Abstract
Objective
To test the potential role of heart-type fatty acid-binding protein (H-FABP) in detecting increased perioperative cardiac risk in comparison with cardiac troponin I (cTnI) in the early postoperative period.
Subjects and Methods
Sixty-seven patients who had clinical risk factors and underwent elective intermediate - or high-risk noncardiac surgery were included in this study. Serum specimens were analyzed for H-FABP and cTnI levels before and at 8 h after surgery. None of the patients had chest pain; 27 had a history of ischemic heart disease, 3 of heart failure, 5 of cerebrovascular diseases, 40 of diabetes and 46 of hypertension.
Results
The mean duration of the operations was 2.33 ± 1.27 h (range 1-6). In the postoperative period, 27 (40.3%) patients had increased H-FABP levels (≥7.5 ng/ml); the median preoperative serum H-FABP level was 0.13 ng/ml (<0.1-5.9) and the median postoperative H-FABP level was 6.86 ng/ml (<0.1-13.7). Only 1 (1.5%) patient had cTnI >0.1 µg/l during the postoperative period. Correlation analysis revealed that the presence of diabetes was associated with an increased H-FABP level (r = 0.30, p = 0.01). Of the 27 patients with H-FABP ≥7.5 ng/ml, 21 (87%) had diabetes. There was no significant correlation with other clinical risk factors, type or duration of surgery.
Conclusion
The H-FABP levels significantly increased in the postoperative period. Most patients with increased postoperative H-FABP levels were diabetic. High H-FABP levels could alert clinicians to increased perioperative cardiovascular risk and could prevent underdiagnosis, especially in diabetic patients.
Key Words: Diabetes mellitus, Fatty acids, Ischemia, Risk factors, Risk management, Troponin
Introduction
The number of patients who undergo noncardiac surgery and the proportion of patients at risk of perioperative cardiac events are increasing worldwide [1]. The risk of perioperative complications are related to the condition of the patient prior to surgery, comorbidities, and the duration and type of the surgery. The type of surgery determines the severity of prolonged hemodynamic and cardiac stress. Recommended clinical risk indices are to be used for preoperative risk stratification to reduce perioperative cardiac complications [1,2,3]. The most commonly used guidelines and recommended cardiac risk stratification tool is Lee's revised cardiac risk index [2]. Lee described six independent risk factors: high-risk type of surgery, history of ischemic heart disease, history of congestive heart failure, history of cerebrovascular disease, insulin-requiring diabetes, and preoperative serum creatinine above 2.0 mg/dl. Several external validation studies have suggested that the Lee index is probably suboptimal for identifying patients with multiple risk factors [4,5,6].
Although less than 1% of patients develop perioperative myocardial infarction (MI) or cardiac arrest, two thirds of these patients die within 30 days of surgery. Apparently, nonfatal perioperative MI is an independent predictor for long-term cardiovascular events following surgery. Hence, determinants of perioperative myocardial injury and early diagnosis are vital [5,6]. Based on previous studies, perioperative cardiac complications occur more frequently in patients with a known diagnosis of diabetes mellitus than in nondiabetic patients due to high preoperative glucose levels [7,8].
Postoperative ischemia is clinically silent in more than 90% of cases and up to half of perioperative MIs may be underdiagnosed if clinicians rely solely on clinical signs or symptoms [1,2,3,9]. Silent ischemia is more prevalent in diabetic patients [8,10,11].
Chest pain and electrocardiographic ST segment changes rarely accompany postoperative myocardial ischemia; hence, the diagnosis of postoperative MI is due mainly to the fluctuations of biochemical markers, especially of cardiac troponin (cTn) [2,3]. Heart-type fatty acid-binding protein (H-FABP) is a small and relatively cardiac-specific cytoplasmic molecule; it appears in the blood as early as 30-90 min after myocardial injury, peaks at around 4-6 h and returns to baseline values within approximately 24 h. Because of its high sensitivity in the early phase of acute MI, H-FABP has been suggested for the initial diagnosis of MI as well as of more subtle degrees of subclinical myocyte injury [12,13,14,15,16,17,18]. It has been reported that H-FABP was more sensitive than cTnT for detecting ongoing myocardial damage in chronic heart failure patients [19]. Hence, the purpose of this study was to investigate the potential role of serum H-FABP and cTnI levels in detecting increased perioperative cardiac risk in patients who have clinical risk factors at an early phase of the postoperative period.
Subjects and Methods
Following institutional review board approval, a total of 67 out of 80 patients who underwent elective intermediate and high-risk noncardiac surgery were enrolled in this study, each providing their informed consent. Thirteen patients were excluded from the study due to inappropriate sample collection or timing. Inclusion criteria were patients aged >18 and <80 years who had one or more clinical risk factors, such as ischemic heart disease, diabetes mellitus, a history of cerebrovascular disease, a history of heart failure but left ventricular ejection fraction (LVEF) >40%, or had minor clinical predictors, such as hypertension or abnormal electrocardiogram, rhythm other than sinus on the electrocardiogram. Patients who underwent intermediate-risk surgery (abdominal, neck and head, carotid, major urologic, major orthopedic, intrathoracic surgery) or high-risk vascular surgery according to the European Society of Cardiology (ESC) guidelines on perioperative cardiovascular evaluation for noncardiac surgery were included [2]. The exclusion criteria were inability or unwillingness to give informed consent, age <18 or >80 years, emergency procedures, acute coronary syndrome, decompensated heart failure and/or severe systolic dysfunction (LVEF <40%), severe valvular heart disease, severe cardiac arrhythmia, renal failure (serum creatinine >1.5 mg/l), pulmonary embolism, obstructive sleep apnea syndrome, chronic obstructive airway disease, and undergoing a low-risk operation, such as breast, eye, reconstructive, minor urologic and minor orthopedic surgery.
Before surgery, patients were evaluated for clinical risk factors, including diabetes mellitus, ischemic heart disease, renal dysfunction, heart failure, stroke and age, and then a physical examination was performed. Prior to surgery, each patient's serum hemoglobin level, serum creatinine level, serum lipid profile, electrocardiography, medications and risk of surgery according to the ESC guideline for perioperative management in noncardiac surgery were reviewed. Echocardiographic evaluation was performed in all patients to exclude severe valvular heart disease and severe systolic dysfunction. All preoperative chronic cardiovascular medications were continued until the day of surgery and resumed as soon as possible postoperatively.
Peripheral venous blood samples drawn in the preoperative and postoperative periods were immediately centrifuged and the serum was stored at −80°C for later analysis. cTnI and H-FABP levels of the serum specimens were analyzed before and at 8 h after surgery as this time period carries the highest risk for silent ischemia and MI. The reasons for this are that the majority of patients receive narcotic therapy or sedation, which could mask clinical symptoms, and troponin sensitivity is at its lowest in the first 6 h. Of equal importance is that the H-FABP sensitivity declines after 8 h of symptom onset at the MI diagnosis, and the H-FABP level peaks approximately at 4-6 h after myocardial injury [13]. Serum cTnI levels were measured using the Elecsys troponin I immunoassay (Roche Diagnostics, Mannheim, Germany), the upper reference limit of which is 0.01 µg/l. Serum H-FABP levels were measured using a human H-FABP enzyme-linked immunosorbent assay kit (Hycult H-FABP Elisa Test Kit; Hycult Biotech, Uden, The Netherlands). The kit has a concentration range of 102-25,000 pg/ml. Serum samples were also obtained from 20 healthy controls to assess the cutoff value for H-FABP, which was measured as 7.5 ng/ml and used to define positive H-FABP levels, based on the 95th percentile for the 20 healthy controls.
Data were analyzed with the software SPSS version 15.0 for Windows (SPSS Inc., Chicago, Ill., USA). Continuous variables are presented as the mean ± standard deviation (SD), and categorical variables as frequencies and percentages. The paired t test was used to compare normally distributed continuous variables and the Mann-Whitney U test for variables without a normal distribution. Median values and their 95% confidence intervals (95% CI) were calculated for continuous variables that were not distributed normally. The χ2 test was used to compare categorical variables. The Spearman and Pearson methods were used for correlation analysis. A p value <0.05 was considered significant.
Results
The patient characteristics are summarized in table 1. The mean ± SD age was 65 ± 11 years, and 41 patients were male and 26 were female. The procedures performed were predominantly intermediate-risk noncardiac surgeries: major orthopedic surgery was performed in 20 patients (28.2%); major urologic surgery in 26 (36.6%), intrathoracic surgery in 1 (1.4%), and other abdominal, neck, head and neurosurgical operations in 17 (25.3%). Also, 3 (4.2%) patients had infrainguinal major vascular surgery. The mean duration of the operations was 2.33 ± 1.27 h. The prevalence of β-blockers and/or statin treatment usage in the patients undergoing noncardiac surgery was 35.8% (24 patients).
Table 1.
Patients’ demographic and clinical characteristics (n = 67)
| Males | 41 (61.1) |
| Age, years | 65 ± 11 |
| History of ischemic heart disease | 27 (40) |
| History of heart failure (LVEF >40%) | 3 (4.4) |
| History of diabetes mellitus | 40 (59.7) |
| History of hypertension | 46 (68.6) |
| History of cerebrovascular disease | 5 (7.5) |
| Hemoglobin, g/dl | 12.90 ± 1.70 |
| Serum creatinine, mg/dl | 0.98 ± 0.30 |
| Fasting serum glucose, mg/dl | 147.55 ± 63.20 |
| Total cholesterol, mg/dl | 199.59 ± 34.10 |
| Triglyceride, mg/dl | 159.72 ± 73.17 |
| High-density lipoprotein, mg/dl | 45.19 ± 12.76 |
| Low-density lipoprotein, mg/dl | 122.61 ± 27.14 |
Values are n (%) or mean ± SD.
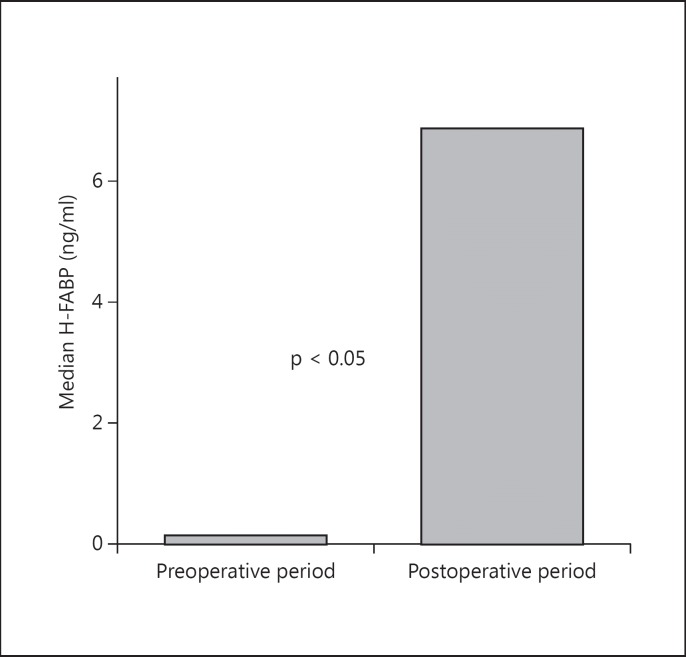
None of the patients had chest pain during the 8 h after surgery. The median H-FABP concentration differed significantly between before and after surgery (fig 1). The median serum H-FABP concentration of patients before surgery was 0.13 ng/ml (<0.1-5.9) and at 8 h after surgery 6.86 ng/ml (<0.1-13.7). The serum H-FABP levels were significantly increased after surgery compared to the preoperative period (p < 0.05). None of the patients had an H-FABP serum level ≥7.5 ng/ml before surgery (fig 2). There was correlation between the preoperative level of H-FABP and age (r = 0.27, p = 0.027). However, at 8 h after surgery, 40% (27/67) of the patients had an H-FABP level ≥7.5 ng/ml. The presence of diabetes was associated with postoperative increased H-FABP levels (r = 0.30, p = 0.01). In the H-FABP ≥7.5 ng/ml group, 78% (21/27) of patients had diabetes, compared with 47.5% (19/40) in the H-FABP <7.5 ng/ml group. There was no association between the use of β-blockers and/or statin therapy, other clinical risk factors, type of surgery or duration of anesthesia and postoperative elevated H-FABP levels. The cTnI level did not differ between before and after surgery [the median serum cTnI measurement before surgery was <0.01 µg/l (<0.01-0.31 µg/l), the median serum cTnI measurement at 8 h after surgery was <0.01 µg/l (<0.01-0.14 µg/l), p = 0.89]. Five out of 67 patients (7%) had serum cTn-I levels ≥0.01 µg/l, and only 1 of them had a level >0.1 µg/l.
Fig. 1.
Preoperative (0.13 ng/ml; 95% CI <0.1-5.9) and postoperative (6.86 ng/ml; 95% CI <0.1-13.7) H-FABP levels.
Fig. 2.
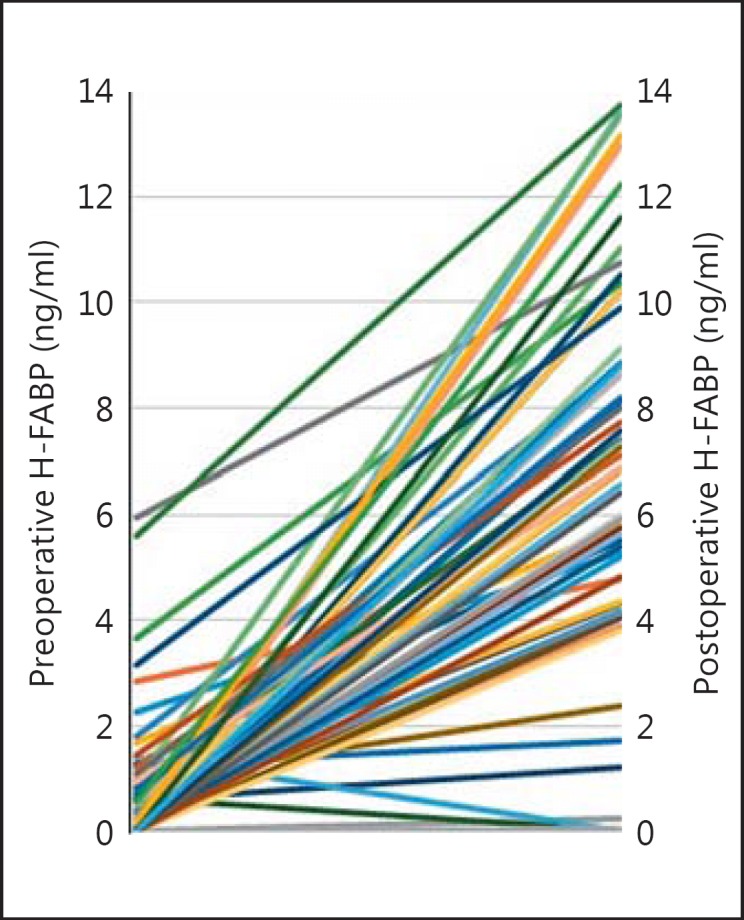
Plot showing every patient's preoperative and postoperative H-FABP levels.
Discussion
The serum H-FABP levels significantly increased after surgery (p < 0.05) but the serum cTnI did not differ between before and at 8 h after surgery. Detection of positive H-FABP or cTnI indicates that the patient might be suffering from myocardial injury and/or be at increased cardiac risk. Higher levels of H-FABP during the early phase of the postoperative period could be a result of secondary myocardial injury induced by surgery. We found that H-FABP is more sensitive than cTnI, which is consistent with previously published studies [20,21,22]. Katrukha et al. [20] reported that in patients with unstable angina pectoris, which involves a relatively small amount of myocardial necrosis, H-FABP was more sensitive than cTnI within 6 h of chest pain. Viswanathan et al. [21] showed that H-FABP predicted long-term mortality and reinfarction in patients with suspected acute coronary syndrome who were troponin negative. O'Donoghue et al. [22] reported that H-FABP identified high-risk patients who were considered to be troponin negative[.] Furthermore, Niizeki et al. [19] reported that, in congestive heart failure patients with negative cTnT, a high H-FABP level was a risk factor for cardiac complications compared to a low H-FABP level; hence, H-FABP was more sensitive in detecting ongoing myocardial damage.
The release and serum kinetics of these markers denoting myocardial ischemia may explain our findings. H-FABP is located in the cytoplasm and is not bound to the sarcomere and, thus, might be released more easily in response to myocardial injury. H-FABP is a small molecule, which further enhances the release. However, cTnI is a part of the troponin-tropomyosin complex in muscle fibers, and is released into the circulation only when the cardiomyocyte is completely damaged. After myocardial injury, the tissue-to-plasma gradient is steeper for H-FABP, thereby making plasma H-FABP rise more during the early phase after myocardial injury than cTnI [12,13,14,15,16,17,18,19].
It has been reported that H-FABP is not solely a cardiac-specific marker and is also present in the skeletal muscle at low concentrations [12,23]. Skeletal muscle damage during surgery may result in leakage of H-FABP, thus the release of H-FABP could be overestimated. Of equal importance, it has been reported that elevated levels of H-FABP, BNP and cTnI in injured adult patients were associated with increased adverse cardiac events and unrelated to direct thoracic injury [24]. Surgery and renal failure causes elevation of H-FABP concentrations [25]. The slow release of proteins from skeletal muscle might be related to lower blood flow during rest and lower permeability of the endothelial barrier in skeletal muscle than in the heart. In our study, it is not known if there is any relationship between an elevated level of H-FABP and cardiac events. Patients were not followed up for cardiovascular events in the following days. We could not determine whether H-FABP rise is the result of a real ischemic event, microvascular ischemia or just nonspecific myocardial damage.
The limitations of this study include the limited number of patients; failure to follow up cardiovascular events occurring in the following days, and lack of objective assessment of ischemia with other cardiovascular tests, such as electrocardiographic ST segment Holter monitoring. Some readers may wonder why we did not research the relationship between the level of HbA1c and H-FABP in diabetic patients. Plasma HbA1c reflects the mean glycemic status over a 2- to 3-month period. It has been demonstrated that a reduction in plasma HbA1c levels below 7% results in a lower incidence of microvascular complications in both type 1 and type 2 diabetes mellitus. However, a reduction in plasma HbA1c levels would result in a similar reduction in the incidence of macrovascular complications in patients with diabetes that failed to reach clinical statistical significance [26,27]. Glycemic control for diabetic patients is especially associated with postoperative infectious complications [28]. Due to these results, we did not research the relationship between the level of HbA1c and H-FABP in patients with diabetes.
Conclusion
H-FABP levels significantly increased in the postoperative period and most patients with increased postoperative H-FABP levels were diabetic. H-FABP could be used in detecting increased perioperative cardiac risk during the early phase of the postoperative period. Further comprehensive research is required to evaluate the value of this marker in the perioperative period for cardiac events and long-term prognosis in larger patient populations.
References
- 1.Devereaux PJ, Goldman L, Yusuf S, et al. Perioperative cardiac events in patients undergoing non-cardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173:627–634. doi: 10.1503/cmaj.050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J. 2009;30:2769–2812. doi: 10.1093/eurheartj/ehp337. [DOI] [PubMed] [Google Scholar]
- 3.Devereaux PJ, Goldman L, Yusuf S, et al. Surveillance and prevention of major perioperative ischemic cardiac events in patients undergoing noncardiac surgery: a review. CMAJ. 2005;173:779–788. doi: 10.1503/cmaj.050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boersma E, Kertai MD, Schouten O, et al. Perioperative cardiovascular mortality in noncardiac surgery: validation of the Lee cardiac risk index. Am J Med. 2005;118:1134–1141. doi: 10.1016/j.amjmed.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 5.Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387. doi: 10.1161/CIRCULATIONAHA.110.015701. [DOI] [PubMed] [Google Scholar]
- 6.Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152:26–35. doi: 10.7326/0003-4819-152-1-201001050-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto J, Suzuki T, Nakahara T, et al. Preoperative risk stratification using stress myocardial perfusion scintigraphy with electrocardiographic gating. J Nucl Med. 2003;44:385–390. [PubMed] [Google Scholar]
- 8.Bai J, Hashimoto J, Nakahara T, et al. Preoperative risk evaluation in diabetic patients without angina. Diabetes Res Clin Pract. 2008;81:150–154. doi: 10.1016/j.diabres.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Mangano DT, Wong MG, London MJ, et al. Perioperative myocardial ischemia in patients undergoing noncardiac surgery - II: incidence and severity during the 1st week after surgery. The Study of Perioperative Ischemia (SPI) Research Group. J Am Coll Cardiol. 1991;17:851–857. doi: 10.1016/0735-1097(91)90864-6. [DOI] [PubMed] [Google Scholar]
- 10.Hoeks S, Flu WJ, van Kuijk JP, et al. Cardiovascular risk assessment of the diabetic patient undergoing major noncardiac surgery. Best Pract Res Clin Endocrinol Metab. 2009;23:361–373. doi: 10.1016/j.beem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Wackers FJ, Young LH, Inzucchi SE, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 12.Alhadi HA, Fox KA. Do we need additional markers of myocyte necrosis: the potential value of heart fatty-acid-binding protein. QJM. 2004;97:187–198. doi: 10.1093/qjmed/hch037. [DOI] [PubMed] [Google Scholar]
- 13.Haltern G, Peiniger S, Bufe A, et al. Comparison of usefulness of heart-type fatty acid binding protein versus cardiac troponin T for diagnosis of acute myocardial infarction. Am J Cardiol. 2010;105:1–9. doi: 10.1016/j.amjcard.2009.08.645. [DOI] [PubMed] [Google Scholar]
- 14.Pasaoglu H, Ofluoglu E, Ilhan MN, et al. The role of heart-type fatty acid-binding protein (H-FABP) in acute myocardial infarction (AMI) compared to conventional cardiac biochemical markers. Turk J Med Sci. 2007;37:61–67. [Google Scholar]
- 15.Liao J, Chan CP, Cheung YC, et al. Human heart-type fatty acid-binding protein for on-site diagnosis of early acute myocardial infarction. Int J Cardiol. 2009;133:420–423. doi: 10.1016/j.ijcard.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 16.Mad P, Domanovits H, Fazelnia C, et al. Human heart-type fatty-acid-binding protein as a point-of-care test in the early diagnosis of acute myocardial infarction. QJM. 2007;100:203–210. doi: 10.1093/qjmed/hcm007. [DOI] [PubMed] [Google Scholar]
- 17.Ruzgar O, Bilge AK, Bugra Z, et al. The use of human heart-type fatty acid-binding protein as an early diagnostic biochemical marker of myocardial necrosis in patients with acute coronary syndrome, and its comparison with troponin-T and creatine kinase-myocardial band. Heart Vessels. 2006;21:309–314. doi: 10.1007/s00380-006-0908-2. [DOI] [PubMed] [Google Scholar]
- 18.Kilcullen N, Viswanathan K, Das R, et al. Heart-type fatty acid-binding protein predicts long-term mortality after acute coronary syndrome and identifies high-risk patients across the range of troponin values. J Am Coll Cardiol. 2007;50:2061–2067. doi: 10.1016/j.jacc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Niizeki T, Takeishi Y, Arimoto T, et al. Heart-type fatty acid-binding protein is more sensitive than troponin T to detect the ongoing myocardial damage in chronic heart failure patients. J Card Fail. 2007;13:120–127. doi: 10.1016/j.cardfail.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Katrukha A, Bereznekiva A, Filatov V, et al. Improved detection of minor ischemic cardiac injury in patients with unstable angina by measurement of TnI and FABP. Clin Chem. 1999;45:A139. [Google Scholar]
- 21.Viswanathan K, Kilcullen N, Morrell C, et al. Heart-type fatty acid-binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol. 2010;55:2590–2598. doi: 10.1016/j.jacc.2009.12.062. [DOI] [PubMed] [Google Scholar]
- 22.O'Donoghue M, de Lemos JA, Morrow DA, et al. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006;114:550–557. doi: 10.1161/CIRCULATIONAHA.106.641936. [DOI] [PubMed] [Google Scholar]
- 23.Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352:15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 24.De'Ath HD, Rourke C, Davenport R, et al. Clinical and biomarker profile of trauma-induced secondary cardiac injury. Br J Surg. 2012;99:789–797. doi: 10.1002/bjs.8728. [DOI] [PubMed] [Google Scholar]
- 25.Górski J, Hermens WT, Borawski J, et al. Increased fatty acid-binding protein concentration in plasma of patients with chronic renal failure. Clin Chem. 1997;43:193–195. [PubMed] [Google Scholar]
- 26.O'Sullivan CJ, Hynes N, Mahendran B, et al. Haemoglobin A1c (HbA1C) in non-diabetic and diabetic vascular patients: is HbA1C an independent risk factor and predictor of adverse outcome? Eur J Vasc Endovasc Surg. 2006;32:188–197. doi: 10.1016/j.ejvs.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Ceyhan K, Altunkaş F. Prediabetes, becoming the equivalent of coronary artery disease. Turk Kardiyol Dern Ars. 2012;40:458–465. doi: 10.5505/tkda.2012.40325. [DOI] [PubMed] [Google Scholar]
- 28.Lamloum SM, Mobasher LA, Karar AH, et al. Relationship between postoperative infectious complications and glycemic control for diabetic patients in an orthopedic hospital in Kuwait. Med Princ Pract. 2009;18:447–452. doi: 10.1159/000235893. [DOI] [PubMed] [Google Scholar]