Abstract
Objective
The aim of this study was to compare the levels of tumor necrosis factor-α (TNF-α) produced by peripheral blood mononuclear cells in normal pregnancies and pregnancies with complications.
Materials and Methods
Maternal peripheral blood mononuclear cells from women with a recurrent spontaneous miscarriage (n = 35), premature rupture of fetal membranes (n = 30), preeclampsia (n = 27) and intrauterine fetal growth retardation (IUGR; n = 36) were stimulated with mitogen or antigen, and the levels of TNF-α produced were compared to those produced by peripheral blood mononuclear cells from a normal pregnancy (n = 35).
Results
The median levels of mitogen-induced TNF-α at the 1st, 2nd and 3rd trimester, and at normal delivery were 1,176.4, 4,320.9, 7,307.4 and 2,463.0 pg/ml, respectively, while those produced in the recurrent spontaneous miscarriage, premature rupture of membranes and preeclampsia cases were 4,159.8, 3,489.5 and 4,149.2 pg/ml, respectively. The differences were statistically significantly higher in these pregnancy complications (p = 0.04, 0.024 and 0.014) as compared to the levels in normal pregnancy. Furthermore, antigen-induced TNF-α levels were produced at statistically significantly higher levels by women with IUGR (120.4 pg/ml) compared to women with normal pregnancies (17.9 pg/ml; p = 0.041).
Conclusion
Higher levels of TNF-α seem to play a role in these pregnancy complications, suggesting its pathogenesis in such conditions.
Key Words: Cytokines, Intrauterine fetal growth retardation, Preeclampsia, Recurrent spontaneous miscarriage, Tumor necrosis factor-α
Introduction
Tumor necrosis factor-α (TNF-α) is a powerful multipotent cytokine with a very impressive range of effects on an enormous variety of cells. Considerable evidence has now accumulated in support of the notion that TNF-α plays essential roles in very early pregnancy [1]. TNF-α has been shown to influence hormone synthesis, placental architecture, embryonic and follicle development, steroidogenesis, uterine cyclicity, placental differentiation and parturition [1]. At the same time, the capacity of TNF-α to be detrimental to pregnancy has been established beyond reasonable doubt [1,2]. TNF-α stimulates the apoptosis of human primary villous trophoblast cells, inhibits the development of the mouse fetus and the proliferation of human trophoblast cells in vitro. The administration of TNF-α to normal pregnant mice causes abortions [1], while anti-TNF-α antibodies reduce resorption rates in a murine model of spontaneous, immunologically mediated abortions [2]. When cocultured with placental cells from abortion-prone murine mating combinations, maternal-strain splenocytes elicit the production of high levels of TNF-α, while coculture with placental cells from normal combinations does not [3]. Thus, while TNF-α is essential for early events in pregnancy, such as implantation, high levels of TNF-α may be deleterious to pregnancy later during gestation [1]. In this study, we compared the levels of TNF-α produced by human maternal peripheral blood mononuclear cells (PBMCs) in different pregnancy complications, including recurrent spontaneous miscarriage (RSM), premature rupture of fetal membranes (PROM), preeclampsia and intrauterine fetal growth retardation (IUGR).
Subjects and Methods
Subjects
This case-controlled prospective study was conducted between 2005 and 2010. The groups of women studied along with the number of patients, clinical history and mean gestational age are summarized in table 1. These included normal pregnant women in the 1st (n = 24), 2nd (n = 16) and 3rd trimester (n = 20), and with normal delivery (ND; n = 35). Pregnancy complications among the subjects included RSM (n = 35), PROM (n = 30), preeclampsia (n = 27) and IUGR (n = 36).
Table 1.
Groups of women studied along with the number of patients, clinical history and mean gestational age
| Description | n | History | Mean gestational age, weeks |
|---|---|---|---|
| ND | 35 | 39.4 ± 1 | |
| 1st trimester | 24 | Women with a history of three or more | 12 ± 2 |
| 2nd trimester | 16 | normal pregnancies | 21.5 ± 0.6 |
| 3rd trimester | 20 | 32.4 ± 4.2 | |
| RSM | 35 | Women having at least a third unexplained miscarriage | 12 ± 3 |
| PROM | 30 | Women with spontaneous rupture of fetal membranes at term | 39 ± 1.1 |
| PIH | 27 | Previously normotensive women who developed hypertension associated with proteinuria during pregnancy | 39 ± 1.4 |
| IUGR | 36 | Pregnant women with an estimated fetal weight less than the 10th percentile | 35.1 ± 3.7 |
| IUGR with placental insufficiency (asymmetric IUGR) IUGR without placental insufficiency (symmetric IUGR) |
19 17 |
Placental insufficiency (raised pulsatility index in the umbilical artery, with either absent or reversed end-diastolic flow) | 34.6 ± 3.3 36.1 ± 4.3 |
The subjects in this study were recruited at the Kuwait Maternity Hospital, which is a tertiary center with 600 beds and currently handles an average of 16,000 deliveries each year. All patients of the different groups were included only after having been evaluated clinically and following laboratory and radiology investigations. Subjects in the normal pregnancy group were those who had previously had at least three normal pregnancies, with no history of abortion, ectopic pregnancy, preterm delivery or stillbirth. The RSM group was composed of women admitted with spontaneous abortion for evacuation who had had at least three previous unexplained miscarriages and who had been clinically investigated for possible anatomical, endocrinological, infectious, genetic and immunological causes of abortion (antinuclear antibodies, lupus anticoagulant and anticardiolipin antibodies). The PROM group consisted of women with spontaneous rupture of fetal membranes at term. Subjects in the preeclampsia group comprised women with pregnancy-induced hypertension (PIH) who were normotensive before pregnancy and during the first 20 weeks of gestation and developed hypertension associated with proteinuria. The women in the IUGR group were further subdivided into IUGR pregnancies with and without placental insufficiency, which was diagnosed if the pulsatility index in the umbilical artery was raised, with either absent or reversed end-diastolic flow. This study was approved by the Ethics Committees of the Faculty of Medicine, Kuwait University, and Kuwait Maternity Hospital, Kuwait
Mitogen-Induced Activation of PBMCs
Peripheral blood samples were obtained by venipuncture within 1 h of a normal or complicated/pathological delivery, to reflect the situation of the cytokine profiles existing in the periphery at that point in time. The PBMCs were separated by Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation, suspended in RPMI medium (Gibco BRL, Grand Island, N.Y., USA) containing 10s% fetal calf serum, aliquoted into 96-well tissue culture plates at a density of 105 cells per well and then stimulated with the mitogen phytohemagglutinin (Sigma Chemicals, St. Louis, Mo., USA) at a concentration of 5 μg/ml for a period of 96 h. Supernatants were harvested for cytokine estimation on day 4 and stored at −80°C.
Trophoblast Antigen-Induced Proliferation
Peripheral blood from the IUGR and ND groups were stimulated with a trophoblast antigen preparation. Antigen extracts were prepared from the human gestational choriocarcinoma cell line JEG-3 (American Type Culture Collection, Rockville, Md., USA), which is of trophoblastic origin. The JEG antigen extracts were prepared essentially as described previously [4]. JEG cells were cultured in RPMI 1640 medium, harvested without trypsinization using a rubber cell scraper, washed three times in medium, and then disrupted in a Dounce homogenizer. The suspension was centrifuged at 1,500 g for 10 min, the trophoblast extract was filtered through a 0.2-mm syringe filter (Nalgene, Rochester, N.Y., USA) and stored at −20°C until use. Maternal PBMCs were stimulated at a density of 105 cells per well with 30 mg/ml of JEG antigen. PBMCs were cultured for 4 days after which supernatants were collected and stored at −80°C. The trophoblast cell line, JEG-3, used to prepare the trophoblast antigen extract has characteristics similar to early normal human trophoblast cells, including invasive characteristics, and endocrine and antigenic features. The JEG preparation was tested by ELISA and found to contain no detectable levels of TNF-α. Thus, we assumed that the TNF-α measured in these cultures were secreted by the stimulated maternal PBMCs, because previous studies using antigen extracts from this cell line had demonstrated higher Th1-type and lower Th2-type reactivity to trophoblast antigens in women with unexplained recurrent miscarriage as compared to women with normal pregnancies [4].
ELISA for Cytokines
The levels of TNF-α were determined by sandwich ELISA (Immunotech SA, Marseille, France). Samples were tested in triplicate, and absorbance values were measured in an ELISA reader. Accurate concentrations of cytokines were determined by comparing their respective absorbencies with those obtained for the reference standards plotted on a standard curve using reference recombinant cytokines. The sensitivity of the assay was 10 pg/ml of TNF-α.
Statistical Analysis
Data analysis was performed using the Statistical Package for the Social Sciences for Windows, version 19.0 (SPSS, Chicago, Ill., USA). The nonparametric Mann-Whitney test for independent samples was used to assess the statistical significance of differences. Data are presented using box plots (median ± 25th and 75th percentiles). A p value <0.05 was considered statistically significant.
Results
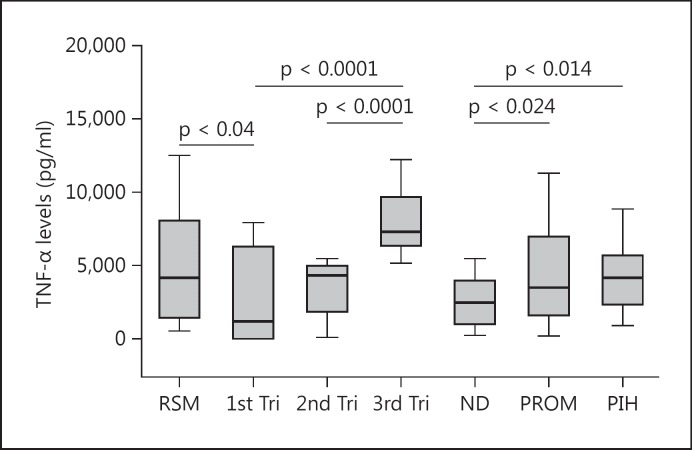
Median TNF-α levels were lowest in the 1st trimester (1,176.4 pg/ml) but not significantly different to the 2nd trimester levels (4,320.9 pg/ml). The median levels of TNF-α produced by PBMCs obtained in the 3rd trimester (7,307.4 pg/ml) were significantly different compared to the 2nd trimester and at delivery (p = 0.0001 in both cases; fig. 1).
Fig. 1.
Median levels of TNF-α produced by mitogen-activated PBMCs from women in their 1st, 2nd and 3rd trimesters (Tri), women with ND, and women with RSM, PROM and preeclampsia (PIH).
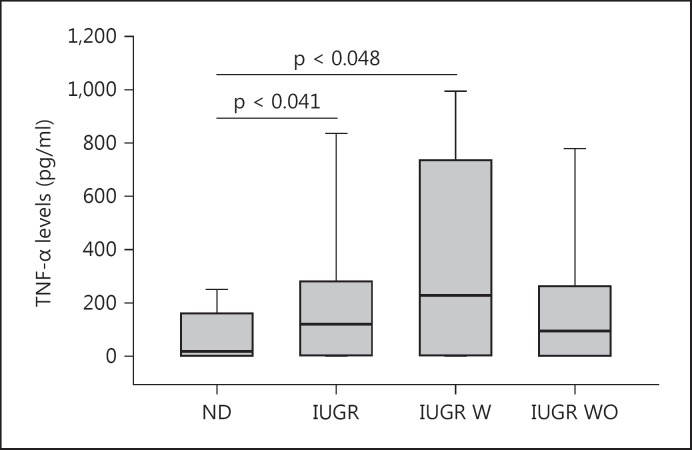
PBMCs from women with RSM produced statistically significantly different median TNF-α levels (4,159.8 pg/ml) compared to the gestationally matched 1st trimester group (p = 0.04). Women with PROM produced statistically significantly different median levels of TNF-α (3,489.55 pg/ml) compared to women in the ND group (2,463.0 pg/ml; p = 0.024). Women with PIH produced statistically significantly higher median levels of TNF-α (4,149.2 pg/ml) compared to women in the ND group (2,463.0 pg/ml; p = 0.014; fig. 1). PBMCs from women with IUGR produced statistically significantly different levels of TNF-α (120.4 pg/ml) compared to women with normal pregnancies (17.9 pg/ml; p = 0.041; fig. 2). Subdividing this group further into IUGR with and without placental insufficiency, the PBMCs from women with placental insufficiency IUGR showed statistically significantly higher median TNF-α levels (227.7 pg/ml) compared to women with normal pregnancies (p = 0.048; fig. 2).
Fig. 2.
Median levels of TNF-α produced by JEG antigen-activated PBMCs from women with ND, all IUGR subjects (IUGR), IUGR with placental insufficiency (IUGR W) and IUGR without placental insufficiency (IUGR WO).
Discussion
In this study the progression of a normal pregnancy from the 1st trimester up to delivery, TNF-α levels were lower in the 1st and 2nd trimesters as compared to the 3rd trimester. While the mediatory roles of TNF-α in late gestation have not been entirely elucidated, it is likely that this proinflammatory cytokine contributes to parturition via increasing prostaglandin levels by manipulating prostaglandin production and/or metabolism [5].
The proper functioning of cytokine networks is essential for normal physiological homeostasis, and this role is emphasized by the fact that cytokine-mediated dysregulations are associated with several pregnancy complications which tend to exhibit changes in both local and systemic cytokine profiles. In the four complications presented in this paper, the overall picture of cytokine production profiles was of a shift towards Th1 or proinflammatory cytokine dominance as compared to normal pregnancies. Women with RSM, PROM, PIH and IUGR had significantly higher TNF-α levels compared to their gestationally matched normal pregnancy controls.
Hunt et al. [6] have demonstrated the expression of TNF-α in placental tissues and the embryo, suggesting that TNF-α has beneficial roles in the development of early pregnancy by enhancing placental maturation and differentiation, embryonic development and parturition [6]. At the same time, TNF-α has been shown to inhibit blastocyst growth and the development of murine pregnancy [3]. A reasonable explanation for this apparent contradiction comes from Guilbert [7], who suggests that the timing and location of cytokines as well as the network of signals and counter-signals are probably responsible. Several researchers suggest that RSM due to Th1 dominance may act via a shift towards a deleterious maternal cell-mediated immune response. This may involve NK cells, which in the presence of proinflammatory cytokines become lymphokine-activated killer (LAK) cells that have been shown to kill trophoblast cells. It has previously been shown that systemic levels of LAK-like cells correlate with high miscarriage rates [8]. Clark et al. [9] propose that maternal ‘rejection’ of the conceptus may be due to the process of ‘vascular autoamputation’ by cytokines like TNF-α and interferon-γ (IFN-γ), involving activation of coagulation mechanisms and leading to vasculitis; this affects the maternal blood supply to the implanted embryo. The pathophysiology of PROM is poorly understood, but it was proposed that many of the pathological effects observed in PROM may be mediated by cytokines. Bowen et al. [10] suggest that whatever the initiating signal (infection or otherwise), an ensuing inflammatory reaction could result in the secretion of cytokines, such as TNF-α, which could lead to the degradation of the membrane matrices, predisposing them to the rupture of fetal membranes either by apoptotic or other mechanisms, such as increased production of prostaglandin E2 and increased uterine activity; this could result in PROM. An important observation in preeclampsia is the generalized activation and/or injury of maternal vascular endothelial cells, leading to microthrombus formation and vasospasm [11]. If, as proposed by several researchers, placental trophoblasts and maternal vascular endothelial cells are the targets of immune aggression in preeclampsia, then maternal inflammatory cytokines are likely to be important effectors of this aggression. Redman and Sargent [12] suggest that the clinical features of preeclampsia are best described as an excessive maternal inflammatory response mediated by cytokines. TNF-α induces activation of and damage to endothelial cells, meaning our observation of increased TNF-α production in the PIH group is pertinent. In fact, serum TNF-α levels are reported to increase even prior to the clinical manifestation of the disorder [13], lending credence to the suggested role of TNF-α in mediating endothelial dysfunction. TNF-α has therefore emerged as one of the key cytokines in PIH given its potent ability to cause endothelial activation and dysfunction.
IUGR with placental insufficiency occurs late in gestation and involves significant placental pathological findings [14]. IUGR with placental insufficiency is believed to be due to maternal diseases that bring about a reduction of uteroplacental blood flow [14], and this feature of IUGR may be caused by TNF-α. Overproduction of TNF-α and other proinflammatory cytokines has been suggested to cause fetal growth restriction in response to hypoxia, possibly by decreasing amino acid uptake by the fetus [15]. TNF-α has other effects on the placenta that may be relevant, including the fact that it inhibits the growth of the trophoblast, interferes with placental development and invasion of the spiral arteries, is directly toxic to endothelium and may damage the decidual vasculature [1]. TNF-α interferes with the anticoagulant system and may induce placental thrombosis [2]. It may also contribute to IUGR by causing apoptosis of trophoblast cells as IUGR has been shown to be characterized by enhanced trophoblast apoptosis, and this has been suggested to lead to abnormal placentation, inadequate spiral artery remodeling and uteroplacental vascular insufficiency [16].
The demonstration of associations between pregnancy complications and TNF-α leads to the prediction that the downregulation of this cytokine may help create a milieu that is more conducive to the success of pregnancy. Winger et al. [17] reported that the use of a TNF-α inhibitor and intravenous immunoglobulin significantly improves the IVF outcome in young infertile women with Th1/Th2 cytokine elevation. Another means by which this may be accomplished is by using a hormone such as progesterone, which has been shown to have anti-inflammatory and immunosuppressive properties [18]. Piccinni et al. [19] demonstrated that progesterone favors the development of human T cells producing Th2 cytokines. Their suggestion is that as the Th1 cytokines IFN-γ and TNF-α promote allograft rejection and may compromise pregnancy, the production of Th2-type cytokines IL-4 and IL-10 may promote allograft tolerance and fetal survival by inhibiting Th1 reactivity. This led us to examine dydrogesterone for possible immunomodulatory activity on lymphocytes from women with RSM. Dydrogesterone (6-dehydro-9β,10α-progesterone) brings about a significantly reduced secretion of TNF-α when added to mitogen-stimulated PBMC cultures from women with unexplained recurrent miscarriage. The decreased production of the Th1 cytokine TNF-α is an indication of the redirection of cytokine profiles from a predominantly proinflammatory bias towards an anti-inflammatory bias which may help avoid a condition that might lead to recurrent miscarriage [20,21].
Conclusion
Considerable evidence has now accumulated in support of the notion that TNF-α is central to normal processes in the placenta, such as inflammation, survival, apoptosis, cell migration, proliferation and differentiation. Along with other inflammatory cytokines, TNF-α may play critical roles in causing pregnancy complications, such as recurrent miscarriage, PROM, preeclampsia and IUGR.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgments
This study was funded by Kuwait University Research Administration Grant MI02/10.
References
- 1.Haider S, Knofler M. Human tumour necrosis factor: physiological and pathological roles in placenta and endometrium. Placenta. 2009;30:111–123. doi: 10.1016/j.placenta.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannubilo SR, Landi B, Pozzi V, et al. The involvement of inflammatory cytokines in the pathogenesis of recurrent miscarriage. Cytokine. 2012;58:50–56. doi: 10.1016/j.cyto.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Raghupathy R, editor. Th1 and Th2 Cytokine Profiles in Successful and Unsuccessful Pregnancy. New Delhi: Narosa Publishing; 1999. pp. 149–158. [Google Scholar]
- 4.Raghupathy R, Makhseed M, Azizieh F, et al. Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999;196:122–130. doi: 10.1006/cimm.1999.1532. [DOI] [PubMed] [Google Scholar]
- 5.Alzamil HA, Pawade J, Fortier MA, et al. Expression of the prostaglandin F synthase AKR1B1 and the prostaglandin transporter SLCO2A1 in human fetal membranes in relation to spontaneous term and preterm labor. Front Physiol. 2014;5:272. doi: 10.3389/fphys.2014.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt JS, Chen HL, Miller L. Tumor necrosis factors: pivotal components of pregnancy? Biol Reprod. 1996;54:554–562. doi: 10.1095/biolreprod54.3.554. [DOI] [PubMed] [Google Scholar]
- 7.Guilbert LJ. There is a bias against type 1 (inflammatory) cytokine expression and function in pregnancy. J Reprod Immunol. 1996;32:105–110. doi: 10.1016/s0165-0378(96)00996-5. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima A, Shima T, Inada K, et al. The balance of the immune system between T cells and NK cells in miscarriage. Am J Reprod Immunol. 2012;67:304–310. doi: 10.1111/j.1600-0897.2012.01115.x. [DOI] [PubMed] [Google Scholar]
- 9.Clark DA, Chaouat G, Arck PC, et al. Cytokine-dependent abortion in CBA × DBA/2 mice is mediated by the procoagulant fgl2 prothrombinase [correction of prothombinase] J Immunol. 1998;160:545–549. [PubMed] [Google Scholar]
- 10.Bowen JM, Chamley L, Keelan JA, et al. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23:257–273. doi: 10.1053/plac.2001.0782. [DOI] [PubMed] [Google Scholar]
- 11.Raghupathy R. Cytokines as key players in the pathophysiology of preeclampsia. Med Princ Pract. 2013;22(suppl 1):8–19. doi: 10.1159/000354200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 13.Omu AE, Al-Azemi MK, Al-Qattan F, et al. Connection between human leucocyte antigens D region and T helper cytokines in preeclampsia. Arch Gynecol Obstet. 2004;269:79–84. doi: 10.1007/s00404-002-0436-y. [DOI] [PubMed] [Google Scholar]
- 14.Creasy RK, Resnik R, Iams JD. Maternal-Fetal Medicine. ed 5. Philadelphia: Saunders; 2004. [Google Scholar]
- 15.Briana DD, Malamitsi-Puchner A. Intrauterine growth restriction and adult disease: the role of adipocytokines. Eur J Endocrinol. 2009;160:337–347. doi: 10.1530/EJE-08-0621. [DOI] [PubMed] [Google Scholar]
- 16.Kilani RT, Mackova M, Davidge ST, et al. Endogenous tumor necrosis factor α mediates enhanced apoptosis of cultured villous trophoblasts from intrauterine growth-restricted placentae. Reproduction. 2007;133:257–264. doi: 10.1530/REP-06-0080. [DOI] [PubMed] [Google Scholar]
- 17.Winger EE, Reed JL, Ashoush S, et al. Treatment with adalimumab (Humira) and intravenous immunoglobulin improves pregnancy rates in women undergoing IVF. Am J Reprod Immunol. 2009;61:113–120. doi: 10.1111/j.1600-0897.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- 18.Halasz M, Szekeres-Bartho J. The role of progesterone in implantation and trophoblast invasion. J Reprod Immunol. 2013;97:43–50. doi: 10.1016/j.jri.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Piccinni MP, Giudizi MG, Biagiotti R, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- 20.Raghupathy R, Kalinka J. Cytokine imbalance in pregnancy complications and its modulation. Front Biosci. 2008;13:985–994. doi: 10.2741/2737. [DOI] [PubMed] [Google Scholar]
- 21.Raghupathy R, Al Mutawa E, Makhseed M, et al. Modulation of cytokine production by dydrogesterone in lymphocytes from women with recurrent miscarriage. BJOG. 2005;112:1096–1101. doi: 10.1111/j.1471-0528.2005.00633.x. [DOI] [PubMed] [Google Scholar]