Abstract
Cancer stem cells (CSCs) are associated with tumor initiation, therapeutic resistance, relapse and metastasis. However, the underlying mechanisms CSCs use to preserve stemness are not yet fully understood. The present study demonstrated that the expression of RAB27A, member RAS oncogene family (Rab27a), which was reported to promote tumor progression by upregulating exocytosis of extracellular vesicles, was higher in mammosphere cells than in adherent MDA-MB-231 breast cancer cells. Downregulation of Rab27A inhibited mammosphere formation by decreasing the proportion of CD44+CD24-/low cells of the MDA-MB-231 cell line. Furthermore, Rab27A overexpression redistributed the cell cycle of breast (b) CSCs. The present study revealed that downregulation of Rab27A enhanced the capacity of metformin, the most widely used oral hypoglycemic drug for the treatment of type II diabetes, to inhibit mammosphere growth. Metformin reduced the expression of Rab27A dose-dependently. These data suggested that Rab27A acts as a mediator of human bCSCs by promoting the growth of mammospheres and that synergistic suppression of Rab27A, alone or in combination with metformin, holds promise for therapeutically targeting bCSCs.
Keywords: RAB27A, member RAS oncogene family, breast cancer stem cell, metformin, mammosphere
Introduction
It has become increasingly evident that a small proportion of cancer cells are cancer stem cells (CSCs), CSC-like cells or tumor initiating cells (TICs), which are associated with tumor initiation, propagation, metastasis and therapy failure (1). CSCs are characterized by a self-renewal capacity, multi-lineage differentiation properties and high tumorigenicity in immunodeficient mice. Such cells have been demonstrated to be resistant to conventional chemotherapy, including 5-fluorouracil, vincristine and cisplatin, or radiotherapy and may cause cancer initiation, metastasis, and recurrence (2–5). Al-Hajj et al (6) first reported that epithelial cell adhesion molecule (ESA)+ cluster of differentiation (CD) 44+CD24−/low lineage-negative (Lin−) human breast cancer cells were significantly enriched for tumor-forming ability in non-obese diabetic/severe combined immunodeficient mice compared with Lin− cells with other phenotypes. Subsequently, breast cancer cells with high aldehyde dehydrogenase 1 activity or a CD61high/CD49fhigh subpopulation were identified as CSCs (7,8). These studies not only provided evidence supporting CSC theory but also established breast (b) CSC markers. These markers, alone or in combination, may act as a signature for defining not only bCSCs but also the progressive state (1,9).
Rab proteins are members of the Ras-related small guanosine triphosphate superfamily and are believed to control certain cellular events, including secretion (10,11). Genomic analysis revealed that the Rab family is composed of 60 members in Homo sapiens (12). RAB27A, member RAS oncogene family (Rab27a), is a key protein for intracellular secretion and contains two isoforms: Rab27A and Rab27B. Loss-of-function mutations in human Rab27A result in Griscelli syndrome, a rare autosomal disorder characterized by partial cutaneous albinism and severe immunodeficiency (13–15). Multiple studies have assessed the functions of Rab27A in the exocytosis of insulin and chromaffin granules in endocrine cells (16–21). These data suggested that Rab27A protein is widely expressed in specialized secretory cells, including exocrine, particularly mucin- and zymogen-secreting cells; endocrine; ovarian and hematopoietic cells, most of which undergo regulated exocytosis (19,22). Previously, Rab27A was reported to promote various types of tumor progression (17,23–28). Rab27A overexpression promotes the growth and metastasis of breast cancer and melanoma in an exosome-dependent or independent manner (29).
Metformin is the most widely used oral hypoglycemic drug for the treatment of type II diabetes (30). Epidemiological studies have previously indicated that metformin may reduce the incidence of cancer in diabetics and improve the outcomes of numerous types of cancer (31–38). Metformin has been revealed to preferentially kill CSCs over non-CSCs in different types of breast tumor, through inhibiting the associated inflammatory response by decreasing the expression of CSC-specific genes (39–42). Metformin was also reported to overcome trastuzumab resistance by specifically killing breast cancer-initiating CD44+CD24−/low cells and by inhibiting erb-b2 receptor tyrosine kinase 2/insulin-like growth factor (IGF)-1R receptor interactions (43–45).
The present study assessed the effect of Rab27A on breast cancer stem cells and examined the underlying molecular mechanisms. The results of the present study demonstrated that decreasing Rab27A expression inhibited the growth of mammospheres by decreasing the proportion of CD44+CD24−/low cells in the MDA-MB-231 cell line, and that a combination of Rab27A downregulation and metformin more effectively eliminated CSCs compared with downregulating Rab27A or using metformin alone. The results of the present study indicated that Rab27A and metformin may be successfully combined to potentiate anti-CSC activities.
Materials and methods
Cell lines and mammosphere culture
The MDA-MB-231 and SK-BR-3 breast cancer cell lines were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and were maintained in complete growth medium according to the manufacturer's protocol. The medium used for the MDA-MB-231 cell line was L-15 medium (Hyclone; GE Healthcare Life Sciences, Chalfont, UK). Complete growth medium included 0.4% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The complete growth medium for the SK-BR-3 cell line was McCoy's 5a medium (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS. Cells were maintained at 37°C in a humidified incubator with 5% CO2. To assay mammosphere formation, single cells were plated in ultralow attachment plates (Corning Incorporated, Corning, NY, USA) at a density of 1,000 viable cells/ml in in serum-free Dulbecco's modified Eagle's medium/F12 (Gibco; Thermo Fisher Scientific, Inc.), supplemented with B27 (Invitrogen; Thermo Fisher Scientific, Inc.), 20 ng/ml human recombinant epidermal growth factor and 10 ng/ml basic fibroblastic growth factor, 0.4% bovine serum albumin (BSA) and 4 mg/ml insulin (all from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Mammospheres were cultured for 6 days in an incubator. Mammospheres were treated with metformin for 6 days (Sigma-Aldrich; Merck KGaA) at the concentration of 1 mM or 5 mM at 37°C. Mammospheres were observed by using a light microscope.
Plasmid construction
The entire open reading frame of human Rab27A (gene ID 5873) was amplified from 293T cells, which were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Total RNA was extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Complementary (c)DNA was synthesized from 0.5–1 mg of total RNA using the SuperScript II reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.), by incubating 2 µg total RNA and 2 µl random primers at 70°C for 5 min, and rapid cooling on ice for 5 min. The following components were added to a nuclease-free microcentrifuge tube: 2 µl dNTP (10 mM), 4 µl 5X First-Strand buffer, 2 µl DTT (0.1 M), 1 µl M-MLV reverse transcriptase, sterile distilled water to 20 µl, and the mixture was incubated at 37°C for 2 h. The Rab27A was cloned by polymerase chain reaction (PCR) using the following gene-specific primers: Rab27A BamHI forward, 5′-CGCGGATCCATGTCTGATGGAGATTATG-3′ (Sangon Biotech Co., Ltd., Shanghai, China) and Rab27A HindIII reverse, 5′-CCCAAGCTTTCAACAGCCACATGCCCCTT-3′ (Sangon Biotech Co., Ltd.) and then fused in-frame into the multiple cloning site of a pENTR3C vector (Invitrogen; Thermo Fisher Scientific, Inc.). Using the LR recombination reaction (Invitrogen; Thermo Fisher Scientific, Inc.). The following components were added to a microcentrifuge tube: 100 ng pENTR-Rab27A plasmid, 1 µl pLenti6/V5-DEST (150 ng/ml), 2 µl LR Clonase II, steriled, distilled TE buffer to 10 µl, and the mixture was incubated at 25°C overnight. Rab27A was subcloned into a destination lentiviral (L.V.) vector (Invitrogen; Thermo Fisher Scientific, Inc.) as recommended by the manufacturer (19), subsequently named L.V.-Rab27A.
The sequence of small interfering (si)RNA-targeting Rab27A (Sangon Biotech Co., Ltd.) was as follows: Sense, 5′-CGGAUCAGUUAAGUGAAGAAA-3′ and antisense, 5′-UUUCUUCACUUAACUGAUCCG-3′. A scrambled siRNA was used as a negative control: Sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense, 5′-ACGUGACACGUUCGGAGAATT-3′.
Virus packaging and infection
293T cells were transfected with the L.V.-Rab27A plasmid in vitro using Lipofectamine® 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.) for 6 h at room temperature. Viruses were subsequently harvested in liquid state after 48 h by centrifugation (157 × g for 5 min at room temperature). A total of 2 ml virus and 10 µg/ml polybrene (Sigma-Aldrich; Merck KGaA) were added to the MDA-MB-231 breast cancer cells, and cells were cultured for 6 h at 37°C. Then the culture medium was replaced by complete medium. After 72 h, infected cells were selected by blasticidin at 37°C (Sigma-Aldrich; Merck KGaA).
RT-quantitative (q)PCR
Total RNA was extracted from 1×106 cells using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. cDNA was synthesized, as aforementioned, from 0.5–1.0 mg total RNA using SuperScript II reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR reactions were performed in a 10 µl reaction volume in triplicate. SYBR-Green II (Invitrogen; Thermo Fisher Scientific, Inc.) was used to detect PCR products. PCR amplification consisted of a 10 min denaturation step at 95°C followed by 45 cycles of PCR at 95°C for 30 sec, 56°C for 30 sec and 72°C for 15 sec. Results were analyzed using the 2−ΔΔCq method (46). Data were presented as the fold difference in gene expression normalized to that of the housekeeping gene human GAPDH and relative to a relevant reference sample. The qPCR primer sequences were as follows: Rab27A sense, 5′-TGGAGGACCAGAGAGTAGTGAAA-3′ and antisense, 5′-AGTTTCAAAGTAGGGGATTCCA-3′; cyclin D sense, 5′-TGTGCATCTACACCGACAAC-3′ and antisense, 5′-AGGAAGTGTTCAATGAAATCGT-3′; CDK4 sense, 5′-TTGCATCGTTCACCGAGATC-3′ and antisense, 5′-CTGGTAGCTGTAGATTCTGGC-3′; p27 sense, 5′-GCTAACTCTGAGGACACGCA-3′ and antisense, 5′-TAGAAGAATCGTCGGTTGCAGG-3′; GAPDH sense, 5′-GACCTGACCTGCCGTCTA-3′ and antisense, 5′-AGGAGTGGGTGTCGCTGT-3′.
Western blot analysis
Cells (1×106) were lysed in 200 µl RIPA buffer (Beyotime Institute of Biotechnology, Haimen, China) for 10 min at 4°C. Lysate was cleared using centrifugation at 2,250 × g for 10 min at 4°C. The supernatant was collected as total proteins and was quantified using the BCA assay (Pierce, Thermo Fisher Scientific, Inc.). SDS-PAGE (12%) was used to separate total proteins (50 µg/lane). Proteins were subsequently transferred to polyvinylidene fluoride membranes (Invitrogen; Thermo Fisher Scientific, Inc.). Membranes were blocked using Blocking Buffer, pH=7.5 (Beyotime Institute of Biotechnology). Membranes were incubated with antibodies raised against Rab27A (cat no. WH0005873M2; dilution, 1:500; Sigma-Aldrich; Merck KGaA), CDK4 (cat no. ab108357; dilution, 1:500), cyclin D (cat no. ab134175; dilution, 1:500), p27 (cat no. ab32034; dilution, 1:500) (all from Abcam, Cambridge, UK) at 4°C overnight. GAPDH (cat no. ab8245; dilution, 1:1,000) or β-actin (cat no. ab8226; dilution, 1:1,000) (both from Abcam) was used as an internal control. The secondary antibodies IRDye® 800CW goat anti-rabbit IgG (H+L; cat no. 925-32211) and IRDye® 800CW goat anti-mouse IgG (H+L; cat no. 925-32210) (both from LI-COR Biosciences, Lincoln, NE, USA) were used at 1:10,000 dilution. Bands were obtained using an Odyssey imaging system (LI-COR Biosciences), and the protein density was quantified in triplicate using Odyssey software version 1.2 (LI-COR Biosciences).
Flow cytometry assay
Cells (1×106/100 ml) were blocked with RPMI-1640 medium containing 3% BSA (Beyotime Institute of Biotechnology) for 30 min on ice. Cells were subsequently incubated with the following primary antibodies: CD44-fluorescein isothiocyanate (FITC) and CD24-phycoerythrin (PE; dilution, 1:100 dilution for 106 cells/100 ml; BD Biosciences, San Jose, CA, USA). Cells were subsequently washed using PBS with 3% BSA. Analysis was performed using a fluorescence-activated cell sorting (FACS) Vantage SE flow cytometer (CellQuest Pro version 6.1 software; BD Biosciences).
Statistical analysis
All in vitro experiments were performed either in triplicate. Results were presented as mean ± standard deviation. Statistical significance of variances between group means was analyzed using either a Student's t-test or one-way analysis of variance. P<0.05 was considered to indicate a significant difference. Statistical analyses were performed using SPSS software version 17.0 (IBM Corp., Armonk, NY, USA).
Results
Expression of Rab27A was upregulated in MDA-MB-231 mammospheres
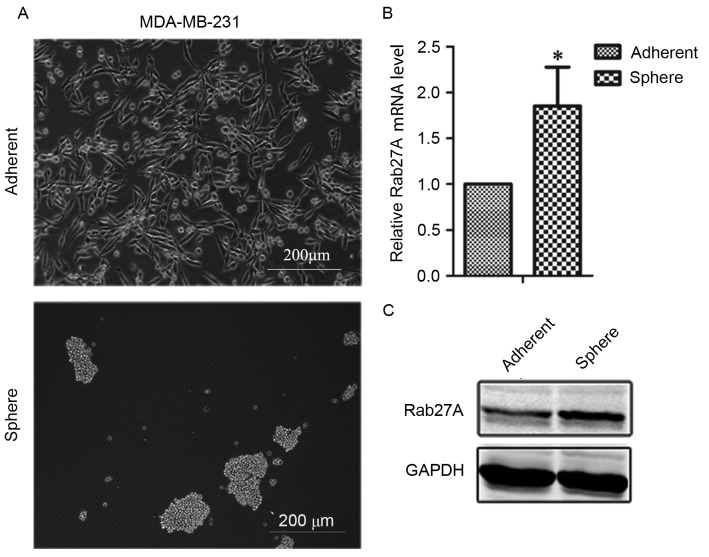
Mammospheres were generated from the estrogen receptor (ER)-negative breast cancer cell line MDA-MB-231 in serum-free media. MDA-MB-231 cells formed aggregated clusters, rather than compact mammospheres (Fig. 1A). The expression of Rab27A was upregulated in MDA-MB-231 spheres compared with adherent cells (Fig. 1B and C), suggesting that Rab27A may serve a function in mammospheres.
Figure 1.
Rab27A expression was upregulated in MDA-MB-231 mammospheres. (A) Representative images of mammospheres and adherent cells of the breast cancer cell line MDA-MB-231. (B) Reverse transcription-quantitative polymerase chain reaction analysis revealed upregulation of Rab27A expression in MDA-MB-231 mammospheres. (C) Western blot analysis demonstrated that Rab27A expression was higher in MDA-MB-231 sphere cells than in adherent cells. Data here presented as the mean ± standard deviation and experiments were performed in triplicates. *P<0.05 vs. control.
Downregulation of Rab27A inhibited mammosphere formation by decreasing the proportion of CD44+/CD24−/low cells
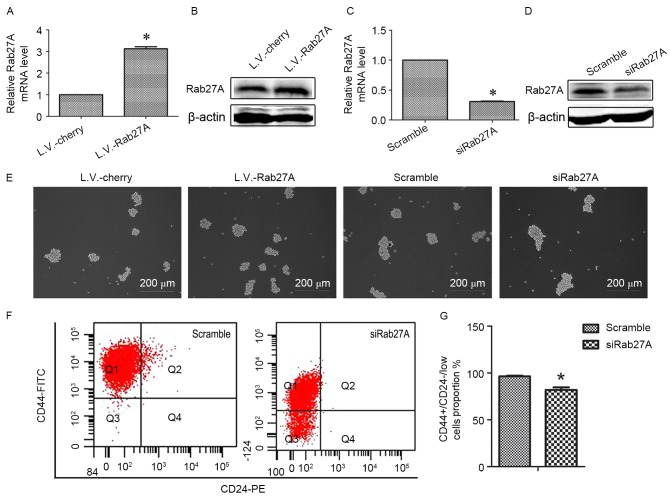
MDA-MB-231 cell lines overexpressing Rab27A (L.V.-Rab27A) were used to assess the function of Rab27A in breast cancer stem cells (Fig. 2). Rab27A was successfully overexpressed in MDA-MB-231 cell lines following transfection with L.V.-Rab27A compared with control group cells which were transfected with the mCherry, a red fluorescence protein (L.V.-Cherry; Fig. 2A and B). L.V.-Rab27A-treated MDA-MB-231 cells formed more mammospheres compared with control L.V.-Cherry-treated cells (Fig. 2E). Rab27A-targeting siRNA sequences were designed (Fig. 2C and D). Following Rab27A knockdown via RNAi, sphere numbers decreased (Fig. 2E). Similar results were revealed in the SK-BR-3 cell line (data not shown).
Figure 2.
Rab27A increased the growth of MDA-MB-231 mammospheres. (A) RT-qPCR analysis of the mRNA level of Rab27A following overexpression of Rab27A. (B) Western blot analysis of the protein level of Rab27A following overexpression of Rab27A. (C) RT-qPCR analysis of the mRNA level of Rab27A following interference with Rab27A expression. (D) Western blot analysis of the protein level of Rab27A following interference with Rab27A expression. (E) Following Rab27A overexpression or knockdown, respectively, mammosphere formation by the MDA-MB-231 cell line was observed using a light microscope. (F) FACS demonstrated the proportion of CD44+/CD24−/low cells of the MDA-MB-231 cell line following Rab27A knockdown. (G) Data of (F) represented as the mean ± SD. Error bars=SD between triplicates. *P<0.05 vs. control. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; FACS, fluorescence-activated cell sorting; CD, cluster of differentiation; SD, standard deviation.
Expression of the prospective bCSC markers CD44-FITC and CD24-PE was assessed using flow cytometry. There was a decreased proportion of CD44+/CD24−/low cells in the basal cell line, MDA-MB-231, following Rab27A downregulation (81.767±4.83%) compared with the scramble group (96.267±1.38%) (Fig. 2F and G) and no difference when Rab27A was overexpressed (data not shown). These data suggested that Rab27A may facilitate cancer development via the expanded self-renewal ability of bCSCs.
Rab27A overexpression redistributed the cell cycle of bCSCs
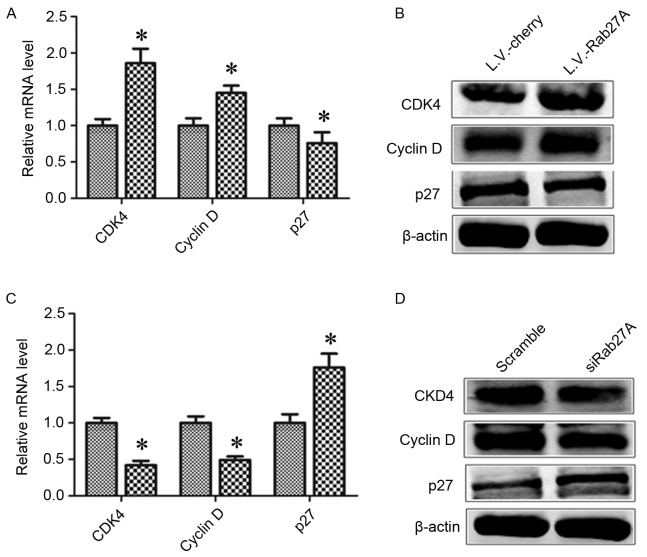
The present study assessed the impact of Rab27A expression on cell cycle distribution. Cell-associated markers were determined, including cyclin D1, cyclin-dependent kinase 4 (CDK4) and cyclin-dependent kinase inhibitor 1B (p27) in MDA-MB-231 spheres. Overexpression of Rab27A increased the expression of CDK4 and cyclin D1 but downregulated the expression of p27. The reverse results were demonstrated following Rab27A knockdown with RNAi (Fig. 3).
Figure 3.
Rab27A overexpression redistributed the cell cycle of breast cancer stem cells. The cell cycles of MDA-MB-231 sphere cells infected with L.V.-Cherry or L.V.-Rab27A were analyzed using (A) RT-qPCR and (B) western blot analysis. The cell cycles of MDA-MB-231 sphere cells infected with scramble siRNA, which was used as a negative control, or siRab27A were analyzed using (C) RT-qPCR and (D) western blot analysis. The expression of CDK4, cyclin D and p27 were revealed following ectopic up or downregulation of Rab27A. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; CDK4, cyclin-dependent kinase 4; p27, cyclin-dependent kinase inhibitor 1B.
Metformin inhibited the growth of mammospheres by downregulating the expression of Rab27A
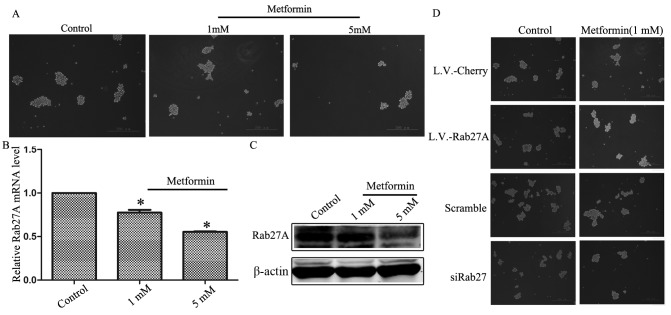
The effect of metformin (1 and 5 mM) on the growth of mammospheres was assessed. Metformin inhibited the growth of mammospheres in a dose-dependent manner when treated with L.V.-cherry (Fig. 4A). Simultaneously, it was revealed that metformin downregulated the expression of Rab27A in a dose-dependent manner (Fig. 4B and C). Thus, metformin inhibited mammosphere growth, and this was associated with decreased expression of Rab27A compared with mammosphere growth in untreated cells.
Figure 4.
Metformin inhibited mammosphere growth via downregulating the expression of Rab27A. (A) Representative images of mammospheres treated with 1 mM and 5 mM metformin, respectively. (B) The mRNA expression levels of Rab27A were analyzed using reverse transcription-quantitative polymerase chain reaction following treatment of MDA-MB-231 cells with metformin. (C) Protein expression levels of Rab27A were analyzed via western blot analysis following treatment of MDA-MB-231 cells with metformin. (D) Rab27A increased the sensitivity of mammospheres to metformin. Error bars=standard deviation between triplicates. *P<0.05 vs. control.
To assess the effect of combining Rab27A expression and metformin treatment, mammospheres overexpressing Rab27A were treated with 1 mM metformin. The growth of mammospheres was not inhibited five days following treatment. However, downregulation of Rab27A increased the capacity of metformin to inhibit mammosphere growth (Fig. 4D).
Discussion
Previous studies have indicated that CSCs are the source of tumor initiation, metastasis and treatment tolerance. However, CSCs account for only ~0.2–1% of cancer cells (1,3,4,5). bCSCs may be enriched by sorting for CD44+CD24−/low cells, by selecting for side-population cells that efflux Hoechst dyes (47) or by isolating mammospheres, spherical clusters of self-replicating cells, from suspension cultures (48). The present study established an assay of bCSC sphere formation. Sphere formation is an index of the self-renewal of CSC-like cells (49). The results of the present study revealed that MDA-MB-231 cells formed stem cell clusters resembling grapes rather than the classic sphere with which the MCF-7 cell line is associated (50). The spherical structure was potentially determined by the genetic characteristics of the cell line.
Expression of Rab27A was higher in mammospheres of the MDA-MB-231 cell line than in adherent cells. These results suggested that Rab27A may serve a function in maintaining the genetic characteristics of CSCs. Rab27A overexpression promoted mammosphere growth. The MDA-MB-231 cell line is highly malignant, and has been associated with tumor initiation, therapeutic resistance, relapse and metastasis (48,50). Rab27A knockdown inhibited mammosphere growth. The results of the present study revealed that Rab27A was involved in regulating the stemness of breast cancer. The molecular mechanisms with which Rab27A is associated were also assessed. The results of FACS demonstrated that the downregulation of Rab27A decreased the proportion of CD44+CD24−/low cells of the MDA-MB-231 cell line.
Cell-cell interactions within the microenvironment are key in the progression of CSCs to local tumor mass and distant metastases. For example, tumors may secrete chemokines or cytokines to modify their environment via signal exchange (51,52). Rab27A is crucial for intracellular secretion in an exosome-dependent or exosome-independent manner (29). Future work will include measuring cytokines secreted in culture media supernatant using a protein microarray and screening out the cytokine for which secretion capacity changed following exogenous expression of Rab27A. The antibody targeting the specifically screened-out cytokine will be prepared and added to the mammosphere culture medium to block the specific cytokine. It can then be assessed whether the antibody inhibits mammosphere growth. These experiments would provide novel insights concerning the therapeutic targeting of CSCs by preventing the secretion of cytokines, and identify an antibody that inhibits CSC growth.
Metformin is the most widely used oral hypoglycemic drug for the treatment of type II diabetes (30). Previously, metformin was reported to target CSCs in numerous types of cancer, including breast, and to regulate the expression of certain microRNAs and CSC-specific genes (44,53,54). The present study demonstrated that metformin inhibited mammosphere growth and reduced the expression of Rab27A in a dose-dependent manner. These results revealed that metformin inhibited mammosphere growth partly by decreasing the expression of Rab27A. The present study also demonstrated that metformin and Rab27A knockdown had a greater inhibitive effect on mammosphere growth when combined than when separate, potentially providing novel routes for clinical and basic research on tumor therapy.
Acknowledgements
The present study was supported by the Scientific and Technological Project of Shaanxi (grant no. 2016SF-177) and the Science Foundation of Shaanxi University of Chinese Medicine (grant no. 2015PY04).
References
- 1.Akbari-Birgani S, Paranjothy T, Zuse A, Janikowski T, Cieślar-Pobuda A, Likus W, Urasińska E, Schweizer F, Ghavami S, Klonisch T, Łos MJ. Cancer stem cells, cancer-initiating cells and methods for their detection. Drug Discov Today. 2016;21:836–842. doi: 10.1016/j.drudis.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Gerweck LE, Wakimoto H. At the crossroads of cancer stem cells, radiation biology, and radiation oncology. Cancer Res. 2016;76:994–998. doi: 10.1158/0008-5472.CAN-15-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: Models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 4.Wicha MS. Cancer stem cells and metastasis: Lethal seeds. Clin Cancer Res. 2006;12:5606–5607. doi: 10.1158/1078-0432.CCR-06-1537. [DOI] [PubMed] [Google Scholar]
- 5.Norton L. Cancer stem cells, EMT, and seeding: A rose is a rose is a rose? Oncology (Williston Park) 2011;25:30. 32. [PubMed] [Google Scholar]
- 6.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo PK, Kanojia D, Liu X, Singh UP, Berger FG, Wang Q, Chen H. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin-TGFβ signaling. Oncogene. 2012;31:2614–2626. doi: 10.1038/onc.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess R, Huang RP. Cancer stem cell biomarker discovery using antibody array technology. Adv Clin Chem. 2016;73:109–125. doi: 10.1016/bs.acc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer SR. Rab GTPases: Specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/S0962-8924(01)02147-X. [DOI] [PubMed] [Google Scholar]
- 11.Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 2002;8:23–30. doi: 10.1016/S1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- 12.Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- 13.Tolmachova T, Ramalho JS, Anant JS, Schultz RA, Huxley CM, Seabra MC. Cloning, mapping and characterization of the human RAB27A gene. Gene. 1999;239:109–116. doi: 10.1016/S0378-1119(99)00371-6. [DOI] [PubMed] [Google Scholar]
- 14.Ménasché G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nature Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 15.Griscelli C, Durandy A, Guy-Grand D, Daguillard F, Herzog C, Prunieras M. A syndrome associating partial albinism and immunodeficiency. Am J Med. 1978;65:691–702. doi: 10.1016/0002-9343(78)90858-6. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda M, Kanno E, Saegusa C, Ogata Y, Kuroda TS. Slp4-a/granuphilin-a regulates dense-core vesicle exocytosis in PC12 cells. J Biol Chem. 2002;277:39673–39678. doi: 10.1074/jbc.M205349200. [DOI] [PubMed] [Google Scholar]
- 17.Yi Z, Yokota H, Torii S, Aoki T, Hosaka M, Zhao S, Takata K, Takeuchi T, Izumi T. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol. 2002;22:1858–1867. doi: 10.1128/MCB.22.6.1858-1867.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, Izumi T. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J Clin Invest. 2005;115:388–396. doi: 10.1172/JCI200522955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, Bracaglia C, Prencipe G, Bemrich-Stolz CJ, Beukelman T, Dimmitt RA, Chatham WW, Zhang K, Li H, Walter MR, et al. A heterozygous RAB27A mutation associated with delayed cytolytic granule polarization and hemophagocytic lymphohistiocytosis. J Immunol. 2016;196:2492–2503. doi: 10.4049/jimmunol.1501284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang S, Shen D, Jia WJ, Han X, Shen N, Tao W, Gao X, Xue B, Li CJ. GGPPS-mediated Rab27A geranylgeranylation regulates β cell dysfunction during type 2 diabetes development by affecting insulin granule docked pool formation. J Pathol. 2016;238:109–119. doi: 10.1002/path.4652. [DOI] [PubMed] [Google Scholar]
- 21.Kowluru A. A lack of ‘glue’ misplaces Rab27A to cause islet dysfunction in diabetes. J Pathol. 2016;238:375–377. doi: 10.1002/path.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolmachova T, Anders R, Stinchcombe J, Bossi G, Griffiths GM, Huxley C, Seabra MC. A general role for Rab27a in secretory cells. Mol Biol Cell. 2004;15:332–344. doi: 10.1091/mbc.E03-07-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JS, Wang FB, Zhang QG, Shen ZZ, Shao ZM. Enhanced expression of Rab27A gene by breast cancer cells promoting invasiveness and the metastasis potential by secretion of insulin-like growth factor-II. Mol Cancer Res. 2008;6:372–382. doi: 10.1158/1541-7786.MCR-07-0162. [DOI] [PubMed] [Google Scholar]
- 24.Dong WW, Mou Q, Chen J, Cui JT, Li WM, Xiao WH. Differential expression of Rab27A/B correlates with clinical outcome in hepatocellular carcinoma. World J Gastroenterol. 2012;18:1806–1813. doi: 10.3748/wjg.v18.i15.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi C, Yang X, Ni Y, Hou N, Xu L, Zhan F, Zhu H, Xiong L, Chen P. High Rab27A expression indicates favorable prognosis in CRC. Diagn Pathol. 2015;10:68. doi: 10.1186/s13000-015-0303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong W, Cui J, Yang J, Li W, Wang S, Wang X, Li X, Lu Y, Xiao W. Decreased expression of Rab27A and Rab27B correlates with metastasis and poor prognosis in colorectal cancer. Discov Med. 2015;20:357–367. [PubMed] [Google Scholar]
- 27.Wang Q, Ni Q, Wang X, Zhu H, Wang Z, Huang J. High expression of RAB27A and TP53 in pancreatic cancer predicts poor survival. Med Oncol. 2015;32:372. doi: 10.1007/s12032-014-0372-2. [DOI] [PubMed] [Google Scholar]
- 28.Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding WQ. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA) Mol Cancer. 2015;14:133. doi: 10.1186/s12943-015-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Théry C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 30.Pierotti MA, Berrino F, Gariboldi M, Melani C, Mogavero A, Negri T, Pasanisi P, Pilotti S. Targeting metabolism for cancer treatment and prevention: Metformin, an old drug with multi-faceted effects. Oncogene. 2013;32:1475–1487. doi: 10.1038/onc.2012.181. [DOI] [PubMed] [Google Scholar]
- 31.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi GN, Gonzalez-Angulo AM. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B, Fan Z, Edgerton SM, Deng XS, Alimova IN, Lind SE, Thor AD. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–2040. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 34.Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, Thor AD. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 35.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Li C, He T, Mao J, Li C, Lyu J, Meng QH. Metformin inhibits prostate cancer cell proliferation, migration, and tumor growth through upregulation of PEDF expression. Cancer Biol Ther. 2016;17:507–514. doi: 10.1080/15384047.2016.1156273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heckman-Stoddard BM, Gandini S, Puntoni M, Dunn BK, DeCensi A, Szabo E. Repurposing old drugs to chemoprevention: The case of metformin. Semin Oncol. 2016;43:123–133. doi: 10.1053/j.seminoncol.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cazzaniga M, Bonanni B. Relationship between metabolic reprogramming and mitochondrial activity in cancer cells. Understanding the anticancer effect of metformin and its clinical implications. Anticancer Res. 2015;35:5789–5796. [PubMed] [Google Scholar]
- 39.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Del Barco S, Martin-Castillo B, Menendez JA. Metformin regulates breast cancer stem cell ontogeny by transcriptional regulation of the epithelial-mesenchymal transition (EMT) status. Cell Cycle. 2010;9:3807–3814. doi: 10.4161/cc.9.18.13131. [DOI] [PubMed] [Google Scholar]
- 41.Deng XS, Wang S, Deng A, Liu B, Edgerton SM, Lind SE, Wahdan-Alaswad R, Thor AD. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle. 2012;11:367–376. doi: 10.4161/cc.11.2.18813. [DOI] [PubMed] [Google Scholar]
- 42.Cazzaniga M, Bonanni B. Breast cancer metabolism and mitochondrial activity: The possibility of chemoprevention with metformin. Biomed Res Int. 2015;2015:972193. doi: 10.1155/2015/972193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cufi S, Corominas-Faja B, Vazquez-Martin A, Oliveras-Ferraros C, Dorca J, Bosch-Barrera J, Martin-Castillo B, Menendez JA. Metformin-induced preferential killing of breast cancer initiating CD44+CD24−/low cells is sufficient to overcome primary resistance to trastuzumab in HER2+ human breast cancer xenografts. Oncotarget. 2012;3:395–398. doi: 10.18632/oncotarget.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu B, Fan Z, Edgerton SM, Yang X, Lind SE, Thor AD. Potent anti-proliferative effects of metformin on trastuzumab-resistant breast cancer cells via inhibition of erbB2/IGF-1 receptor interactions. Cell Cycle. 2011;10:2959–2966. doi: 10.4161/cc.10.17.16359. [DOI] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 47.Guha P, Bandyopadhyaya G, Polumuri SK, Chumsri S, Gade P, Kalvakolanu DV, Ahmed H. Nicotine promotes apoptosis resistance of breast cancer cells and enrichment of side population cells with cancer stem cell-like properties via a signaling cascade involving galectin-3, α9 nicotinic acetylcholine receptor and STAT3. Breast Cancer Res Treat. 2014;145:5–22. doi: 10.1007/s10549-014-2912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saadin K, White IM. Breast cancer stem cell enrichment and isolation by mammosphere culture and its potential diagnostic applications. Expert Rev Mol Diagn. 2013;13:49–60. doi: 10.1586/erm.12.117. [DOI] [PubMed] [Google Scholar]
- 49.Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Ishige N. Proliferation of sphere-forming hepatocellular carcinoma cells is suppressed in a medium without glucose and arginine, but with galactose and ornithine. Oncol Lett. 2017;13:1264–1268. doi: 10.3892/ol.2017.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manuel Iglesias J, Beloqui I, Garcia-Garcia F, Leis O, Vazquez-Martin A, Eguiara A, Cufi S, Pavon A, Menendez JA, Dopazo J, Martin AG. Mammosphere formation in breast carcinoma cell lines depends upon expression of E-cadherin. PLoS One. 2013;8:e77281. doi: 10.1371/journal.pone.0077281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andriani F, Bertolini G, Facchinetti F, Baldoli E, Moro M, Casalini P, Caserini R, Milione M, Leone G, Pelosi G, et al. Conversion to stem-cell state in response to microenvironmental cues is regulated by balance between epithelial and mesenchymal features in lung cancer cells. Mol Oncol. 2016;10:253–271. doi: 10.1016/j.molonc.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacina L, Plzak J, Kodet O, Szabo P, Chovanec M, Dvorankova B, Smetana K., Jr Cancer microenvironment: What can we learn from the stem cell niche. Int J Mol Sci. 2015;16:24094–24110. doi: 10.3390/ijms161024094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al Hilli MM, Bakkum-Gamez JN, Mariani A, Cliby WA, McGree ME, Weaver AL, Dowdy SC, Podratz KC. The effect of diabetes and metformin on clinical outcomes is negligible in risk-adjusted endometrial cancer cohorts. Gynecol Oncol. 2016;140:270–276. doi: 10.1016/j.ygyno.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Camacho L, Dasgupta A, Jiralerspong S. Metformin in breast cancer-an evolving mystery. Breast Cancer Res. 2015;17:88. doi: 10.1186/s13058-015-0598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]