Abstract
Objective
This study was designed to identify the effect of rivaroxaban, a direct factor Xa inhibitor, on trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats.
Materials and Methods
Twenty-four female Wistar rats were divided into 4 groups of 6 each. Group 1 received TNBS + rivaroxaban, group 2 received TNBS + methylprednisolone, group 3 received TNBS and group 4 received a saline enema. Colitis was induced in the rats by the intracolonic administration of TNBS. Rivaroxaban and methylprednisolone were given by oral gavage daily for 7 days. The rats were killed 7 days after the induction of colitis.
Results
Rivaroxaban and methylprednisolone significantly reduced gross damage and histopathological scores. Rivaroxaban was more effective than methylprednisolone in terms of microscopic mucosal healing. Rivaroxaban attenuated the accumulation of malonyldialdehyde (MDA) and transforming growth-factor β1 (TGF-β1) and the activites of myeloperoxidase (MPO), matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1. Methylprednisolone reduced only the activity of MPO and the accumulation of MDA and TGF-β1. Superoxide dismutase activity showed a restoration to normal levels after rivaroxaban and methylprednisolone administration.
Conclusions
Rivaroxaban showed a therapeutic effect in the TNBS model of experimental colitis, and it seemed to be at least as effective as methylprednisolone. This effect may be brought about by the inhibition of oxidative stress and metalloproteinase activity associated with tissue injury and remodeling.
Key Words: Blood coagulation, Factor Xa, Inflammatory bowel disease, Proteinase-activated receptor 2, Rivaroxaban
Introduction
Inflammatory bowel disease (IBD) is a group of inflammatory conditions of the colon and small intestine. Crohn's disease and ulcerative colitis (UC) are the principal types of IBD [1]. The contribution of genetic predisposition and immune dysregulation to IBD pathogenesis has been well established [1]. On the other hand, clinical experience and bench research have clearly demonstrated an intimate link between inflammation and coagulation in IBD [2]. Various inflammatory mediators and the signaling pathways that they activate induce a hypercoagulable state and initiate clotting, by reducing the activity of natural anticoagulant pathways and impairing the fibrinolytic system. There is also emerging evidence that cells and molecules classically implicated in the physiological process of coagulation exert an influence on the inflammatory response [3]. In other words, the interaction between inflammation and coagulation seems to be bidirectional.
The identification of the proteinase-activated receptors (PARs), a novel family of transmembrane receptors, was an important step in the understanding of the pathogenesis of several tissue fibrosis and remodeling disorders in which activation of the coagulation cascade was observed. When stimulated, PARs couple to various G proteins and activate signal transduction pathways, resulting in the rapid transcription of genes that are involved in inflammation. To date, four PARs (PAR-1-4) have been identified, with distinct N-terminal cleavage sites and tethered ligand pharmacology. PAR-2 is expressed throughout the gastrointestinal tract on several cell types involved in immune responses and inflammation, the regulation of endothelial-leukocyte interactions and the modulation of the secretion of inflammatory mediators [4]. In an experimental model, activation of PAR-2 in the mouse colon induced inflammation and disruption of the integrity of the intestinal barrier [5].
Factor Xa, besides possessing properties for blood coagulation, induces intracellular signaling via the proteolytic cleavage of PAR-1 and PAR-2 [6]. Interestingly, factor Xa triggers signaling pathways involved in the regulation of cell growth and extracellular matrix deposition: it stimulates the proliferation of fibroblasts and smooth-muscle cells and induces the expression of interleukin 6, interleukin 8, monocyte chemotactic protein and transforming growth-factor β (TGF-β1) [7,8,9]. The aim of this study was to assess the ability of rivaroxaban, a direct factor Xa inhibitor, to reduce the severity of colitis in an animal model, and to evaluate its possible mechanism of action.
Materials and Methods
Animals
Twenty-four female Wistar rats, weighing 200–250 g, were kept at a constant temperature (22°C) and humidity with 12-hour dark/light cycles and allowed standard laboratory animal chow and water ad libitum throughout the experimental period.
Induction of Colitis and Experimental Groups
Trinitrobenzene sulfonic acid (TNBS), used previously in several studies to induce experimental colitis [10,11], was also used in this study to induce colitis in rats, based on the procedure described by Morris et al. [10]. Briefly, rats that had fasted for 24 h were anesthetized with ketamine hydrochloride and then had an 8-french polyethylene catheter inserted rectally up to the splenic flexure (8 cm from the anus). Next, 30 mg of TNBS (Sigma, France), dissolved in a volume of 0.15 ml of ethanol 50%, was administered through the catheter. The TNBS was retained in the colon for 1 min, after which the fluid was withdrawn. Control animals received only vehicle (0.9% saline).
The rats were randomly divided into 4 groups with 6 in each. Group 1 received TNBS + rivaroxaban (n = 6), group 2 received TNBS + methylprednisolone, group 3 received TNBS and group 4 received a saline enema. Rivaroxaban tablets (10 mg) were dissolved in 20 ml of distilled water and the solution was administered via oral gavage at a dose of 3 mg/kg. Methylprednisolone tablets (8 mg) were dissolved in 20 ml of distilled water and the solution was administered via oral gavage at a dose of 2 mg/kg. These 2 drugs were started in the relevant groups after the induction of colitis and were continued once daily until the end of the study on day 7.
The rats were killed 7 days after the induction of colitis, and the distal 8 cm of the colon was excised and then opened by longitudinal incision. Tissue samples were prepared for histopathological examination and the remaining mucosa was immediately snap-frozen in liquid nitrogen and stored at −80°C for the determination of the activities of superoxide dismutase (SOD), myeloperoxidase (MPO), matrix metalloproteinase-3 (MMP-3), tissue inhibitor of metalloproteinases-1 (TIMP-1) and the levels of malonyldialdehyde (MDA) and TGF-β1.
The study protocol was approved by the Gazi University Ethics Committee.
Assessment of Colitis
Morphological examination was performed by an experienced pathologist (O.E.) unaware of the experimental details. The macroscopic appearance of the colonic mucosa was scored on a scale adapted from Morris et al. [10]: 0 = no macroscopic change, 1 = mucosal erythema alone, 2 = mild mucosal edema, slight bleeding or small erosions, 3 = moderate edema, bleeding ulcers or erosions and 4 = severe ulceration/erosions, edema and tissue necrosis. For the microscopic examination, tissue samples were fixed in phosphate-buffered formaldehyde and embedded in paraffin, and routine 5-µm sections were prepared. Tissues were routinely stained with hematoxylin and eosin and evaluated with light microscopy. The microscopic appearance of the colonic mucosa was scored on a scale adapted from Ackerman et al. [12]: depth of necrosis: 0 = none, 1 = mucosal, 2 = mucosal and submucosal, 3 = mucosal, submucosal and muscularis propria and 4 = full thickness; extent of necrosis: 0 = none, 1 = a small area, 2 = a moderate area, 3 = a large area and 4 = extensive; inflammation: 0 = none, 1 = minimal, 2 = mild, 3 = moderate and 4 = severe; extent of inflammation: 0 = none, 1 = mucosal, 2 = mucosal and submucosal, 3 = mucosal, submucosal and muscularis propria and 4 = the full thickness.
The scores for each category examined were calculated for each specimen in the different groups. These were then added to obtain the total score, which was then divided by the number of rats' colons examined in each group to obtain the average histological score of induced colitis for the group.
Assessment of Tissue Biomarkers
The sample tissues were homogenized (50 g/l) in 50 mmol/l of ice-cold potassium phosphate buffer (pH 6.0) containing 0.5% of hexadecyltrimethylammonium bromide. The homogenate was frozen and thawed 3 times, then centrifuged at 15,000 rpm for 15 min at 4°C. The MPO activity in the supernatant was measured with an assay kit (Eastbiopharm, China) according to the provider's instructions and using the O-dianisidine method described by Xia et al. [13]. The MDA level and SOD activities in the supernatant were measured likewise. TGF-β1 levels and TIMP-1 and MMP-3 activites (Eastbiopharm) were assessed with the ELISA technique according to its provider's instructions.
Statistical Analysis
All statistical analyses were performed with SPSS version 11.5 for Windows (Chicago, Ill., USA). Normality was assessed using the Shapiro-Wilks test. Accordingly, data were expressed as a median (min-max). A comparison between the groups was performed using the Kruskal-Wallis nonparametric test or one-way ANOVA. The correlation of tissue biomarkers with each other and with the histological scores was assessed using Spearman rank correlation. A two-sided p value ≤0.05 was considered statistically significant.
Results
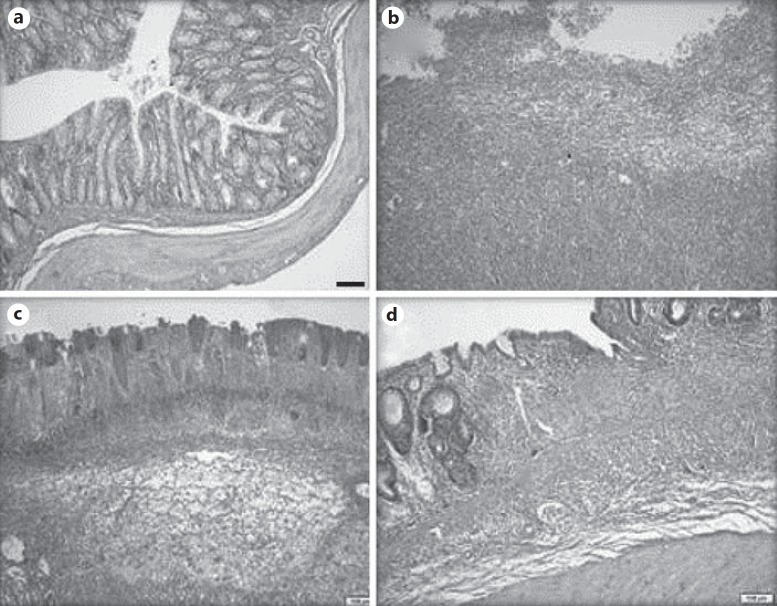
There was no mortality of animals with colitis (groups 1–3). The TNBS-treated animals (groups 1–3) exhibited a severe macroscopic inflammation of the colon 7 days after rectal administration, assessed from the colonic damage score. Treatment with rivaroxaban (group 1) and methylprednisolone (group 2) similarly and significantly decreased the severity of macroscopic damage (table 1). The microscopic features of the colon from the sham-treated rats (group 4) were typical of a normal structure. The inflamed colon of the rats with TNBS-induced colitis (groups 1–3) showed evidence of mucosal edema, crypt distortion, thickening of the colon wall, necrosis and a high level of leukocyte and polymorphonuclear infiltration (fig. 1). Treatment with rivaroxaban and methylprednisolone significantly decreased the severity of microscopic damage and rivaroxaban was more effective than methylprednisolone in terms of microscopicmucosal healing (table 1; fig. 1, 2).
Table 1.
Effect of treatment with rivaroxaban on macroscopic and microscopic pathological scores for the colonic tissue from rats with TNBS-induced colitis
| Group 1 TNBS + R | Group 2 TNBS + MP | Group 3 TNBS | Group 4 controls | |
|---|---|---|---|---|
| Macroscopic scores | 2 (1–3)a | 2 (2–3)b | 3.5 (2–4)d | 0 (0–0) |
| Microscopic scores | 8 (4–8)a, c | 9 (8–9)b | 13 (10–16)d | 0 (0–0) |
Scores are expressed as a median (min–max). MP = Methylprednisolone; R = rivaroxaban.
p < 0.05 compared with group 3.
p < 0.05 compared with group 3.
p < 0.05 compared with group 2.
p < 0.05 compared with group 4 for all parameters.
Fig. 1.
Sections of colonic samples taken at 7 days from rats receiving saline (a), TNBS (b), TNBS + methylprednisolone (c) and TNBS + rivaroxaban (d). a Colonic segment with normal morphology. b Severe and full thickness inflammation, extensive and full thickness necrosis and a large superficial ulceration. c Moderate inflammation involving mucosa and submucosa and moderate necrosis. d Moderate inflammation involving mucosa and submucosa and crypt distortion as an indicator of chronic epithelial cell damage. HE. ×25.
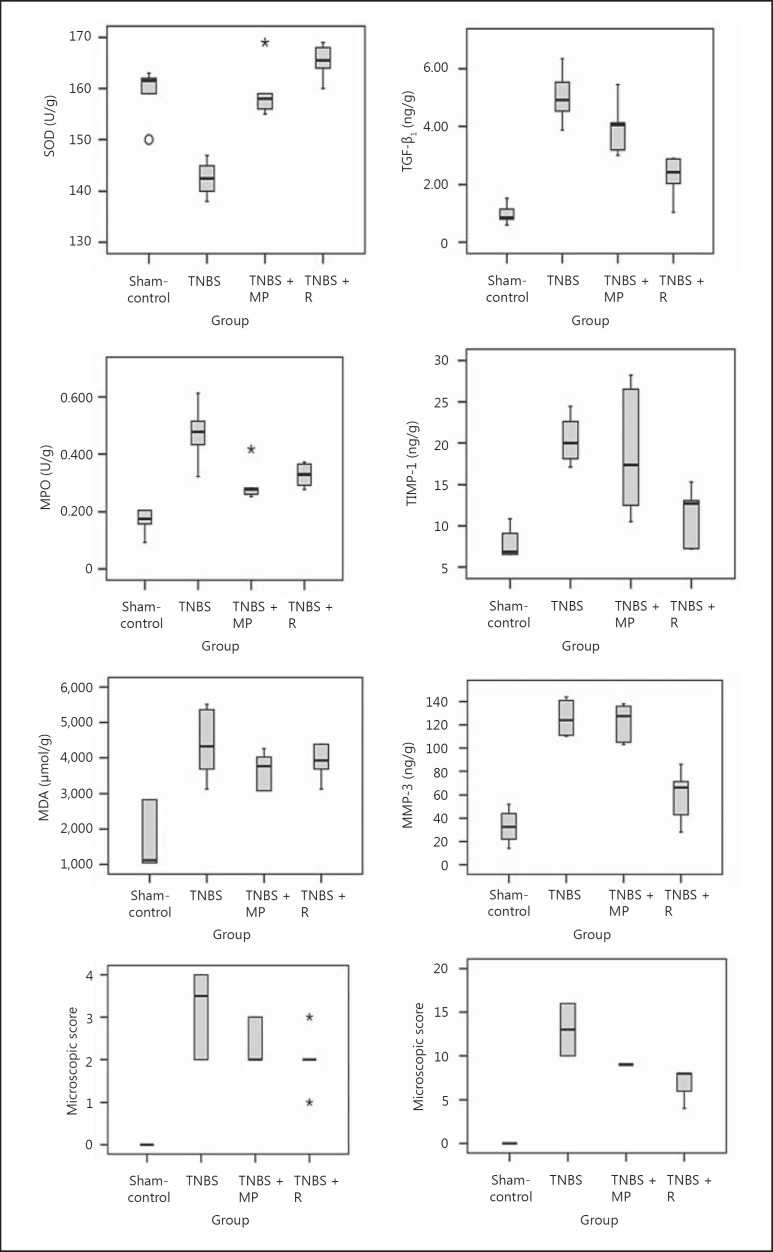
Fig. 2.
Effect of treatment with rivaroxaban on the macroscopic and microscopic pathological scores for colonic tissue and various tissue biomarkers from rats with TNBS-induced colitis. The p values represented by asterisks are expressed in detail in table 2.
MPO activity, which is directly related to the number and activity of infiltrating myeloid cells, was significantly increased 7 days after TNBS instillation. Administration of rivaroxaban and methylprednisolone reduced the accumulation of MPO in the colons of animals receiving TNBS (table 2; fig. 2). These results were consistent with the histological findings.
Table 2.
Effect of treatment with rivaroxaban on various tissue biomarkers from rats with TNBS-induced colitis
| Group 1 TNBS + R | Group 2 TNBS + MP | Group 3 TNBS | Group 4 controls | |
|---|---|---|---|---|
| MPO, U/g | 0.33 (0.25–0.47)a | 0.29 (0.25–0.32)a,b | 0.47 (0.31–0.61)d | 0.17 (0.1–0.21) |
| MDA, μmol/g | 3.9 (3.1–4.4)a | 3.6 (3.1–4.3)a,b | 4.3 (3.1–5.5)d | 1.6 (1–2.8) |
| TGF-β1, ng/g | 2.3 (1–2.9)a, c | 4 (3–5.5)a | 5 (3.9–6.4)d | 1 (0.6–1.5) |
| MMP-3, ng/g | 67 (28–86)a,c | 128 (103–138) | 124 (110–144)d | 33 (15–53) |
| TIMP-1, ng/g | 11.3 (7.2–15.3)a,c | 18.7 (10.5–28.2) | 20.4 (17.1–24.4)d | 7.8 (6.5–10.9) |
| SOD, U/g | 165 (160–169)a,c | 158 (155–168)a | 142 (137–147)d | 159 (150–162) |
MP = Methylprednisolone; R = rivaroxaban.
p < 0.05 compared with group 3.
p < 0.05 compared with group 1.
p < 0.05 compared with group 2.
p < 0.05 compared with group 4 for all parameters.
Compared with the controls, the MDA, TGF-β1, MMP-3 and TIMP-1 levels in the colonic tissues of rats with TBNS-induced colitis were significantly increased (table 2; fig. 2). Rivaroxaban attenuated the accumulation of MDA and TGF-β1 and the activities of MMP-3 and TIMP-1, but methylprednisolone could only reduce the accumulation of MDA and TGF-β1 (table 1; fig. 2). Rivaroxaban was more effective than methylprednisolone at decreasing the accumulation of TGF-β1 in the rat colonic tissues. Compared to the controls, SOD activity was significantly decreased in the colonic tissue of rats exposed to TNBS, with a restoration to normal levels after methylprednisolone. In the rivaroxaban group, SOD activity was even higher than in the control group and in the methylprednisolone-treated rats (table 2; fig. 2).
Histopatological scores showed a significant positive correlation with the measurements of the MPO, MMP-3, TIMP-1 activities and the MDA and TGF-β1 levels. The microscopic scores had a significant negative correlation with SOD activity (table 3).
Table 3.
Correlation of histopathological scores with the biomarkers in the colonic tissue of rats exposed to TNBS
| Biomarker | Correlation coefficient | p value |
|---|---|---|
| MPO | –0.62 | <0.001 |
| MDA | 0.42 | <0.05 |
| TGF-β1 | 0.80 | <0.001 |
| MMP-3 | 0.87 | <0.001 |
| TIMP-1 | 0.67 | <0.001 |
| SOD | 0.62 | <0.001 |
Discussion
In our model of experimental colitis, treatment with rivaroxaban attenuated damage to the colon, as verified by our macroscopic and histological findings. Rivaroxaban was found to be better than methylprednisolone in terms of histological improvement.
The influence of hemostasis on inflammation is supported by numerous reports that describe how the different components of coagulation-anticoagulation pathways can regulate inflammation by exerting an influence on the endothelial cells, platelets and/or leukocytes [2]. Considering the cross-talk between coagulation and inflammation, it seems reasonable to hypothesize that anticoagulants or coagulation-related drugs can affect inflammation and so could be considered for the treatment of IBD.
The influence of anticoagulant strategies on the severity of gut inflammation has been examined previously in animal models of IBD. For example, the thrombin-directed drugs, heparin and argatroban, have significantly reduced macroscopic and histological damage, mucosal MPO activity and mucosal leukotriene B4 levels (as a proinflammatory mediator) in rats with TNBS-induced colitis [14,15]. Similar protection against gut inflammation has been demonstrated following intracolonic administration of the low-molecular-weight heparin, CB-01-05, in rats with dinitrobenzene-induced colitis [16]. Data from animal studies and reports suggesting an improvement in UC symptoms while on anticoagulant therapy led to randomized controlled studies of heparin and low-molecular-weight heparin in active UC. Although the outcome of most clinical trials does not support the use of subcutaneous heparin in active UC, Celasco et al. [16] showed a statistically significant benefit with low-molecular-weight heparin versus placebo in achieving clinical remission and improvement, endoscopic improvement and reduced rectal bleeding, when administered in the form of extended-release colon tablets [17,18,19].
There is strong evidence that factor Xa is more than a passive intermediate in the coagulation cascade, and that it might orchestrate fundamental processes during tissue remodeling and fibrosis due to its pleiotropic cellular effects [20]. Factor Xa is well-known to elicit an inflammatory response that occurs mainly via PAR-2 activation [7,8,9]. PAR-2 has been identified as a potentially crucial receptor for the pathogenesis of IBD and for sustaining fibrosis in Crohn's disease. PAR-2 inhibition seems amenable to pharmacological intervention [21]. The inhibiton of factor Xa, as a PAR-2 agonist, seems to be an attractive therapeutic strategy in IBD, as it has specifically been shown to be beneficial in animal models of chronic inflammatory disorders and fibrotic disease [21,22].
Among the novel, direct, oral factor Xa inhibitors developed in recent years, rivaroxaban was the first to gain regulatory approval for clinical use (in 2008) [23]. It effectively prolongs prothrombin time, inhibits thrombin generation and reduces both collagen- and tissue factor-induced endogenous thrombin potential [23]. To shed light on the mechanisms responsible for the possible beneficial effects of rivaroxaban, we undertook the measurement of several tissue biomarkers. MPO is the most abundant proinflammatory enzyme stored in the azurophilic granules of neutrophilic granulocytes, accounting for approximately 5% of their dry mass, and it is widely used to quantify neutrophil accumulation in tissues [24]. Lipid peroxidation, a type of oxidative degeneration of polyunsaturated fatty acids, is linked with altered membrane structure and decreased activity of antioxidant enzymes. Increased lipid peroxidation by-products in the colorectal biopsy specimens of patients with UC have been reported [25]. We measured MDA, an end-product of lipid peroxidation, as an indicator of this pathological process [26]. Animal models of IBD provide compelling evidence for the fundamental role of TGF-β1 in triggering and sustaining intestinal fibrogenesis [27]. MMPs (including MMP-3 and TIMP-1), that are involved in the remodeling and degradation of the ECM, were shown to be increased in inflamed colons of IBD patients compared with noninflamed tissue samples [28]. Lastly, SOD was included in our group of tissue biomarkers because it converts the superoxide anion into hydrogen peroxide, and is considered a primary defense against oxidative stress which has a role in mediating intestinal damage in IBD [29,30].
We observed a significant correlation between the histopathological scores and all of the tissue biomarkers mentioned above in our experimental model of rat colitis (table 3). The finding that rivaroxaban prevented an increase in MPO, MMP-3 and TIMP-1 activities and the accumulation of MDA and TGF-β1 supports the concept of an inhibition of inflammatory response, oxidative stress and tissue remodeling as rivaroxaban's contribution to the attenuation of macroscopic and microscpoic colonic damage. The restoration of SOD activity to the normal levels (even higher than normal) following treatment with rivaroxaban is also consistent with its antioxidant potential. Steroids did not have a similar effect to rivaroxaban on MMPs, so rivaroxaban may indeed be more beneficial with respect to the remodeling and fibrosis-related complications of IBD, e.g. stricture.
Conclusion
This study showed that treatment with rivaroxaban attenuates the outcome of TNBS-induced colitis, resulting in a significant histological improvement, reducing oxidative stress and cytokine production and impairing the inflammatory response. Rivaroxaban seems to be at least as effective as steroids. Pharmacological modification of rivaroxaban to obtain an enhanced efficacy in the targeting gastrointestinal lesions and to exert a weaker systemic antithrombotic effect may allow this drug to be used in the treatment of IBD in the future.
References
- 1.Danese S, Papa A, Saibeni S, et al. Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am J Gastroenterol. 2007;102:174–186. doi: 10.1111/j.1572-0241.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida H, Granger DN. Inflammatory bowel disease: a paradigm for the link between coagulation and inflammation. Inflamm Bowel Dis. 2009;15:1245–1255. doi: 10.1002/ibd.20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1:1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 4.Kong W, McConalogue K, Khitin LM, et al. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci USA. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cenac N, Coelho AM, Nguyen C, et al. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riewald M, Kravchenko VV, Petrovan RJ, et al. Gene induction by coagulation factor Xa is mediated by activation of protease-activated receptor 1. Blood. 2001;97:3109–3116. doi: 10.1182/blood.v97.10.3109. [DOI] [PubMed] [Google Scholar]
- 7.Borensztajn KS, Bijlsma MF, Groot AP, et al. Coagulation factor Xa drives tumour cells into apoptosis through BH3-only protein Bim up-regulation. Exp Cell Res. 2007;313:2622–2633. doi: 10.1016/j.yexcr.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Koo BH, Chung KH, Hwang KC, et al. Factor Xa induces mitogenesis of coronary artery smooth muscle cells via activation of PAR-2. FEBS Lett. 2002;523:85–89. doi: 10.1016/s0014-5793(02)02948-4. [DOI] [PubMed] [Google Scholar]
- 9.Rauch BH, Millette E, Kenagy RD, et al. Thrombin and factor Xa-induced DNA synthesis is mediated by transactivation of fibroblast growth factor receptor-1 in human vascular smooth muscle cells. Circ Res. 2004;94:340–345. doi: 10.1161/01.RES.0000111805.09592.D8. [DOI] [PubMed] [Google Scholar]
- 10.Morris GP, Beck PL, Herridge MS. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 11.Jragh DM, Khan I, Oriowo MA. Colonic inflammation increases the contribution of muscarinic M2 receptors to carbachol-induced contraction of the rat colon. Med Princ Pract. 2011;20:530–537. doi: 10.1159/000328419. [DOI] [PubMed] [Google Scholar]
- 12.Ackerman Z, Karmeli F, Cohen P, et al. Experimental colitis in rats with portal hypertension and liver disease. Inflamm Bowel Dis. 2003;9:18–24. doi: 10.1097/00054725-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y, Zweier JL. Measurement of myeloperoxidase in leukocyte containing tissues. Anal Biochem. 1997;245:93–96. doi: 10.1006/abio.1996.9940. [DOI] [PubMed] [Google Scholar]
- 14.Onomura M, Tsukada H, Fukuda K, et al. Effect of argatroban on trinitrobenzene sulfonic acid induced colitis. J Gastroenterol Hepatol. 2000;15:931–938. doi: 10.1046/j.1440-1746.2000.02279.x. [DOI] [PubMed] [Google Scholar]
- 15.Fries W, Pagiaro E, Canova E, et al. The effect of heparin on trinitrobenzene sulphonic acid induced colitis in the rat. Aliment Pharmacol Ther. 1998;12:229–236. doi: 10.1046/j.1365-2036.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- 16.Celasco G, Moro L, Bozzella R, et al. Efficacy of intracolonic administration of low-molecular-weight heparin CB-01-05, compared to other low-molecular-weight heparins and unfractionated heparin, in experimentally induced colitis in rat. Dig Dis Sci. 2008;53:3170–3175. doi: 10.1007/s10620-008-0299-6. [DOI] [PubMed] [Google Scholar]
- 17.Bloom S, Kiilerich S, Lassen MR, et al. Low molecular weight heparin (tinzaparin) vs. placebo in the treatment of mild to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2004;19:871–878. doi: 10.1111/j.1365-2036.2004.01926.x. [DOI] [PubMed] [Google Scholar]
- 18.de Bievre MA, Vrij AA, Schoon EJ, et al. Randomized, placebo-controlled trial of low molecular weight heparin in active ulcerative colitis. Inflamm Bowel Dis. 2007;13:753–758. doi: 10.1002/ibd.20085. [DOI] [PubMed] [Google Scholar]
- 19.Celasco G, Papa A, Jones R, et al. Clinical trial: oral colon-release parnaparin sodium tablets (CD-01-05 MMX) for active left-sided ulcerative colitis. Aliment Pharmacol Ther. 2010;31:375–386. doi: 10.1111/j.1365-2036.2009.04194.x. [DOI] [PubMed] [Google Scholar]
- 20.Borensztajn K, Spek CA. Blood coagulation factor Xa as an emerging drug target. Expert Opin Ther Targets. 2011;15:341–349. doi: 10.1517/14728222.2011.553608. [DOI] [PubMed] [Google Scholar]
- 21.Nomura K, Liu N, Nagai K, et al. Roles of coagulation pathway and factor Xa in rat mesangioproliferative glomerulonephritis. Lab Invest. 2007;87:150–160. doi: 10.1038/labinvest.3700502. [DOI] [PubMed] [Google Scholar]
- 22.Shinagawa K, Martin JA, Ploplis VA, et al. Coagulation factor Xa modulates airway remodeling in a murine model of asthma. Am J Respir Crit Care Med. 2007;175:136–143. doi: 10.1164/rccm.200608-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubitza D, Perzborn E, Berkowitz SD. The discovery of rivaroxaban: translating preclinical assessments into clinical practice. Front Pharmacol. 2013;25:145. doi: 10.3389/fphar.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultz J, Kaminker K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- 25.D'Odorico A, Bortolan S, Cardin R, et al. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1289–1294. doi: 10.1080/003655201317097146. [DOI] [PubMed] [Google Scholar]
- 26.Kim KS, Paik IY, Woo JH, et al. The effect of training type on oxidative DNA damage and antioxidant capacity during three-dimensional space exercise. Med Princ Pract. 2010;19:133–141. doi: 10.1159/000273075. [DOI] [PubMed] [Google Scholar]
- 27.Vallance BA, Gunawan MI, Hewlett B, et al. TGF-beta1 gene transfer to the mouse colon leads to intestinal fibrosis. Am J Physiol Gastrointest Liver Physiol. 2005;289:116–128. doi: 10.1152/ajpgi.00051.2005. [DOI] [PubMed] [Google Scholar]
- 28.Von Lampe B, Barthel B, Coupland SE, et al. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63–73. doi: 10.1136/gut.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keshavarzian A, Banan A, Farhadi A, et al. Increases in free radicals and cytoskeletal protein oxidation and nitration in the colon of patients with inflammatory bowel disease. Gut. 2003;52:720–728. doi: 10.1136/gut.52.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]