Abstract
Objective
We report the second case of gastric adenocarcinoma associated with type B lactic acidosis.
Clinical Presentation and Intervention
An 81-year-old man presenting with upper gastrointestinal bleeding was found to have an advanced gastric adenocarcinoma. He had persistently elevated serum lactate attributed to malignancy-associated type B lactic acidosis as a diagnosis of exclusion. As he remained clinically stable with a near-normal pH, his elevated lactate was not specifically treated.
Conclusion
This patient had an unusual type B lactic acidosis associated with gastric cancer. In the absence of signs and symptoms of other etiologies of lactic acidosis, physicians should consider malignancy-associated type B lactic acidosis.
Key Words: Type B lactic acidosis, Gastric adenocarcinoma, Liver metastases
Introduction
Type B lactic acidosis is a rare complication of nonhematologic malignancies. We report the second case of gastric cancer associated with type B lactic acidosis [1]. In the cancer patient, it is important to identify type B lactic acidosis, as it represents a life threatening and potentially treatable complication. Several treatment modalities, including bicarbonate infusion and thiamine supplementation, have been employed in such cases with varying success, but removal of the malignancy remains the gold standard therapy.
Case Report
An 81-year-old man with a medical history of chronic kidney disease secondary to hypertension (stage III/IV) and peptic ulcer disease presented with epigastric pain, a weight loss of 25 pounds over several months, melena for 2 weeks and hematemesis for 2 days. He had a remote history of alcohol abuse, but had remained abstinent for 4 decades. His medications included 20 mg of omeprazole twice daily and 20 mg of furosemide daily. He had no known allergies.
His vital signs on admission were temperature: 36.3°C, heart rate: 90 bpm, respiratory rate: 20 breaths/min and blood pressure: 173/63 mm Hg. Physical examination findings included generalized jaundice and a distended abdomen tender to palpation in the right upper quadrant with no peritoneal signs. There was profound hepatomegaly without splenomegaly. A rectal examination revealed guaiac-positive melenic stools. No palmar erythema, spider angiomas, asterixis or fluid wave were appreciated. The patient was started on intravenous fluids and a pantoprazole drip, and he remained clinically stable for the 4-day hospital course.
Initial laboratory tests showed hemoglobin of 11.6 g/dl (decreased from 13.6 g/dl 1 month previously), a white blood cell count of 10.8 × 109 cells/l, a platelet count of 342 × 109/l, total bilirubin of 10.1 mg/dl, direct bilirubin of 6.1 mg/dl, aspartate aminotransferase 727 U/l, alanine aminotransferase 333 U/l and alkaline phosphatase 333 U/l. Electrolytes revealed levels of sodium: 141 mEq/l, potassium: 4.9 mEq/l, chloride: 107 mEq/l, bicarbonate: 16 mEq/l, blood urea nitrogen: 68 mg/dl, creatinine: 2.2 mg/dl, glucose: 81 mg/dl, phosphorous: 6.0 mg/dl and calcium: 9.2 mg/dl. Electrolytes showed no trend toward significant acute kidney injury or tumor lysis syndrome during the hospital course. Lactate was elevated at 6.6 mmol/l. Urinalysis was unremarkable. P-lipase was normal. Urine drug screen, hepatitis panel and blood and urine cultures were negative. Arterial blood gas revealed two primary acid-base disorders: metabolic acidosis and respiratory alkalosis (pH: 7.43, pCO2: 24.6 mm Hg, pO2: 85.5 mm Hg and HCO3-: 16.1 mmol/l).
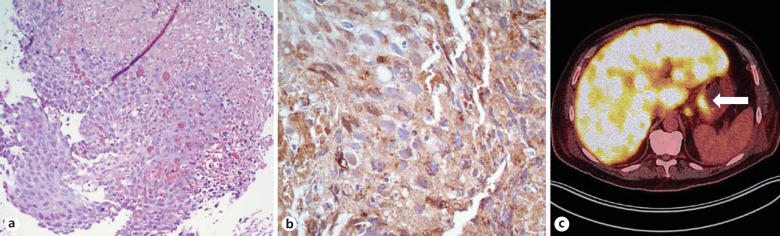
Diagnostic procedures included right upper quadrant abdominal ultrasound, which did not show common bile duct dilatation or gallstones. Esophagogastroduodenoscopy revealed a 2-cm, ulcerated mass on the retroflexion of the cardia. Biopsies of the mass revealed a poorly differentiated α-fetoprotein (AFP)-positive adenocarcinoma (fig. 1a, 1b). A diagnosis of gastric adenocarcinoma was made. Positron emission tomography/computed tomography showed uptake of fluorodeoxyglucose in the cardia of the stomach, diffuse and intense uptake in the liver and focal uptake in several pulmonary nodules and the pelvis (fig. 1c). Lactate remained persistently elevated throughout the hospital course (range 4.0-6.6 mmol/l). Tumor marker tests were significant for the levels of AFP (896 ng/ml, normal range: 0-8) and cancer antigen 19-9 (100 U/ml, normal range: 0-35). Due to the extent of his disease and his poor performance status, the patient was thought to be a poor candidate for antineoplastic intervention. The findings were discussed with the patient and his family, and he opted for discharge to hospice care. He died several days later.
Fig. 1.
Gastric tumor with extensive hepatic metastasis. a Histopathology demonstrates a poorly differentiated adenocarcinoma. HE stain. b Immunohistochemical staining demonstrates an AFP-positive adenocarcinoma (dark stain). c Abdominal positron emission tomography/computed tomography (transverse section). Diffuse hepatic disease and primary gastric tumor (arrow) demonstrate a fluorodeoxyglucose uptake signal.
Discussion
Our patient had persistent lactic acidosis over his 4-day hospital course, with lactate levels ranging from 4.0 to 6.6 mM, in the absence of hypotension or other clinical signs of ongoing end-organ dysfunction. This ruled out type A lactic acidosis (secondary to hypoperfusion). The absence of other reported etiologies of type B lactic acidosis, such as diabetes or active alcoholism [2], strongly supported a diagnosis of type B lactic acidosis secondary to malignancy.
A particularly unusual aspect of this case was the patient's initial arterial blood gas, as two primary acid-base disorders - an anion gap metabolic acidosis and a respiratory alkalosis - resulted in a near-normal pH. In such cases, patients usually present with severe systemic acidosis with high anion gaps and low carbon dioxide levels [1,3,4]. In this case, despite the significant lactic acidemia and increased anion gap, the patient's pH was borderline alkaline. By Winter's formula, the expected pCO2 was 30-34 mm Hg (actual 24.6 mm Hg). As there was no base excess, there was a concurrent primary respiratory alkalosis. This was most likely a central respiratory alkalosis from net-respiratory stimulatory substances (e.g. progesterone) that can accumulate in the setting of liver failure and/or from increased chemosensitivity to carbon dioxide [5].
There are several plausible pathophysiologic mechanisms for malignancy-associated type B lactic acidosis. (1) Malignancy can indirectly cause lactic acidosis by inducing thiamine deficiency, especially in the context of rapidly proliferating hematologic malignancies and total parenteral nutrition [4,6]. Thiamine is an essential cofactor for the pyruvate dehydrogenase complex, which converts pyruvate to acetyl-CoA and allows aerobic metabolism to proceed. In at least 1 case, thiamine supplementation was reported to improve lactic acidosis associated with an acute leukemia [6]. (2) Another hypothesis involves the Warburg effect - the tendency for tumor cells to favor glycolysis even in the presence of sufficient oxygen. It is thought that certain hematologic malignancies proliferate so rapidly that lactate production overwhelms the body's carbonic acid-bicarbonate buffer system [6,7]. An interesting component of this case was the AFP-positive tumor (fig. 1b), which is associated with aggressive tumor behavior [8]. Given the extensive tumor burden and aggressive tumor subtype, it is possible that the tumor's overwhelming anaerobic metabolism approximated that of a hematologic malignancy. (3) Reduced lactate clearance may be an important pathophysiologic factor, as was likely in our case. Normally, the liver is responsible for the clearance of 80-90% of lactate via the Cori cycle [2], and extensive hepatic disease is therefore hypothesized to impact lactate metabolism. The kidney is responsible for clearing the remaining lactate. As this patient's serum lactate levels did not improve upon resolution of his mild acute kidney injury shortly after admission, it is possible that his baseline stage III/IV CKD may have contributed to the systemic lactate accumulation by impairing the secondary mechanism for lactate clearance.
Conclusion
This was a rare case of gastric carcinoma with extensive hepatic metastases causing a type B lactic acidosis. Physicians should be aware that type B lactic acidosis can occur in a variety of nonhematologic tumors, including gastric carcinoma.
Acknowledgements
The authors thank Dr. Prema Gogate for obtaining the pathology figures shown.
References
- 1.Permpalung N, Ammannagari N, Price CD, et al. Refractory lactic acidosis in CD30 positive gastric cancer. Ann Hematol. 2014;93:1805–1806. doi: 10.1007/s00277-014-2011-6. [DOI] [PubMed] [Google Scholar]
- 2.Emmet M. Causes of lactic acidosis. In: Sterns RH, Forman JP, editors. UpToDate. Waltham: Wolters Kluwer; 2014. [Google Scholar]
- 3.Ruiz JP, Singh AK, Hart P. Type B lactic acidosis secondary to malignancy: case report, review of published cases, insights into pathogenesis, and prospects for therapy. ScientificWorldJournal. 2011;11:1316–1324. doi: 10.1100/tsw.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine. 2007;86:225–232. doi: 10.1097/MD.0b013e318125759a. [DOI] [PubMed] [Google Scholar]
- 5.Passino C, Giannoni A, Mannucci F, et al. Abnormal hyperventilation in patients with hepatic cirrhosis: role of enhanced chemosensitivity to carbon dioxide. Int J Cardiol. 2012;154:22–26. doi: 10.1016/j.ijcard.2010.08.066. [DOI] [PubMed] [Google Scholar]
- 6.Svahn J, Schiaffino M, Caruso U, et al. Severe lactic acidosis due to thiamine deficiency in a patient with B-cell leukemia/lymphoma on total parenteral nutrition during high-dose methotrexate therapy. J Pediatr Hematol Oncol. 2003;25:965–968. doi: 10.1097/00043426-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Silos EM, Shenep JL, Burghen GA, et al. Lactic acidosis: a metabolic complication of hematologic malignancies. Cancer. 2001;92:2237–2246. doi: 10.1002/1097-0142(20011101)92:9<2237::aid-cncr1569>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Cheng Y, Sheng W, et al. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249–255. doi: 10.1002/jso.21624. [DOI] [PubMed] [Google Scholar]