204:1273–1280. doi: 10.1084/jem.20062481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLay RN, Kimura M, Banks WA, Kastin AJ. Granulocyte-macrophage colony-stimulating factor crosses the blood–brain and blood–spinal cord barriers. Brain. 1997;120(Pt 11):2083–2091. doi: 10.1093/brain/120.11.2083. [DOI] [PubMed] [Google Scholar]
- 43.Reddy PH, Manczak M, Zhao W, Nakamura K, Bebbington C, Yarranton G, Mao P. Granulocyte-macrophage colony-stimulating factor antibody suppresses microglial activity: implications for anti-inflammatory effects in Alzheimer’s disease and multiple sclerosis. J Neurochem. 2009;111:1514–1528. doi: 10.1111/j.1471-4159.2009.06432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 45.Schneider UC, Schilling L, Schroeck H, Nebe CT, Vajkoczy P, Woitzik J. Granulocyte-macrophage colony-stimulating factor-induced vessel growth restores cerebral blood supply after bilateral carotid artery occlusion. Stroke. 2007;38:1320–1328. doi: 10.1161/01.STR.0000259707.43496.71. [DOI] [PubMed] [Google Scholar]
- 46.Schabitz WR, Kruger C, Pitzer C, Weber D, Laage R, Gassler N, Aronowski J, Mier W, Kirsch F, Dittgen T, Bach A, Sommer C, Schneider A. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF) J Cereb Blood Flow Metab. 2008;28:29–43. doi: 10.1038/sj.jcbfm.9600496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.