Abstract
Objective
To compare specific characteristics and clinical outcomes of monomicrobial necrotizing fasciitis caused by Aeromonas hydrophila and Klebsiella pneumoniae.
Material and Methods
Cases of monomicrobial necrotizing fasciitis caused by A. hydrophila (n = 11) and K. pneumoniae (n = 7) over an 8-year period were retrospectively reviewed. Differences in mortality, patient characteristics, clinical presentations, and laboratory data were compared between the A. hydrophila and the K. pneumoniae groups.
Results
The clinical signs and symptoms at the time of presentation did not differ significantly (p > 0.05) between the two groups. The A. hydrophila group had a significantly shorter interval between contact and admission (1.55 ± 0.52 vs. 5.14 ± 2.12 days, p < 0.001) and significant lower total white blood cell counts (10,245 ± 5,828 vs. 19,014 ± 11,370 cells/mm3, p < 0.045) than the K. pneumoniae group in the emergency room. Hepatic dysfunction was associated with mortality in patients with A. hydrophila infection, while diabetes mellitus was associated with mortality in patients with K. pneumoniae infection. Overall, 5 (45.5%) patients in the A. hydrophila group and 3 (42.8%) in the K. pneumoniae group died.
Conclusion
The initial clinical course of A. hydrophila monomicrobial necrotizing fasciitis was characterized by more rapidly progressive disease than that of the K. pneumoniae infection. Patients with hepatic dysfunction and necrotizing fasciitis should be suspected of having A. hydrophila infection, and diabetic patients with necrotizing fasciitis should be suspected of having K. pneumoniae infection initially.
Key Words: Necrotizing fasciitis, Aeromonas hydrophila, Klebsiella pneumoniae
Introduction
Necrotizing fasciitis is a rapidly progressive, life-threatening soft-tissue infection that is a true medical and surgical emergency needing early diagnosis, emergent surgical debridement, and broad-spectrum antibiotic therapy when patients present to the emergency department [1,2,3,4]. Although necrotizing fasciitis is often caused by polymicrobial infection, the prevalence of monomicrobial necrotizing fasciitis has recently been reported to be as high as 60–80% [4,5,6,7]. Our previous study revealed that the clinical course of necrotizing fasciitis caused by Gram-negative microorganisms was more rapid and fulminant than that of Gram-positive infection, and Gram-negative aerobic pathogens, such as Vibrio vulnificus, Klebsiella pneumoniae, Aeromonas hydrophila, and Escherichia coli, were the most frequently isolated microorganisms causing necrotizing fasciitis [4].
Aeromonas spp. are Gram-negative bacilli that thrive in aquatic environments, especially in sewage, fresh or brackish water, soil, tap water, and nonfecal organic materials [8,9,10,11]. Necrotizing fasciitis caused by A. hydrophila often occurs after soft-tissue trauma with associated exposure to contaminated water or nonfecal organic materials and can produce skin lesions similar to those observed in necrotizing fasciitis caused by Vibrio species [8,9,10,11,12]. A. hydrophila is frequently associated with polymicrobial infections and can cause synergistic necrotizing fasciitis in patients coinfected with Clostridium species or Gram-negative bacilli, of which Klebsiella spp. have recently been reported to be the most common copathogens [11,12,13,14].
K. pneumoniae, a member of the Enterobacteriaceae, is a common cause of Gram-negative bacteremia and has frequently been described as a facultative organism in polymicrobial necrotizing fasciitis [15,16]. Monomicrobial necrotizing fasciitis caused by K. pneumoniae is extremely rare in the Western hemisphere [16], but community-acquired infections have been increasingly reported in the past decade, especially in the Asian regions, including Taiwan, Hong Kong, Japan, Singapore, and Malaysia, with fatality rates of nearly 50% [16,17,18,19,20,21].
Our previous studies [4,8,22,23] revealed that mortality rates in patients with Vibrio necrotizing fasciitis have decreased from 38 to 13% due to an effective program that includes early recognition, application of a treatment algorithm for emergency fasciotomy or amputation, treatment with a third-generation cephalosporin plus tetracycline or gentamicin, and an intensive unit care. Patients with Vibrio necrotizing fasciitis can be identified early because of a history of contact with seawater or raw seafood. However, patients with A. hydrophila and K. pneumoniae necrotizing fasciitis still have the highest mortality rates (50 and 60%, respectively) even after early diagnosis and surgical intervention in the emergency room [4]. Monomicrobial necrotizing fasciitis caused by A. hydrophila and K. pneumoniae are rarely reported [12,17,18]. To our knowledge, no publication or literature review has to date described or compared these two causative pathogens of fatal monomicrobial necrotizing fasciitis. Therefore, the purpose of this study was to compare the initial clinical features of A. hydrophila and K. pneumoniae necrotizing fasciitis, and the risk factors related to the outcomes.
Subjects and Methods
Study Design and Patient Selection
We reviewed the medical records of 33 patients with surgically confirmed necrotizing fasciitis caused by A. hydrophila and K. pneumoniae who were admitted to the emergency department of our hospital from June 2004 to December 2012. The enrolled patients were categorized into 2 groups: an A. hydrophila group and a K. pneumoniae group. Eight patients with A. hydrophila and 7 patients with K. pneumoniae infection who had confirmed polymicrobial necrotizing fasciitis were excluded (these patients survived after surgery).
Broad-spectrum antibiotics were initially administered to all patients, and excisional debridement of the necrotic fascia or immediate limb amputation was performed in all 33 patients diagnosed with necrotizing fasciitis.
Microbiology Laboratory Procedures
The identification of A. hydrophila and K. pneumoniae was based on standard phenotypic tests used in clinical microbiology laboratories, and the identification of A. hydrophila was further confirmed using the Api20E test (BioMerieux, Marcy l'Etoile, France). The antimicrobial susceptibility of A. hydrophila and K. pneumoniae was evaluated in the hospital microbiology laboratory using the standard disk diffusion technique at our institute. The susceptibility interpretative criteria for Enterobacteriaceae and Aeromonas spp. in our microbiological laboratory were established as recommended by the Clinical and Laboratory Standards Institute [24].
Clinical Assessment
Age, gender, comorbidities, signs and symptoms, infection site, results of bacteriological tests, predisposing factors, laboratory findings at the time of admission, interval between contact and admission, interval between diagnosis and first surgery, length of stay, and clinical outcomes were reviewed for each patient.
Differences in mortality, patient characteristics, clinical presentation, underlying chronic diseases, infection site, first operative procedure, laboratory data and hospital course were compared between the A. hydrophila group and the K. pneumoniae group.
Statistical Analysis
Statistical analyses were performed using SPSS version 12.0 statistical software (SPSS, Chicago, Ill., USA). Student's t test was used for continuous variables and Fisher's exact test was used for categorical variables to examine significant relationships between risk factors and outcomes in the two groups. p < 0.05 was considered statistically significant.
Results
Of the 18 patients, 15 were men and 3 were women, and the mean age was 63.6 ± 14.9 years (range 40–90). The most common complaints of patients with necrotizing fasciitis were hypotension and pain and swelling of the involved limbs with edematous, patchy, erythematous, and hemorrhagic bullous skin lesions at the time of admission to the emergency room or at the time of consultation in the hospital ward.
Culture findings confirmed that the cause of monomicrobial infection was A. hydrophila in 11 patients and K. pneumoniae in 8 patients. Eight patients died (5 in the A. hydrophila group and 3 in the K. pneumoniae group), resulting in an all-cause in-hospital mortality rate of 44.4%. A. hydrophila specimens were isolated from wounds in 8 cases, and from blood and wounds in 3 cases. K. pneumoniae was isolated from wounds in 5 cases and from blood and wounds in 2 cases. Broad-spectrum antibiotics were initially administered to these patients with necrotizing fasciitis in the emergency room. These antibiotics were continued after surgery and changed to antibiotics specifically targeting cultured bacteria a few days later.
Characteristics of the Patients in the A. hydrophila Group
Of the 11 patients in the A. hydrophila group, 9 were men and 2 were women, with a mean age of 65.9 ± 11.4 years (range 47–85). One patient reported having handled fish, 1 had acquired abrasion wounds while working, 1 had had contact with dirty water in a drain, and 1 had fallen into a ditch during a motor vehicle accident. Two patients had cutting wounds and 1 had been injured while working with bamboo on a farm. Four patients were farmers and did not recall any injuries. Five patients died (a mean of 6.20 ± 5.76 days after admission), resulting in an all-cause in-hospital mortality rate of 45.5% (tables 1, 2).
Table 1.
Characteristics of patients with monomicrobial necrotizing fasciitis caused by A. hydrophila
| Patient No. | Age, years | Gender | Chronic underlying diseases | Site | Interval A, days | Contact mechanism | Interval B, h | Operations (first, final) | Interval C, days | Result | Duration of hospitalization, days | Systolic blood pressure ≤90 mm Hg in the ER | Intensive care unit stay | Body temperature >38.5°C in the ER |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | M | HCC, LC | left leg | 2 | abrasion | 18 | Fas | 0 | death | 16 | Y | Y | Y |
| 2 | 68 | F | HB, steroid use | both legs | 2 | bamboo | 2 | Fas | 0 | death | 2 | Y | Y | N |
| 3 | 55 | M | LC, gout | left thigh | 1 | unclear | 2 | Fas | 0 | death | 2 | Y | Y | N |
| 4 | 47 | M | LC, HCC, DM | right leg | 1 | unclear | 5 | Fas | 4 | death | 5 | N | Y | N |
| 5 | 58 | M | Alcoholism | right leg | 2 | unclear | 10 | Fas, AK | 4 | death | 6 | Y | Y | N |
| 6 | 64 | M | Gout | left leg | 1 | cutting wound | 10 | Fas, STSG | 20 | discharge | 63 | N | N | N |
| 7 | 77 | M | Hypertension | right foot | 2 | drain | 5 | Fas | 0 | discharge | 12 | N | N | N |
| 8 | 85 | M | DM, CRI, steroid use | right leg | 1 | unclear | 2 | Fas, AK | 11 | discharge | 90 | Y | Y | N |
| 9 | 73 | M | DM, HB | left forearm | 2 | cutting wound | 2 | Fas, STSG | 28 | discharge | 37 | N | Y | Y |
| 10 | 62 | F | DM | left forearm | 1 | fish | 2 | Fas, Flap | 20 | discharge | 14 | N | Y | N |
| 11 | 58 | M | ESRD | right leg | 2 | fell into ditch | 7 | Fas, AK | 10 | discharge | 24 | N | Y | N |
| Mean | 65.9 | 1.55 | 5.91 | 8.81 | 24.6 | |||||||||
M = Male; F = female; HCC = hepatic cell carcinoma; LC = liver cirrhosis; HB = hepatitis B; CRI = chronic renal insufficiency; DM = diabetes mellitus; ESRD = end-stage renal disease; Interval A = time from contact to presentation in the emergency room; Interval B = time from the first consultation to the first operation; Interval C = time from the first operation to the final operation; Fas = fasciotomy; AK = above-the-knee amputation; STSG = split-thickness skin graft; Y = yes; N = no; ER = emergency room.
Table 2.
Laboratory data of patients with monomicrobial necrotizing fasciitis caused by A. hydrophila
| Patient No. | Result | White blood cell count, n/mm3 | Banded forms, % | Segmented forms, % | Lymphocyte forms, % | Platelet count, n/mm3 | Albumin, g/dl | Creatinine, μmol/l | Alanine aminotransferase, U/l | C-reactive protein, mg/l | Positive culture |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | death | 16,500 | 16 | 78 | 2 | 151,000 | 2.2 | 1.61 | 30 | 43.5 | B and W |
| 2 | death | 5,600 | 15 | 67 | 6 | 150,000 | 1.7 | 2.08 | 45 | 93.4 | W |
| 3 | death | 3,100 | 27 | 57 | 8 | 57,000 | 2.9 | 2.12 | 23 | 65.77 | B and W |
| 4 | death | 5,100 | 34 | 45 | 8 | 64,000 | 1.6 | 1.4 | 121 | 30 | W |
| 5 | death | 2,600 | 9 | 61 | 6 | 90,000 | 1.7 | 2.8 | 189 | 100 | W |
| 6 | discharge | 11,200 | 4 | 88 | 4 | 158,000 | 3 | 1.7 | 27 | 26.2 | B and W |
| 7 | discharge | 18,800 | 2 | 81.5 | 7.5 | 131,000 | 2.5 | 1.14 | 25 | 115 | W |
| 8 | discharge | 7,200 | 4 | 71 | 18 | 140,000 | 2.1 | 2.33 | 35 | 27.5 | W |
| 9 | discharge | 12,100 | 9 | 85 | 3 | 170,000 | 2.4 | 1.49 | 20 | 140 | W |
| 10 | discharge | 13,500 | 1 | 80 | 13 | 79,000 | 2.5 | 0.88 | 72 | 49.5 | W |
| 11 | discharge | 17,000 | 12.5 | 71 | 9.5 | 150,000 | 1.5 | 5.7 | 19 | 89 | W |
| Mean | 10,245 | 12.1 | 71.3 | 7.73 | 121,818 | 2.19 | 2.11 | 55.1 | 70.9 | ||
W = Wound; B = blood.
The estimated period from exposure or injury to presentation at the emergency room was 1–2 days prior to admission. The mean interval from treatment in the emergency room to the first operation was 5.91 ± 5.1 h.
Two patients had upper-limb skin lesions and 9 had lower-limb skin lesions. All of the patients initially underwent fasciotomy and debridement. Three patients underwent above-the-knee amputation after a few days due to progressive skin involvement following fasciotomy. Two patients received skin grafts, 1 patient underwent flap reconstruction, 1 patient underwent debridement, and 1 received only wound care after the initial fasciotomy (fig. 1). Three patients did not undergo any surgery following fasciotomy; these patients died. Five (53.3%) patients were hypotensive with a systolic blood pressure ≤90 mm Hg. The mean hospital stay of the patients was 24.6 ± 28.3 days (range 2–90).
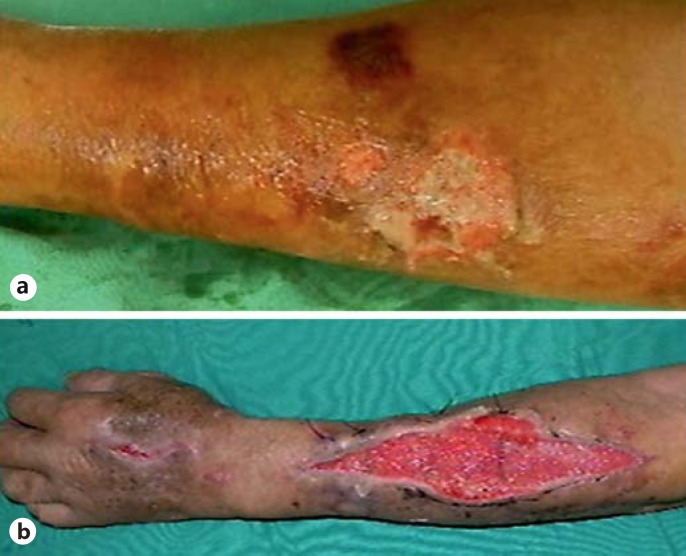
Fig. 1.
A 73-year-old male with a history of hepatitis B and diabetes mellitus had left forearm and hand pain on the second day after a cutting injury. a Preoperative photographs of the left hand revealed skin erosion, vesicles, and subcutaneous bleeding. b After an emergency fasciotomy, a wound culture confirmed the presence of A. hydrophila. He received a skin graft on the thirtieth day after fasciotomy.
All of the A. hydrophila isolates were susceptible to ampicillin, amikacin, ceftazidime, ceftriaxone, cefuroxime, ciprofloxacin, gentamicin, imipenem, piperacillin, and tetracycline. Broad-spectrum antibiotic therapy with third-generation cephalosporin plus vancomycin or teicoplanin was initially administered to 9 patients, and antibiotic therapy with oxacillin plus gentamicin was administered to 2 patients (cases 7 and 9).
Characteristics of the Patients in the K. pneumoniae Group
Of the 7 patients in the K. pneumoniae group, 6 were men and 1 was a woman, with a mean age of 59.9 ± 19.6 years (range 40–90). Three patients had acquired abrasion wounds while working, and 1 had previous chronic ulcers. One patient had had contact with seawater, and 2 did not recall any injuries. Three patients died a mean of 27.7 ± 27.4 days after admission, and the all-cause in-hospital mortality rate was 42.8% (tables 3, 4).
Table 3.
Characteristics of patients with monomicrobial necrotizing fasciitis caused by K. pneumoniae
| Patient No. | Age, years | Gender | Chronic underlying diseases | Site | Interval A, days | Contact mechanism | Interval B, h | Operations (first, final) | Interval C, days | Result | Duration of hospitalization, days | Systolic blood pressure ≤90 mm Hg in the ER | Intensive care unit stay | Body temperature >38.5°C in the ER |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 84 | M | DM, MI | right forearm | 7 | unknown | 5 | Fas | 0 | death | 8 | Y | Y | N |
| 2 | 90 | F | DM, gout, steroid use | left leg | 2 | abrasion | 3 | AK, debridement | 12 | death | 16 | Y | Y | N |
| 3 | 58 | M | DM, steroid use | left leg | 4 | abrasion | 7 | Fas, debridement | 14 | death | 59 | Y | Y | N |
| 4 | 49 | M | DM, LC, HC | right leg | 7 | seawater | 12 | Fas, STSG | 30 | discharge | 44 | N | Y | N |
| 5 | 55 | M | DM, HC, gout | right arm | 6 | abrasion | 6 | Fas, STSG | 13 | discharge | 26 | N | Y | N |
| 6 | 43 | M | DM | left leg | 7 | chronic ulcer | 5 | Fas, debridement | 7 | discharge | 10 | N | Y | N |
| 7 | 40 | M | DM, LC | right forearm | 3 | unknown | 14 | Fas, STSG | 17 | discharge | 21 | N | N | N |
| Mean | 59.9 | 5.14 | 7.43 | 13.3 | 26.3 | |||||||||
M = Male; F = female; DM = diabetes mellitus; MI = myocardial infarction; LC = liver cirrhosis; HC = hepatitis C; Interval A = time from contact to presentation to the emergency room; Interval B = time from the first consultation to the first operation; Interval C = time from the first operation to the final operation; AK = above-the-knee amputation; STSG = split-thickness skin graft; Y = yes; N = no; Fas = fasciotomy; ER = emergency room.
Table 4.
Laboratory data of patients with monomicrobial necrotizing fasciitis caused by K. pneumoniae
| Patient No. | Result | White blood cell count, n/mm3 | Banded forms, % | Segmented forms, % | Lymphocyte forms, % | Platelet count, n/mm3 | Albumin, g/dl | Creatinine, μmol/l | Alanine aminotransferase, U/l | C-reactive protein, mg/l | Positive culture |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | death | 15,100 | 18 | 65.5 | 13.5 | 124,000 | 1.5 | 1.86 | 68 | 70 | W |
| 2 | death | 36,600 | 4 | 90 | 25 | 197,000 | 1.7 | 1.16 | 15 | 93 | W |
| 3 | death | 3,000 | 9 | 27.5 | 8 | 54,000 | 2 | 1.3 | 33 | 156 | B and W |
| 4 | discharge | 31,300 | 10.5 | 80 | 5 | 44,000 | 2.6 | 1.73 | 64 | 128 | B and W |
| 5 | discharge | 15,400 | 0 | 87 | 9 | 144,000 | 3 | 1.25 | 92 | 10 | W |
| 6 | discharge | 18,200 | 0 | 86 | 11 | 235,000 | 1.8 | 0.88 | 24 | 139 | W |
| 7 | discharge | 13,500 | 2 | 95 | 1 | 125,000 | 2 | 0.8 | 28 | 178 | W |
| Mean | 19,014 | 6.2 | 75.86 | 10.36 | 131,857.14 | 2.08 | 1.28 | 46.3 | 110.6 | ||
W = Wound; B = blood.
The interval from injury to presentation at the emergency room ranged from 2 to 7 days (mean 5.14 ± 2.12). The mean interval between treatment in the emergency room and the first operation was 7.43 ± 4.04 h.
Three patients had upper-limb skin lesions and 4 had lower-limb skin lesions. Six patients initially underwent fasciotomy with debridement and 1 patient underwent an immediate above-the-knee amputation due to progressive uncontrolled initial sepsis (fig. 2). Three patients received skin grafts, and 3 underwent debridement with direct closure. No Klebsiella patient was febrile. Three (14.5%) patients had a systolic blood pressure ≤90 mm Hg at presentation to the emergency room; these patients died. The mean duration of hospital stay was 26.3 ± 18.8 days (range 8–59).
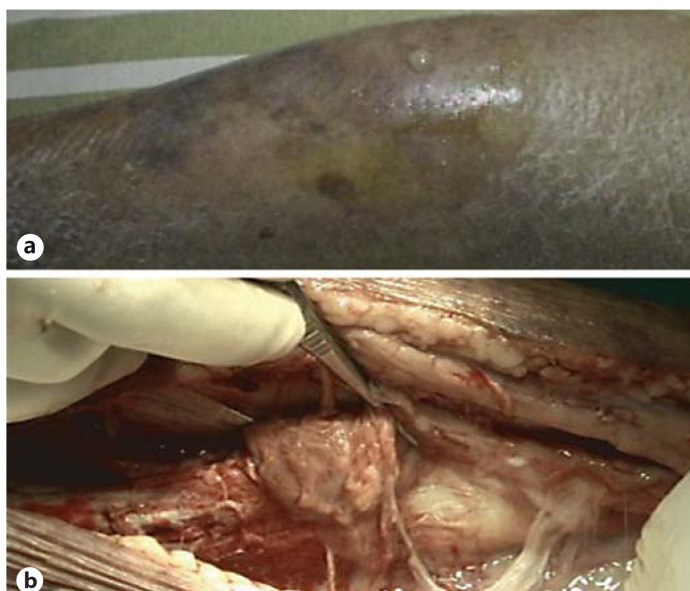
Fig. 2.
A 90-year-old female with a history of diabetes mellitus and chronic renal insufficiency had left lower leg pain for 2 days. a The left lower leg revealed patchy purpura and edema in the emergency room. b After fasciotomy, the lower leg showed yellowish pus accumulated in the fascia and muscular layer. Above-the-knee amputation was performed immediately. The cultured specimen confirmed K. pneumoniae; however, this patient died on the sixteenth day after admission owing to progressive septic shock and multiple organ failure.
All K. pneumoniae isolates were susceptible to amikacin, ceftazidime, ceftriaxone, cefuroxime, ciprofloxacin, aztreonam, gentamicin, and imipenem. Broad-spectrum antibiotics were administered initially in the emergency room to patients with K. pneumoniae infection; ceftriaxone alone was given to 3, cefazolin plus gentamicin to 2, and ceftriaxone plus vancomycin to 2 patients.
Comparison of the A. hydrophila and K. pneumoniae Groups
Age, sex, wound location, degree of hypotension, fever, nature of the first surgery, laboratory data and hospital course, and interval between diagnosis and the first surgery did not differ significantly (p > 0.05) between the two groups. However, the patients with Aeromonas infection had a significantly shorter interval between contact and admission (1.55 ± 0.52 vs. 5.14 ± 2.12 days, p < 0.001) and significantly lower total white blood cell counts (10,245 ± 5,828 vs. 19,014 ± 11,370 cells/mm3, p < 0.045) than patients with K. pneumoniae infection in the emergency room (tables 5, 6).
Table 5.
Comparison between the A. hydrophila group and the K. pneumoniae group for characteristics at the first consultation and treatment
| Variable | A. hydrophila group | K. pneumoniae group | p value |
|---|---|---|---|
| Patients, n | 11 | 7 | |
| Age, years | 65.9 ± 11.4 | 59.9 ± 19.6 | 0.42 |
| Sex, n | |||
| Male | 9 | 6 | 0.47 |
| Female | 2 | 1 | |
| Mortality rate, % | 45.5 | 42.8 | 0.65 |
| Death | 5 | 3 | |
| Survival | 6 | 4 | |
| Timing from contact to presentation to the ER, days | 1.55 ± 0.52 | 5.14 ± 2.12 | <0.001a |
| Death | 1.6 ± 0.55 | 4.33 ± 2.52 | 0.049a |
| Survival | 1.5 ± 0.55 | 5.75 ± 1.89 | 0.0007a |
| Timing from the first consultation to the first operation, h | 5.91 ± 5.1 | 7.43 ± 4.04 | 0.52 |
| Death | 7.43 ± 6.77 | 5 ± 2 | 0.58 |
| Survival | 4.67 ± 3.33 | 9.25 ± 4.43 | 0.097 |
| Underlying chronic disease, n | |||
| Hepatic dysfunction and DM | 2 (1) | 3 (0) | |
| Hepatic dysfunction with or without other conditions | 4 (4) | 0 (0) | 0.048b |
| Diabetes mellitus with or without other conditions | 2 (0) | 4 (3) | 0.01c |
| End-stage renal disease | 1 (0) | 0 (0) | |
| Hypertension | 1 (0) | 0 (0) | |
| Gout | 1 (0) | 0 (0) | |
| Wound location, n | |||
| Upper extremity | 2 (0) | 3 (1) | |
| Lower extremity | 9 (5) | 4 (2) | |
| Hospital stay, days | 24.6 ± 28.3 | 26.3 ± 18.8 | 0.89 |
| Death | 6.20 ± 5.76 | 27.7 ± 27.4 | 0.13 |
| Survival | 40 ± 30.8 | 25.2 ± 14.2 | 0.4 |
Values are presented as means ± SD unless otherwise stated. Values in parentheses represent the number of deaths.
Mean p < 0.05 and the difference was significant.
Mean p < 0.05 and the difference was significant (association with mortality for hepatic dysfunction with or without other conditions, e.g. diabetes, gout, or steroid use).
Mean p < 0.05 and the difference was significant (association with diabetes mellitus with or without other conditions).
Table 6.
Comparison between the A. hydrophila group and the K. pneumoniae group for laboratory data at the first consultation in the emergency room or ward
| White blood cell count, n/mm3 | Banded forms, % | Segmented forms, % | Lymphocyte forms, % | Platelet count, n/mm3 | Albumin, g/dl | Creatinine, μmol/l | Alanine aminotransferase, U/l | C-reactive protein, mg/l | |
|---|---|---|---|---|---|---|---|---|---|
| All patients (n = 18) | |||||||||
| A. hydrophila group (n = 11) | 10,245 ± 5,828 | 12.1 ± 10.5 | 71.3 ± 13.1 | 7.73 ± 4.6 | 121,818 ± 41,080 | 2.19 ± 0.52 | 2.11 ± 1.31 | 55.1 ± 53.8 | 70.9 ± 39 |
| K. pneumoniae group (n = 7) | 19,014 ± 11,370 | 6.21 ± 6.64 | 75.9 ± 23.3 | 10.4 ± 7.62 | 131,857 ± 69,420 | 2.09 ± 0.53 | 1.28 ± 0.4 | 46.3 ± 28.5 | 110.6 ± 57.4 |
| p value | 0.045a | 0.2 | 0.6 | 0.37 | 0.7 | 0.68 | 0.13 | 0.7 | 0.098 |
Mean p < 0.05 and the difference was significant.
Hepatic dysfunction with or without other conditions (diabetes, gout, or steroid use) was associated with mortality in patients with A. hydrophila infection (p = 0.048), while diabetes mellitus was associated with patients with mortality in K. pneumoniae infection (p = 0.01).
Discussion
In the present study, the interval between exposure and admission to the emergency room for patients with A. hydrophila infection was significantly shorter than that for patients with K. pneumoniae infection. The initial clinical course of A. hydrophila infection was characterized by more rapidly progressive disease than that of the K. pneumoniae infection. A. hydrophila infection often occurred in patients with liver cirrhosis, hepatitis, and hepatic malignancies, while K. pneumoniae was often associated with diabetes mellitus.
The more rapid progression of A. hydrophila compared to K. pneumoniae could be due to the fact that A. hydrophila can produce many virulence factors, including hemolysin, cytotoxin, aerolysin, enterotoxin, endotoxin, protease, adhesion, and lipases [12,14,25,26]. These factors are associated with extensive muscular necrosis and cause damage to the liver, kidneys, and pulmonary system, resulting in septic shock and multiple organ failure [25,26]. However, the virulence and pathogenicity of K. pneumoniae have been linked to its polysaccharide capsule envelope, and K1 and K2 are the most virulent serotypes [17,18,19,20]. These capsular serotypes and hypermucoviscosity phenotypes are highly resistant to serum killing and phagocytosis and have been implicated in the development of disseminated infection [18,27]. Necrotizing fasciitis caused by K. pneumoniae may be a consequence of transient bacteremia, or of gut bacterial translocation, followed by bacterial seeding at the extremities [17,18,27]. Equally important, Ko et al. [28] reported that A. hydrophila caused more rapid and intense local accumulation of inflammatory cells than K. pneumoniae and induced a more robust proinflammatory cytokine response when inoculated intramuscularly in mice [28].
The finding that patients with Aeromonas infection had a higher amount of banded leukocyte forms and significantly lower total white blood-cell counts than patients with K. pneumoniae infection in the emergency room in this study confirms the results of the animal study of Ko et al. [28]. They reported that the response of suppurative inflammation and aggregates of neutrophils might occur faster in mice with A. hydrophila infection than in those with K. pneumoniae infection [28]. We speculated that the response of suppurative inflammation might occur later with K. pneumoniae infection than that with A. hydrophila infection, and that the host can produce much more white blood cells to resist K. pneumoniae infection.
Finally, the finding of previous studies [10,12,14] that monomicrobial A. hydrophila infections such as necrotizing fasciitis and bacteremia have commonly been reported to be associated with liver cirrhosis and malignancy and impaired phagocytic activity of the reticuloendothelial system [10,12,14] confirms previous reports that cases of K. pneumoniae necrotizing fasciitis have shown a significant association with diabetes mellitus, present in 62.5–100% of such patients [17,18,27]. In this study, we also observed that hepatic dysfunction was associated with mortality in patients with A. hydrophila infection and that diabetes mellitus was associated with mortality in patients with K. pneumoniae infection. Although patients with K. pneumoniae necrotizing fasciitis have been reported to have a higher incidence of concomitant distant abscess, we did not find any distant abscesses in our cases [15,16,17,18,19,20].
In our previous studies, the clinical course of necrotizing fasciitis caused by Gram-negative microorganisms was more rapid and fulminant than that of Gram-positive infection, and the patients with diabetes mellitus alone had significant associations with Staphylococcus aureus necrotizing fasciitis [4,29]. The mortality rate of necrotizing fasciitis caused by S. aureus in diabetic patients was 18.7% (6/32) [29]. The 7 cases of K. pneumoniae necrotizing fasciitis were associated with diabetes mellitus, resulting in a mortality rate of 42.8% in this study. The clinical signs and symptoms of necrotizing fasciitis caused by K. pneumoniae and S. aureus in diabetic patients are characteristically indistinguishable at the time of presentation, and it takes 3–4 days to obtain the results of microbiological analysis and antimicrobial sensitivity of the specimens. Due to the high mortality rate and fulminant clinical course, K. pneumoniae infection as well as S. aureus infection should be initially suspected in diabetic patients with necrotizing fasciitis.
In this study, there were several limitations. First, we did not detect the capsular serotype K1/K2. Genotype K1 strains are significantly virulent and predominant in the East; however, we need to identify the roles of these genotypes in necrotizing fasciitis. The second limitation was that we could not reduce the mortality associated with monomicrobial A. hydrophila and K. pneumoniae necrotizing fasciitis, and we did not identify the causes of death even though we performed early diagnosis and surgical intervention.
Conclusion
Monomicrobial necrotizing fasciitis caused by A. hydrophila and K. pneumoniae had similar clinical courses and high mortality rates. The initial clinical course of A. hydrophila infection was characterized by more rapidly progressive disease than that of K. pneumoniae infection. Patients with hepatic dysfunction and necrotizing fasciitis should be suspected of having A. hydrophila infection, and diabetic patients with necrotizing fasciitis should be suspected of having K. pneumoniae infection initially if they have no history of contact with seawater or raw seafood.
References
- 1.Bellapianta JM, Ljungquist K, Tobin E, et al. Necrotizing fasciitis. J Am Acad Orthop Surg. 2009;17:174–182. doi: 10.5435/00124635-200903000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Morgan MS. Diagnosis and management of necrotizing fasciitis: a multiparametric approach. J Hosp Infect. 2010;75:249–257. doi: 10.1016/j.jhin.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Angoules AG, Kontakis G, Drakoulakis E, et al. Necrotizing fasciitis of upper and lower limb: a systematic review. Injury. 2007;38S:18–25. doi: 10.1016/j.injury.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Tsai YH, Huang KC, Shen SH, et al. Microbiology and surgical indicators of necrotizing fasciitis in a tertiary hospital of Southwest Taiwan. Int J Infect Dis. 2012;16:e159–e165. doi: 10.1016/j.ijid.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Anaya DA, Bulger EM, Kwon YS, et al. Predicting death in necrotizing soft tissue infections: a clinical score. Surg Infect. 2009;10:517–522. doi: 10.1089/sur.2008.112. [DOI] [PubMed] [Google Scholar]
- 6.Bair MJ, Chi H, Wang WS, et al. Necrotizing fasciitis in Southeast Taiwan: clinical features, microbiology, and prognosis. Intern J Infect Dis. 2009;13:255–260. doi: 10.1016/j.ijid.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Oncul O, Erenoglu C, Top C, et al. Necrotizing fasciitis: a life-threating clinical disorder in uncontrolled type 2 diabetic patients. Diabetes Res Clin Pract. 2008;80:218–223. doi: 10.1016/j.diabres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Tsai YH, Hsu RW, Huang TJ, et al. Necrotizing soft tissue infections and sepsis caused by Vibrio vulnificus compared with those caused by Aeromonas species. J Bone Joint Surg Am. 2007;89:631–636. doi: 10.2106/JBJS.F.00580. [DOI] [PubMed] [Google Scholar]
- 9.Tsai YH, Huang KC, Huang TJ, et al. Fatal necrotizing fasciitis caused by Aeromonas sobria in two diabetic patients. Clin Orthop Relat Res. 2009;467:846–849. doi: 10.1007/s11999-008-0504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CJ, Chen PL, Tang HJ, et al. Incidence of Aeromonas bacteremia in Southern Taiwan: Vibrio and Salmonella bacteremia as comparators. J Microbiol Immunol Infect. 2014;47:145–148. doi: 10.1016/j.jmii.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Ko WC, Chuang YC. Aeromonas bacteremia: review of 59 episodes. Clin Infect Dis. 1995;20:1298–1304. doi: 10.1093/clinids/20.5.1298. [DOI] [PubMed] [Google Scholar]
- 12.Ko WC, Lee HC, Chuang YC, et al. Clinical features and therapeutic implications of 104 episodes of monomicrobial Aeromonas bacteraemia. J Infect. 2000;40:267–273. doi: 10.1053/jinf.2000.0654. [DOI] [PubMed] [Google Scholar]
- 13.Furusu A, Yoshizuka N, Abe K, et al. Aeromona hydrophila necrotizing fasciitis and gas gangrene in a diabetic patient on hemodialysis. Nephrol Dial Transplant. 1997;12:1730–1734. doi: 10.1093/ndt/12.8.1730. [DOI] [PubMed] [Google Scholar]
- 14.Chao CM, Lai CC, Tang HJ, et al. Skin and soft-tissue infections caused by Aeromonas species. Eur J Clin Microbiol Infect Dis. 2013;32:543–547. doi: 10.1007/s10096-012-1771-y. [DOI] [PubMed] [Google Scholar]
- 15.Dalal A, Ahluwalia M, Urban C. Klebsiella pneumoniae necrotizing fasciitis associated with lung abscess. Infect Dis Clin Pract. 2009;17:48–51. [Google Scholar]
- 16.Kohler JE, Hutchens MP, Sadow PM, et al. Klebsiella pneumoniae necrotizing fasciitis and septic arthritis: an appearance in the Western hemisphere. Surg Infect (Larchmt) 2007;8:227–232. doi: 10.1089/sur.2006.007. [DOI] [PubMed] [Google Scholar]
- 17.Cheng NC, Yu YC, Tai HC, et al. Recent trend of necrotizing fasciitis in Taiwan: focus on monomicrobial Klebsiella pneumoniae necrotizing fasciitis. Clin Infect Dis. 2012;55:930–939. doi: 10.1093/cid/cis565. [DOI] [PubMed] [Google Scholar]
- 18.Lee SS. Klebsiella pneumoniae is an emerging major pathogen in necrotizing fasciitis. Clin Infect Dis. 2012;55:940–942. doi: 10.1093/cid/cis571. [DOI] [PubMed] [Google Scholar]
- 19.Ho PL, Tang WM, Yuen KY. Klebsiella pneumoniae necrotizing fasciitis associated with diabetes and liver cirrhosis. Clin Infect Dis. 2000;30:989–990. doi: 10.1086/313791. [DOI] [PubMed] [Google Scholar]
- 20.Mita N, Narahara H, Okawa M, et al. Necrotizing fasciitis following psoas muscle abscess caused by hypermucoviscous Klebsiella pneumoniae. J Infect Chemother. 2012;18:565–568. doi: 10.1007/s10156-011-0338-7. [DOI] [PubMed] [Google Scholar]
- 21.Mazita A, Abdullah A, Primuharsa SH. Cervical necrotizing fasciitis due to Klebsiella. Med J Malaysia. 2005;60:657–659. [PubMed] [Google Scholar]
- 22.Tsai YH, Hsu RW, Hung KC, et al. Systemic Vibrio infection presenting as necrotizing fasciitis and sepsis: a series of thirteen cases. J Bone Joint Surg Am. 2004;86:2497–2502. doi: 10.2106/00004623-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Tsai YH, Huang TJ, Hsu RW, et al. Necrotizing soft-tissue infections and primary sepsis caused by Vibrio vulnificus and Vibrio cholerae non-O1. J Trauma. 2009;66:899–905. doi: 10.1097/TA.0b013e31816a9ed3. [DOI] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: Nineteenth Informational Supplement–M100-S19. Wayne: CLSI; 2009. [Google Scholar]
- 25.Brenden RA, Huizinga HW. Pathophysiology of experimental Aeromonas hydrophila infection in mice. J Med Microbiol. 1986;21:311–317. doi: 10.1099/00222615-21-4-311. [DOI] [PubMed] [Google Scholar]
- 26.Wu CJ, Wu JJ, Yan JJ, et al. Clinical significance and distribution of putative virulence markers of 116 consecutive clinical Aeromonas isolates in Southern Taiwan. J Infect. 2007;54:151–158. doi: 10.1016/j.jinf.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Chang CM, Lee HC, Lee NY, et al. Community-acquired Klebsiella pneumoniae complicated skin and soft-tissue infections of extremities: emphasis on cirrhotic patients and gas formation. Infect. 2008;36:328–334. doi: 10.1007/s15010-008-7272-3. [DOI] [PubMed] [Google Scholar]
- 28.Ko WC, Chiang SR, Yan JJ, et al. Comparative pathogenicity of bacteraemic isolates of Aeromonas hydrophila and Klebsiella pneumoniae. Clin Microbiol Infect. 2005;11:553–558. doi: 10.1111/j.1469-0691.2005.01181.x. [DOI] [PubMed] [Google Scholar]
- 29.Tsai YH, Hsu RW, Hung KC, et al. The comparison of necrotizing fasciitis and sepsis caused by Vibrio vulnificus and Staphylococcus aureus. J Bone Joint Surg Am. 2011;93:274–284. doi: 10.2106/JBJS.I.01679. [DOI] [PubMed] [Google Scholar]