Abstract
Objective
The efficacy of oral administration of Melissa officinalis essential oil (MOEO) on hyperalgesia was investigated using the formalin test in streptozotocin (STZ)-induced diabetic rats.
Materials and Methods
Animals were divided into control, MOEO-treated control (0.01, 0.02 and 0.04 mg/day), diabetic and MOEO-treated diabetic (0.01, 0.02 and 0.04 mg/day) groups. Nociceptive testing was performed on male adult Wistar rats 4 weeks after the onset of hyperglycemia. At the end of the experiment, all rats were weighed and plasma glucose measurements were performed.
Results
Diabetes was associated with significant hyperalgesia during both phases of the formalin test. MOEO (0.04 mg/day) completely reversed hyperalgesia in diabetic rats, while MOEO (0.02 and 0.04 mg/day) caused less intensive nociceptive behaviors during both phases of the test in control rats. MOEO at both high doses restored euglycemia and reduced the body weight of treated diabetic animals compared to untreated diabetic animals. The 0.01-mg dose of MOEO did not alter pain responses in the control or diabetic groups compared to their respective controls.
Conclusions
This study shows that chronic administration of MOEO displays efficacy in an experimental model of diabetic hyperalgesia. MOEO may therefore show promise as a treatment for painful diabetic neuropathy.
Key Words: Hyperalgesia, Diabetes, Melissa officinalis, Rats
Introduction
Diabetes mellitus is a major public health problem that affects approximately 5% of the world's population [1]. Hypersensitivity to painful stimuli (hyperalgesia) is one of the most common complications of diabetes mellitus [2]. It is known that diabetic rats display hyperalgesic behavior in response to noxious stimuli and this may serve as a model of painful diabetic neuropathy in humans [3]. Streptozotocin (STZ)-diabetic rats have been increasingly used as a model of painful diabetic neuropathy to assess the efficacy of potential analgesic agents [4]. Increased flinch responses following the injection of formalin into the paw are abnormal behavioral responses to nociceptive stimuli in diabetic rats and have proven to be a useful model for investigating diabetic hyperalgesia [5,6].
Recently, treatment of diabetes mellitus and its complications has focused on the use of plant products. The WHO had estimated that approximately 80% of the global population rely on traditional medicine for their primary health-care needs, and most of this therapy involves the use of plant extracts and essential oils [7].
Melissa officinalis is a well-known medicinal plant species used in perfumes, cosmetics, tea and food products in many countries, and has been reported to possess sedative, spasmolytic and antibacterial properties [8,9]. Herbal essential oils generally contain a variety of volatile compounds, which may have medicinal properties [10,11,12,13]. It has been reported that M. officinalis essential oil (MOEO) has antimicrobial, antioxidative and antitumor properties [14]. Previous papers have also reported on the antidiabetic effects of M. officinalis in diabetic mice [15,16]. However, there is no reported information, to our knowledge, regarding the effects of MOEO on pain-associated behaviors in diabetic rats. We therefore investigated the effects of various doses of MOEO in an experimental model of hyperalgesia using STZ-diabetic rats.
Materials and Methods
Drugs
STZ (Pharmacia & Upjohn, Kalamazoo, Mich., USA) and ketamine hydrochloride (Rotexmedica, Trittau, Germany) were used in this study. STZ was dissolved in 1 ml of normal saline immediately before use. All injections (except for STZ) were administered intraperitoneally. The drugs were injected at a volume of 1 ml/kg.
Animals
Sixty-four locally bred male Wistar rats (250-280 g) were used in these experiments. All animals were maintained at a constant temperature (22 ± 0.5°C) with a 12-hour light and 12-hour dark cycle. The animals were divided into the following groups (n = 8): controls, MOEO-treated controls (0.01, 0.02 and 0.04 mg/day), diabetics and MOEO-treated diabetics (0.01, 0.02 and 0.04 mg/day). Diabetes was induced by a single intravenous injection of STZ (40 mg/kg) into the lower portion of the tail, approximately a third of the distance from the tip. Fasting blood glucose levels were determined 3 days later. Animals were considered diabetic if plasma glucose levels exceeded 250 mg/dl. Rats were fed normal chow or chow with MOEO (0.01, 0.02 or 0.04 mg/day) for 4 weeks after the onset of hyperglycemia. After feeding, nociceptive testing was performed in the various experimental groups. Animals were handled in accordance with the criteria outlined in the Guide for the Care and Use of Laboratory Animals[17]. All the protocols were also approved by the institutional ethics committee of Bu-Ali Sina University.
Nociceptive Testing
The ambient temperature of the room was maintained at 24 ± 0.5°C at all times. Rats were acclimated to the testing environment by placing them in the formalin test apparatus, a Perspex box (20 × 30 × 20 cm) with a mirror positioned at an angle of 45° to permit unhindered observation of the animals’ paws for 1 h on each of the 3 days prior to the test day. Each animal was given a single formalin injection (2.5%, 50 μl) under the plantar surface of one hind paw at 60 min after MOEO administration, before being placed in the formalin test apparatus. Scoring of the nociceptive behaviors started immediately and was continued for 60 min.
Subcutaneous formalin injection to the hind paw induces a biphasic pain-related response. The biphasic response is characterized by an early acute pain period (phase 1; 0-5 min), a brief quiescent period, and a second period of sustained ‘tonic’ pain (phase 2; 10-60 min) [18]. A weighted-score or rating-scale method of scoring for formalin-induced nociceptive responses was employed to quantify the nociceptive effects of the drugs [19]. Nociception was quantified by assigning weights to the following pain-related behaviors: the animal walks or sits normally without favoring the injected paw (weight = 0); the animal walks or sits while placing some, but not full, pressure on the injected paw (weight = 1); the animal walks or sits while maintaining the paw completely elevated off the floor (weight = 2); the animal licks, bites or vigorously shakes the injected paw (weight = 3). A weighted average nociceptive score was obtained for each 5-min test interval by multiplying the number of seconds the animal spent in each category by its assigned weight, summing these products and dividing the result by the total time (300 s):
Nociceptive score = (t₀ × 0) + (t1 × 1) + (t2 × 2) + (t3 × 3) / t₀ + t1 + t2 + t3.
By utilizing the above-mentioned method, an ordinal scale of nociceptive scores was generated with a range of 0-3. The operator was unaware of the treatment group to which an animal belonged when scoring formalin-induced nociceptive behaviors.
Measurement of Plasma Glucose Levels
At the end of the experiment, all rats were weighed and decapitated under ketamine HCl anesthesia (50 mg/kg, i.p.) after blood sampling. Plasma glucose from blood samples was measured using a kit (Zistshimi, Tehran, Iran) and spectrophotometer (UV3100; Shimadzu, Kyoto, Japan).
Statistical Analysis
All data are expressed as the mean ± SEM. Differences among groups were statistically tested by one-way analysis of variance (ANOVA) with the Tukey post hoc test. Probability values <0.05 were considered significant.
Results
Effects of Diabetes on the Formalin Test
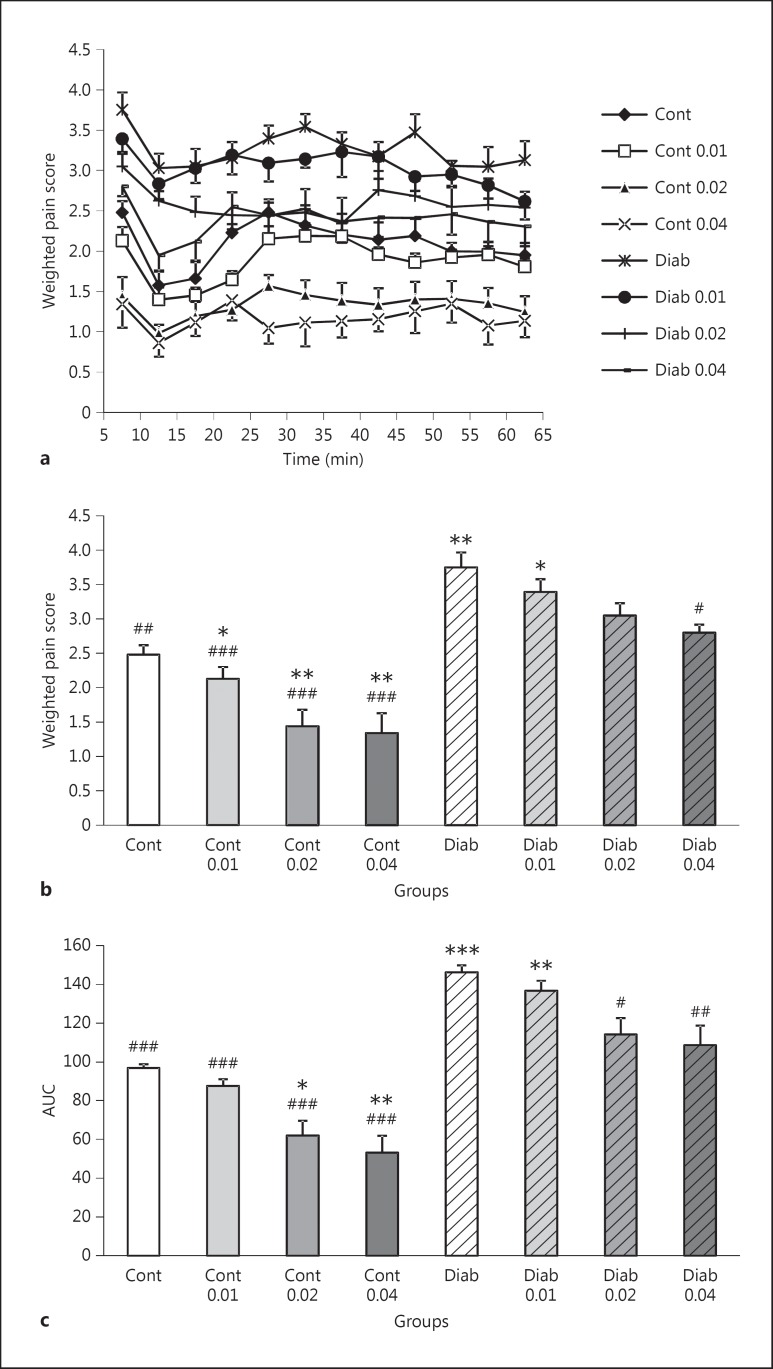
The results of the formalin test in 4-week-old diabetic rats are shown in figure 1. Formalin injection into the hind paw produced a marked biphasic response in the rats of all groups. The scores in phase 1 and the area under the curve (AUC) of scores in phase 2 of the formalin test in untreated diabetics were greater (3.75 ± 0.21 and 146.2 ± 3.6, respectively) than those of control rats (2.48 ± 0.13 and 96.8 ± 1.9, respectively). This indicates that there is a significant hyperalgesia in untreated diabetic animals compared to controls in both phases of the formalin test (p < 0.01, p < 0.001, respectively; fig. 1a-c).
Fig. 1.
a Time-course of formalin-induced pain behavior by administration of MOEO (0, 0.01, 0.02 and 0.04 mg/day) in control (Cont, Cont 0.01, Cont 0.02 and Cont 0.04, respectively) and diabetic (Diab, Diab 0.01, Diab 0.02 and Diab 0.04, respectively) groups. Data are expressed as mean ± SEM (n = 8). b The effects of various doses of MOEO (0.01, 0.02 and 0.04 mg/day) on the weighted pain scores in the first (early) phase of the formalin test. Data are expressed as mean ± SEM (n = 8). * p < 0.05, ** p < 0.01 (as compared to the control group); # p < 0.05, ## p < 0.01, ### p < 0.001 (as compared to the diabetic group). c The AUCs of pain scores using the time-response curves shown in a for rats receiving various doses of MOEO (0.01, 0.02 and 0.04 mg/day) in the second (late) phase of the formalin test. Data are expressed as mean ± SEM (n = 8). * p < 0.05, ** p < 0.01, *** p < 0.001 (as compared to the control group); # p < 0.05, ## p < 0.01, ### p < 0.001 (as compared to the diabetic group).
Effects of Long-Term MOEO Administration on the Pain Threshold in the Formalin Test
Oral administration of MOEO (0.02 and 0.04 mg/day) resulted in lower nociceptive scores compared to the untreated control group in both phase 1 (p < 0.01, p < 0.01, respectively) and phase 2 (p < 0.05, p < 0.001, respectively) of the formalin test. The scores in phase 1 (1.44 ± 0.24 and 1.34 ± 0.29, respectively) and the AUC of scores in phase 2 (62 ± 7.5 and 53.1 ± 8.7, respectively) of the formalin test in MOEO-treated control rats (0.02 and 0.04 mg/day) were smaller than those for untreated controls (2.48 ± 0.13 and 96.8 ± 1.9, respectively; fig. 1b, c). In addition, there was a significant difference in the pain scores in phase 1 of the formalin test between the MOEO-treated diabetic (0.04 mg/day) and untreated diabetic groups (p < 0.05; fig. 1b). There were also significant differences in the AUC of scores in phase 2 of the formalin test between the MOEO-treated diabetic (0.02 and 0.04 mg/day) and untreated diabetic groups (p < 0.05, p < 0.01, respectively; fig. 1c). However, weighted pain scores for diabetic rats treated with MOEO (0.02 or 0.04 mg/day) did not differ significantly in either phase of the test from those of untreated controls (fig. 1b, c). Weighted pain scores for control and diabetic rats administered MOEO (0.01 mg/day) did not differ significantly from those of the respective controls in either phase of the test (fig. 1a-c).
Effects of Long-Term MOEO Administration on Body Weights and Plasma Glucose Levels
The effects of long-term MOEO (0.01, 0.02 and 0.04 mg/day) administration on the body weights and plasma glucose levels are shown in table 1. There were no significant differences in body weight and plasma glucose levels between the groups prior to the intervention. At the end of 4 weeks of diabetes, the body weight of untreated diabetics (231 ± 9) and MOEO-treated diabetic rats (0.01 mg/day; 225 ± 8) were significantly (p < 0.001, p < 0.001, respectively) lower than those of control rats (300 ± 7). There were no significant differences in the body weights of the MOEO-treated controls (0.02 and 0.04 mg/day) and untreated control animals (table 1). However, MOEO treatment (0.02 or 0.04 mg/day) significantly increased the body weights of diabetic animals compared to untreated diabetic animals (p < 0.05, p < 0.05, respectively).
Table 1.
Body weights and plasma glucose levels
| Body weight, g |
Plasma glucose, mg/dl |
|||
|---|---|---|---|---|
| week 4 | week 0 | week 4 | week 0 | |
| Cont | 272 ± 2 | 300 ± 7 | 95.7 ± 5 | 91.6 ± 6.8 |
| Cont 0.01 | 270 ± 2 | 321 ± 6 | 88.6 ± 5.2 | 93.5 ± 6.3 |
| Cont 0.02 | 265 ± 4 | 323 ± 9 | 100 ± 8 | 102 ± 6.5 |
| Cont 0.04 | 268 ± 3 | 280 ± 9 | 86.7 ± 8 | 95.1 ± 6.1 |
| Diab | 267 ± 3 | 231 ± 9*** | 93.3 ± 7.7 | 366.1 ± 8.6*** |
| Diab 0.01 | 264 ± 3 | 225 ± 8*** | 87.8 ± 6.8 | 350.7 ± 4.4*** |
| Diab 0.02 | 267 ± 3 | 266 ± 2 | 96.6 ± 4.6 | 120.3 ± 2.6 |
| Diab 0.04 | 264 ± 4 | 268 ± 4* | 87.1 ± 7.6 | 119.7 ± 7.9 |
Body weights and plasma glucose levels of control (Cont), MOEO-treated control (0.01 mg/day, Cont 0.01; 0.02 mg/day, Cont 0.02; 0.04 mg/day, Cont 0.04), diabetic (Diab) and MOEO-treated diabetic (0.01 mg/day, Diab 0.01; 0.02 mg/day, Diab 0.02; 0.04 mg/day; Diab 0.04) rats at the beginning and 4 weeks after the intervention.
Significant difference compared to the control group.
p < 0.001, ANOVA, Tukey's test for post hoc comparisons.
Regarding plasma glucose levels, untreated diabetics (366.1 ± 8.6) and MOEO-treated diabetic animals (0.01 mg/day; 350.7 ± 4.4) had significantly (p < 0.001, p < 0.001, respectively) elevated plasma glucose levels compared to those of control rats (91 ± 6). Although administration of MOEO (0.02 and 0.04 mg/day) to control rats did not alter their plasma glucose levels, MOEO treatment (0.02 or 0.04 mg/day) in diabetic animals significantly (p < 0.001, p < 0.001, respectively) decreased levels of plasma glucose as compared with untreated diabetic animals. There were no significant differences in the plasma glucose levels between MOEO-treated diabetic animals (0.02 and 0.04 mg/day) and untreated controls (table 1). MOEO treatment (0.01 mg/day) did not affect the body weight or plasma glucose levels of controls or diabetics compared to their respective controls at the end of 4 weeks after the intervention (table 1).
Discussion
The results presented here extend literature data and clearly demonstrate for the first time that long-term orally administered MOEO elicits a significant antihyperalgesic effect in an animal model of diabetic hyperalgesia. The results also demonstrate that there is an intensified nociceptive response in both phases of the formalin test in diabetic rats. It has been shown that diabetic rats display hyperalgesic behavior in response to noxious stimuli that may model aspects of painful diabetic neuropathy in humans. For this reason, STZ-diabetic rats have been increasingly used as a model of painful diabetic neuropathy to assess the efficacy of potential analgesic agents [5].
Some studies with various herbal formulations containing M. officinalis have demonstrated analgesic activity, especially against visceral pain [20,21]. It has also recently been shown that M. officinalis extracts produce antinociceptive effects in the visceral nociceptive response induced by acetic acid injection in mice [22]. Our current findings extend previous observations and demonstrate that oral administration of MOEO (0.02 and 0.04 mg/day) leads to decreased nociceptive scores in control animals and prevents hyperalgesia in an experimental model of diabetic neuropathy. Therefore, MOEO given by the oral route was effective in inhibiting both phases (neurogenic and inflammatory) of formalin-induced nociception. The neurogenic phase of the test is elicited by direct activation of nociceptive terminals; on the other hand, the inflammatory phase is mediated by a combination of peripheral and central mechanisms [23,24]. Other agents, such as bicuculline [25], gabapentin [26] and URB597 [27] that are effective in animal models of neuropathic pain arising from physical nerve injury have also been shown to attenuate hyperalgesia in the formalin test in diabetic rats. Such pharmacological studies are useful in examining the potential of agents for treating painful diabetic neuropathy as well as providing insights into the underlying etiology of abnormal sensory processing during diabetes.
Phytochemical analysis for determination of the components in MOEO responsible for the observed effects in this study was not conducted. However, based on previously published papers [8,22,28], we can speculate on the probable components of MOEO. Phytochemical studies carried out with M. officinalis have demonstrated the presence of many classes of constituents, including polyphenolic compounds (rosmarinic acid, caffeic acid and protocatechuic acid), essential oils (citral), monoterpenoid aldehydes, sesquiterpenes, flavonoids (luteolin) and tannins. However, rosmarinic acid is the major phenol constituent of M. officinalis, which possesses strong antinociceptive and antioxidant effects [8]. For example, oral administration of rosmarinic acid can produce significant inhibition of the nociceptive response caused by intraplantar injection of glutamate into the mouse hind paw [22]. Furthermore, according to a recent paper, rosmarinic acid induces antinociception by inhibiting acetic acid-induced abdominal constriction in mice [28]. Based on these studies, the antinociceptive effects of MOEO in the present study could be mainly related to rosmarinic acid.
In the present study, MOEO treatment significantly reduced plasma glucose levels and improved the reduced body weight of diabetic rats compared with control animals. These results are consistent with those of previous reports describing the hypoglycemic effects of MOEO administration for 6 weeks in diabetic mice [15]. It has been suggested that hyperglycemia contributes to the development and maintenance of painful diabetic neuropathy. Hyperalgesia in diabetic animals may result from focal injury caused by a direct toxic effect of glucose in the peripheral nervous system [29]. Since oral MOEO administration in diabetic rats reduced plasma glucose levels, the antihyperalgesic effects may be related to the normalization of glucose plasma levels.
On the other hand, it has been reported that M. officinalis is a readily accessible source of natural antioxidants because of its high radical scavenging and strong antioxidant activity [14]. Since oxidative stress could be involved in the pathogenesis of diabetes and its complications, such as hyperalgesia [30], the antioxidant activity of MOEO may be involved in the observed effects.
In this study we focused on the antinociceptive and antihyperglycemic effects of MOEO in experimental diabetes. It has been recently reported that MOEO also has anti-inflammatory properties, as demonstrated using carrageenan- and experimental trauma-induced hind paw edema in rats [31]. M. officinalis also has a wider range of biological effects which may attract attention for its more extensive use. For instance, M. officinalis is a traditional herbal medicine with mild sedative, spasmolytic, antibacterial, anti-inflammatory, hepatoprotective, antilipidemic and anxiolytic effects [8,22]. It has also been suggested, in light of its memory-enhancing properties, that it may effectively enhance cognition in healthy individuals as well as those with cognitive deficits associated with Alzheimer's disease [31]. Therefore, MOEO may be a candidate for evaluation of its memory-enhancing, hepatoprotective and antilipidemic effects in experimental diabetes.
Conclusions
Taken together, our data indicate that long-term oral administration of MOEO (at an effective dose of 0.04 mg/day) can suppress chemical hyperalgesia in diabetic rats. The efficacy of agents attenuating hyperalgesia in the formalin test in diabetic rats further supports the validity of this model for investigating potential therapeutic agents for painful diabetic neuropathy and the etiology of diabetes-induced sensory disorders. Since there is an emerging interest in the treatment of diabetes mellitus and its complications using plant products, it may be suggested that appropriate intake of MOEO could be beneficial in preventing or ameliorating diabetic symptoms or complications such as hyperalgesia.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgements
The authors wish to thank Dr. Mohan Kumar for her valuable suggestions in editing the manuscript.
References
- 1.Taylor SI. Deconstructing type 2 diabetes. Cell. 1999;97:9–12. doi: 10.1016/s0092-8674(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 2.Obrosova IG. Update on the pathogenesis of diabetic neuropathy. Curr Diab Rep. 2003;3:439–445. doi: 10.1007/s11892-003-0005-1. [DOI] [PubMed] [Google Scholar]
- 3.Rocha-González HI, Ramírez-Aguilar M, Granados-Soto V, et al. Antineuropathic effect of 7-hydroxy-3,4-dihydrocadalin in streptozotocin-induced diabetic rodents. BMC Complement Altern Med. 2014;14:129–141. doi: 10.1186/1472-6882-14-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Hernández L, Navarrete-Vázquez G, González-Trujano ME, et al. Antihyperalgesic activity of a novel synthesized analogue of lidocaine in diabetic rats. J Pharm Pharmacol. 2013;65:689–696. doi: 10.1111/jphp.12025. [DOI] [PubMed] [Google Scholar]
- 5.Courteix C, Bourget P, Caussade F, et al. Is the reduced efficacy of morphine in diabetic rats caused by alterations of opiate receptors or of morphine pharmacokinetics? J Pharmacol Exp Ther. 1998;285:63–70. [PubMed] [Google Scholar]
- 6.Nacitarhan C, Minareci E, Sadan G. The effect of benfotiamine on mu-opioid receptor mediated antinociception in experimental diabetes. Exp Clin Endocrinol Diabetes. 2014;122:173–178. doi: 10.1055/s-0033-1363977. [DOI] [PubMed] [Google Scholar]
- 7.Quintans JS, Antoniolli AR, Almeida JR, et al. Natural products evaluated in neuropathic pain models - a systematic review. Basic Clin Pharmacol Toxicol. 2014;114:442–450. doi: 10.1111/bcpt.12178. [DOI] [PubMed] [Google Scholar]
- 8.Bisset NG, Wichtl M. Herbal Drugs. Stuttgart: Medpharm GmbH Scientific Publishers; 1994. [Google Scholar]
- 9.Canadanović-Brunet J, Cetković G, Djilas S, et al. Radical scavenging, antibacterial, and antiproliferative activities of Melissa officinalis L. extracts. J Med Food. 2008;11:133–143. doi: 10.1089/jmf.2007.580. [DOI] [PubMed] [Google Scholar]
- 10.Chung MJ, Park KW, Kim KH, et al. Asian plantain (Plantago asiatica) essential oils suppress 3-hydroxy-3-methylglutaryl co-enzyme-A reductase expression in vitro and in vivo and show hypocholesterolaemic properties in mice. Br J Nut. 2008;99:67–75. doi: 10.1017/S0007114507798926. [DOI] [PubMed] [Google Scholar]
- 11.Clegg RJ, Middleton B, Bell GD, et al. The mechanism of cyclic monoterpene inhibition of hepatic 3-hydroxy-3-methylglutaryl co-enzyme-A reductase in vivo in the rat. J Biol Chem. 1982;257:2294–2299. [PubMed] [Google Scholar]
- 12.von Bergmann K, Beck A, Engel C, et al. Administration of a terpene mixture inhibits cholesterol nucleation in bile from patients with cholesterol gallstones. Klin Wochenschr. 1987;65:458–462. doi: 10.1007/BF01712838. [DOI] [PubMed] [Google Scholar]
- 13.Chung MJ, Kang AY, Park SO, et al. The effect of essential oils of dietary wormwood (Artemisia princeps), with and without added vitamin E, on oxidative stress and some genes involved in cholesterol metabolism. Food Chem Toxicol. 2007;45:1400–1409. doi: 10.1016/j.fct.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 14.de Sousa AC, Alvino DS, Blank AF, et al. Melissa officinalis L. essential oil: antitumoral and antioxidant activities. J Pharm Pharmacol. 2004;56:677–681. doi: 10.1211/0022357023321. [DOI] [PubMed] [Google Scholar]
- 15.Chung MJ, Cho SY, Bhuiyan MJ, et al. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br J Nutr. 2010;104:180–188. doi: 10.1017/S0007114510001765. [DOI] [PubMed] [Google Scholar]
- 16.Weidner C, Wowro SJ, Freiwald A, et al. Lemon balm extract causes potent antihyperglycemic and antihyperlipidemic effects in insulin-resistant obese mice. Mol Nutr Food Res. 2014;58:903–907. doi: 10.1002/mnfr.201300477. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health Guide for the Care and Use of Laboratory Animals. NIH publication 86-23. 1985. http://oacu.od.nih.gov/regs/
- 18.Gutierrez T, Nackley AG, Neely MH, et al. Effects of neurotoxic destruction of descending noradrenergic pathways on cannabinoid antinociception in models of acute and tonic nociception. Brain Res. 2003;987:176–185. doi: 10.1016/s0006-8993(03)03324-9. [DOI] [PubMed] [Google Scholar]
- 19.Coderre TJ, Fundytus ME, McKenna JE, et al. The formalin test: a validation of the weighted-scores method of behavioural pain rating. Pain. 1993;54:43–50. doi: 10.1016/0304-3959(93)90098-A. [DOI] [PubMed] [Google Scholar]
- 20.Vejdani R, Shalmani HR, Mir-Fattahi M, et al. The efficacy of an herbal medicine, Carmint, on the relief of abdominal pain and bloating in patients with irritable bowel syndrome: a pilot study. Dig Dis Sci. 2006;51:1501–1507. doi: 10.1007/s10620-006-9079-3. [DOI] [PubMed] [Google Scholar]
- 21.Capasso R, Savino F, Capasso F. Effects of the herbal formulation ColiMil on upper gastrointestinal transit in mice in vivo. Phytother Res. 2007;21:999–1101. doi: 10.1002/ptr.2192. [DOI] [PubMed] [Google Scholar]
- 22.Guginski G, Luiz AP, Silva MD, et al. Mechanisms involved in the antinociception caused by ethanolic extract obtained from the leaves of Melissa officinalis (lemon balm) in mice. Pharmacol Biochem Behav. 2009;93:10–16. doi: 10.1016/j.pbb.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Hajhashemi V, Zolfaghari B, Yousefi A. Antinociceptive and anti-inflammatory activities of Satureja hortensis seed essential oil, hydroalcoholic and polyphenolic extracts in animal models. Med Princ Pract. 2012;21:178–182. doi: 10.1159/000333555. [DOI] [PubMed] [Google Scholar]
- 24.Zakaria ZA, Somchit MN, Mat Jais AM, et al. In vivo antinociceptive and anti-inflammatory activities of dried and fermented processed virgin coconut oil. Med Princ Pract. 2011;20:231–236. doi: 10.1159/000323756. [DOI] [PubMed] [Google Scholar]
- 25.Jolivalt CG, Lee CA, Ramos KM, et al. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain. 2008;140:48–57. doi: 10.1016/j.pain.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ceseña RM, Calcutt NA. Gabapentin prevents hyperalgesia during the formalin test in diabetic rats. Neurosci Lett. 1999;262:101–104. doi: 10.1016/s0304-3940(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 27.Hasanein P, Parviz M, Keshavarz M, et al. URB597, an inhibitor of fatty acid amide hydrolase, reduces hyperalgesia in diabetic rats. Can J Physiol Pharmacol. 2009;87:432–439. doi: 10.1139/y09-026. [DOI] [PubMed] [Google Scholar]
- 28.Lucarini R, Bernardes WA, Ferreira DS, et al. In vivo analgesic and anti-inflammatory activities of Rosmarinus officinalis aqueous extracts, rosmarinic acid and its acetyl ester derivative. Pharm Biol. 2013;51:1087–1090. doi: 10.3109/13880209.2013.776613. [DOI] [PubMed] [Google Scholar]
- 29.Dobretsov M, Hastings SL, Romanovsky D, et al. Mechanical hyperalgesia in rat models of systemic and local hyperglycemia. Brain Res. 2003;960:174–183. doi: 10.1016/s0006-8993(02)03828-3. [DOI] [PubMed] [Google Scholar]
- 30.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 31.Bounihi A, Hajjaj G, Alnamer R, et al. In vivo potential anti-inflammatory activity of Melissa officinalis L. essential oil. Adv Pharmacol Sci. 2013;2013:101759. doi: 10.1155/2013/101759. [DOI] [PMC free article] [PubMed] [Google Scholar]