Abstract
Objective
To investigate the prevalence of thyroid autoimmunity among children and adolescents with type 1 diabetes in Kuwait.
Subjects and Methods
In a mixed cross-sectional and longitudinal study, anti-thyroid peroxidase (anti-TPO) and anti-thyroglobulin (anti-TG) were measured in 232 subjects (118 males and 114 females) with type 1 diabetes.
Results
The mean age of the total study population was 10.9 ± 3.6 years (range 1–21), and the median diabetes duration was 3.9 years (range 0–16). At the initial screening, 57 out of 232 (24.6s%) patients had positive antibodies, and of the remaining 175 patients, who were antibody negative,131 (74.3s%) were followed up for 4–9 years. 23 out of these 131 (17.7s%) patients became antibody positive, with a cumulative prevalence of elevated antibodies of 34.5s%. Anti-TPO was present in 34 (14.7s%), anti-TG in 23 (9.9s%) and both antibodies in 23 (9.9s%) patients. Thyroid antibodies presented early within the first 5 years of the onset of diabetes (63.2 vs. 36.8s%, p < 0.05). The prevalence of elevated thyroid antibodies increased after the onset of puberty in both females and males (p < 0.0001). A total of 58.7s% of the patients with positive antibodies were females compared to 41s% males (p < 0.0001). The basal thyroid-stimulating hormone was higher in subjects with positive antibodies (5.1 ± 10.7 mIU/l) compared to those who were antibody negative (1.79 ± 0.87 mIU/l, p < 0.001). Furthermore, 30 out of 232 (12.9s%) patients developed thyroid dysfunction.
Conclusion
In this study, a high prevalence of thyroid autoimmune antibodies was found in patients either at the onset of type 1 diabetes or within the 4–9 years of follow-up.
Key Words: Thyroid autoimmune prevalence, Type 1 diabetes, Thyroid dysfunction, Anti-thyroid peroxidase, Anti-thyroglobulin
Introduction
Autoimmune thyroid diseases are the most common form of autoimmune disorders occurring in patients with type 1 diabetes [1] and the most common autoimmune endocrinopathy occurring in their families [2,3]. The frequency of thyroid autoimmune disease and the prevalence of thyroid autoantibodies (TAA) in patients with type 1 diabetes varies widely between 3 and 50s% [4,5] and is often related to age, gender or ethnic origin, whereas thyroid dysfunction has been reported in approximately 8s% of patients [6]. Hashimoto's thyroiditis (chronic autoimmune thyroiditis) and Graves' disease (thyromegaly and hyperthyroidism) are the major autoimmune thyroid diseases that occur with increased frequency in patients with type 1 diabetes.
TAA are specific markers for autoimmune thyroid diseases with expression typically higher in Hashimoto's thyroiditis than in Graves' disease [7]. They occur in 17–40s% of pediatric patients with type 1 diabetes [7,8] appearing early before any clinical or biochemical signs of thyroid dysfunction. However, studies have shown that 50s% of the subjects with positive antibodies may progress to develop autoimmune thyroid disease [4].
Antimicrosomal antibodies, whose specific antigen has been identified as thyroid peroxidase (anti-TPO), an enzyme that catalyzes the iodination of tyrosine residues on thyroglobulin, are more often seen in type 1 diabetes with a prevalence of 25–50s% of cases, and thyroglobulin antibodies (anti-TG) are present in 20–30s% of cases [1]. Only few longitudinal studies have assessed the risk of thyroid autoimmunity (Th-AA) and diabetes [4,5] and the optimal method or frequency of screening for Th-AA in patients with type 1 diabetes is still controversial [2,3,5].
Kuwait has a high and increasing incidence of type 1 diabetes compared to other Arab countries [9,10,11,12]. The age-standardized incidence in children aged 0–14 years in Kuwait between 1992 and 1999 was reported as 20.9/100,000 [10]. Studies on the prevalence of Th-AA in Arab children with type 1 diabetes are scarce [6,13,14], and longitudinal studies assessing the risk of Th-AA are lacking. Therefore, we undertook this study to (1) investigate the prevalence and natural history of Th-AA among Arab children and adolescents with type 1 diabetes in Kuwait and (2) determine the clinical significance of the detection of TAA as a marker for autoimmune thyroid disease in children with type 1 diabetes.
Subjects and Methods
This study was done in the Pediatric Diabetes Unit, established at the Amiri Hospital, Kuwait, in 1998. Between1992 and 1999, a total of 266 children and adolescents with type 1 diabetes were registered in the diabetes outpatient clinic using a computer-based program (MS access). The study population included 232 patients (114 males and 118 females) below the age of 22 years (range 1–21) [15] in whom thyroid antibodies and thyroid-stimulating hormone (TSH) were measured. All patients were diagnosed with type 1 diabetes according to standard criteria, which included polyuria, polydipsia and unexplained weight loss as well as a casual plasma glucose concentration of ≥11.1 mmol/l (200 mg/dl) [3]. All subjects were screened annually for thyroid disease by measuring the TAA: anti-TPO, anti-TG, free thyroxine (FT4) and sensitive thyroid-stimulating hormone (sTSH). The diagnosis of autoimmune thyroid disease was based on the clinical evaluation and detection of thyroid antibodies and abnormal thyroid function tests including sTSH and FT4.
Assays of anti-TPO and anti-TG were measured in the serum at the first clinic visit after the initiation of the study and repeated annually thereafter until a positive antibody test was found [2]. UniCAP™ (Pharmacia and Upjohn Company LLC, Kalamazoo, Mich., USA) anti-TG and anti-TPO are enzyme immunoassays for the qualitative and quantitative determination of anti-TG or anti-TPO in human serum or plasma, respectively. The cutoff values to enable clinical discrimination were: <60 IU/ml (normal), 60–100 IU/ml (equivocal) and >100 IU/ml (positive) for anti-TPO, and <280, 280–344 and >344 IU/ml for anti-TG. The negative control was <100 IU/ml. Thyroid function tests, sTSH and FT4 were also measured in all the patients at their first clinic visit. These tests were repeated at a 6-month interval if TAA were detected and annually during the 4–9 years of follow-up if they were absent. The sTSH assay was determined by a continuous random access chemiluminescent immunoassay system (Abbott IMX). The test is a third-generation TSH assay having a functional sensitivity in the range of 0.01–0.02 µIU/ml, usually expressed in mIU/l in accordance with the international system of units. The strategy is to test third-generation TSH as a first-line test and then to measure FT4, if abnormal. The normal values for sTSH and FT4 are 0.490–4.676 mIU/l and 9.14–23.81 pmol/l, respectively. Overt clinical hypothyroidism was defined as elevated TSH levels and triiodothyronine, thyroxine or FT4 levels under the lower limit of normal. Subclinical hypothyroidism was defined as an elevated TSH level with normal triiodothyronine, thyroxine or FT4 levels.
Statistical Analysis
The statistical analysis was carried out using descriptive statistics, including means and frequencies, and inferential statistics, that included using Student's t test and χ2 test. Student's t test was used to test the significance of the differences between the mean values of two continuous variables. χ2 analysis was performed to test the differences in proportions of categorical variables between ≥2 groups. In 2 × 2 tables, the Fisher exact test replaced the χ2 test if the assumptions underlying the χ2 test were violated. The level of p < 0.05 was considered as the cutoff value for significance. Data analysis was done using SAS software, version 9.2.
Results
The mean age of the study population was 10.9 ± 3.6 years (range 1–21), and the median diabetes duration was 3.9 years (range 0–16). Of the 232 pediatric subjects, 57 (24.6s%) had positive TAA (23 males and 34 females), while 175 (75.4s%) were antibody negative. The mean age of the subjects with type 1 diabetes who exhibited Th-AA was 11.75 ± 3.46 years (range 4–21) versus those without 10.63 ± 3.39 years (range 1–21), and the median duration of diabetes was 3 4.5 ± 3.73 and 3.79 ± 3.09 years in those with and without Th-AA, respectively. The clinical characteristics of the patients with positive and negative TAA are summarized in table 1.
Table 1.
Clinical characteristics of the cross-sectional group of patients with type 1 diabetes with positive and negative TAA
| Group with positive TAA (n = 57) | Group with negative TAA (n = 175) | p value | |
|---|---|---|---|
| Age, years | |||
| Means ± SD | 11.75 ± 3.46 | 10.63 ± 3.57 | n.s. |
| Ranges | 4 – 21 | 1 – 20 | |
| Duration, years | |||
| Means ± SD | 4.94 ± 3.62 | 4.30 ± 3.09 | n.s. |
| Ranges | 0.57 – 16.13 | 0.16 – 13.8 | |
| Males, n | 23 | 91 | n.s. |
| Females, n | 34 | 84 | |
| Puberty stage I, n | 17 | 77 | n.s. |
| Puberty stages II–V, n | 40 | 98 | |
| TSH, nmol/l | |||
| Means ± SD | 5.2 ± 10.6 | 1.79 ± 0.87 | <0.005 |
| Ranges | 0.5 – 59.6 | 0.47 – 4.94 |
n.s. = Not significant.
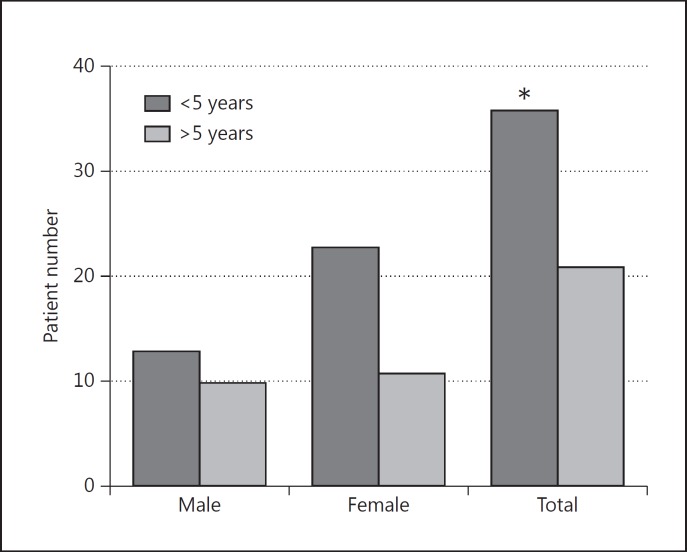
Of the 232 patients, at least 1 thyroid antibody was elevated in 57 (24.6s%) patients, 20s% (23/114) males and 29s% (34/118) females. Of 57 patients with elevated thyroid antibody, 36 (63s%) developed positive TAA within 5 years of the onset of diabetes and 21 (37s%) after a 5-year duration of diabetes. The difference was statistically significant (p < 0.05) (fig. 1).
Fig. 1.
Association of positive Th-AA with duration of diabetes among 23 males and 34 females with type 1 diabetes in the cross-sectional group. * p ≤ 0.05.
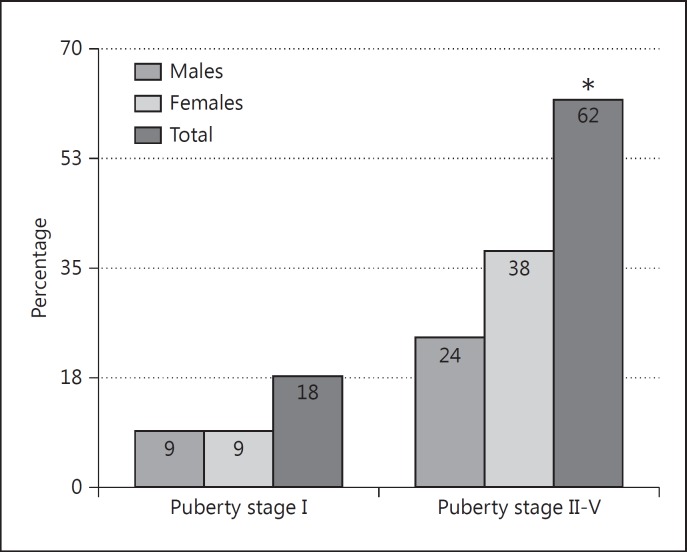
A subset of the study population (131 subjects) was prospectively followed for 4–9 years: 68 (51.9s%) and 63 (48.1s%) patients for 4 and 9 years: respectively, during which time 17.5s% of them became TAA positive. Females were significantly more likely to develop Th-AA (58.7s%, 47/80) than males (41s%, 33/80, p < 0.0001). The TAA appeared more often after the onset of puberty in both sexes 77.5s% (62/80) versus their appearance before puberty (22.5s%, 18/80, p < 0.0001) (fig. 2).
Fig. 2.
The association between thyroid antibodies and puberty stage (Tanner white-staging method) in both cross-sectional and longitudinal groups. * p ≥ 0.0001.
The cumulative prevalence rate for positive Th-AA from both cross-sectional and longitudinal studies was 34.5s% (80/232). Anti-TPO was present in 34 (14.7s%) patients, anti-TG in 23 (9.9s%) and both antibodies in 23 (9.9s%) patients, with a statistically significant p value of ≤0.05. Furthermore, 12.9s% (30/232) had laboratory evidence of thyroid dysfunction. Among these 30 patients, subclinical hypothyroidism was the most common diagnosis in 17 (7.3s%) patients, i.e. 56.7s%, followed by overt hypothyroidism that required replacement therapy with L-thyroxine in 10 (4.3s%) patients, i.e. 33.3s%, and hyperthyroidism in 3 (1.2s%) patients, i.e. 10s% (table 2).
Table 2.
Cumulative frequency of TAA in both cross-sectional and longitudinal groups of patients with positive Th-AA
| Males | Females | Total | |
|---|---|---|---|
| Anti-TPO | 16 (6.8) | 18 (7.78) | 34 (14.65)* |
| Anti-TG | 8 (3.4) | 15 (6.5) | 23 (9.9) |
| Both | 12 (5.2) | 14 (6) | 23 (9.9) |
| Total | 36 (15.5) | 47 (20.3) | 80 (34.5) |
Values represent n (s%).
p ≤ 0.05 (χ2 test).
Discussion
In this study, the prevalence of TAA observed in children with type 1 diabetes was high (34.5s%) compared to other studies [7,16,17]. However, it is similar to the prevalence found in Iranian children (39.6s%) [17]. Our results are considerably higher than those found in either Libyan [6], Jordanian [9], Saudi Arabian [13] and Sudanese children [14].
It is well known that genetic predisposition contributes to the risk of developing type 1 diabetes, and the HLA class II locus contributes to the shared risk for both type 1 diabetes and autoimmune thyroid disease. The major haplotype contributing to this association is DR3DQB1*0201 [6], which was significantly increased in Kuwaiti Arab children with type 1 diabetes [18] and may explain the high prevalence of Th-AA in the present study.
The frequency of thyroid antibodies in healthy Kuwaiti individuals in the general population was previously reported as 3.1s%, being slightly higher in females [19] and increasing with other autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus and primary Sjögren's syndrome. In contrast, a high frequency of thyroid dysfunction (55s%) and of Th-AA (52s%) was found in Kuwaiti adults with Down syndrome [20]. No studies examined the prevalence of Th-AA in healthy children in the general population in Kuwait.
The overall prevalence of Th-AA appeared to increase over time as reported by different authors [1,6]. However, in this study, 63s% of autoantibody-positive children developed Th-AA within 5 years of the onset of diabetes, a finding that emphasizes the importance of screening for thyroid antibodies early after the diagnosis of type 1 diabetes.
Our data showed a significant increase in positive autoantibodies in females, similar to other studies in the literature [8,12,17,21,22] where female sex was reported as an important risk factor for Th-AA. Furthermore, TAA appeared significantly more often after the onset of puberty in both sexes, 77.5 versus 22.5s% before puberty. It is well accepted that in children at genetic risk of type 1 diabetes, seroconversion to anti-TPO peaks around the time of puberty, at which time the thyroid gland undergoes remodeling [6].
Biochemical thyroid dysfunction may be present at the diagnosis of type 1 diabetes [4,23] or may be detected after several decades of diabetes [4,21,23]. A strong association exists between Th-AA and the risk of thyroid dysfunction in people with type 1 diabetes with a risk ratio of 49 in children (95s% CI 16–120) [24]. Furthermore, a prolonged period of autoimmunity makes the clinical diagnosis of thyroid autoimmune disease difficult, as it often occurs in a subclinical state. Measurements of thyroid function tests in clinically suspected cases or in those who have positive TAA would confirm the disease [17].
Previous studies have shown a clear association of type 1 diabetes and autoimmune thyroiditis, often requiring treatment with L-thyroxine. The prevalence of autoimmune thyroiditis in our study as shown by abnormal thyroid function tests was 12.9s% and compares well to other studies [8,14,25,26]. The presence of subclinical hypothyroidism, which varies between 3 and 11s% worldwide [6], was found to be the most common dysfunction in our study (7.3s%), followed by overt hypothyroidism (4.3s%) and hyperthyroidism (1.2s%). The latter finding is low compared to the prevalence reported in other studies (3–6s%) [24]].
Despite the striking evidence that Th-AA with subclinical hypothyroidism [23,26,27,28,29] is a frequent finding in children and adolescents with type 1 diabetes, there is still controversy concerning the necessity of therapeutic intervention in these patients [30]. Mild thyroid failure has been extensively evaluated as a cardiovascular risk factor in adults, and neurobehavioral changes, myocardial dysfunction and dyslipidemia have frequently been described in this population [26]. Children with diabetes and subclinical hypothyroidism had reduced growth rates, particularly in those with TSH values of >10 IU/l, and therapy, when started early, reduced the risk of hyperlipidemia and atherosclerotic heart disease [30]. Although our decision was only to treat patients with TSH levels of >10 IU/l, this strategy would entail a close follow-up, as previous studies have shown that progression to clinical hypothyroidism occurs in 3–18s% of patients per year [26,28,29,31]. It has been recommended to start treatment with L-thyroxine if TSH increases ≥4.5 mIU/l on two subsequent measurements and/or if a thyroid gland enlargement with diffuse parenchymal hypoechogenicity on ultrasound examination was present [4]. However, although thyroid imaging may be helpful, it is not a strict criterion for diagnosis.
Recently, the majority of studies have recommended annual screening for thyroid dysfunction and/or autoimmunity from diagnosis, while others recommend screening after the onset of puberty. However, the optimal methods and frequency of screening are yet to be established [24].
Conclusion
The cumulative incidence of Th-AA from this mixed cross-sectional and longitudinal study was high, and subclinical hypothyroidism was the most common disorder diagnosed. The appearance of Th-AA early after diagnosis strongly supports the recommendation of screening all patients at the onset of diabetes, as early detection has the potential to prevent significant morbidity related to unrecognized disease.
References
- 1.Witek PR, Witek J, Pańkowska E. Type 1 diabetes-associated autoimmune diseases: screening, diagnostic principles and management (in Polish) Med Wieku Rozwoj. 2012;16:23–34. [PubMed] [Google Scholar]
- 2.Hanas R, Donaghue KC, Klingensmith G, et al. ISPAD clinical practice consensus guidelines 2009 compendium. Introduction. Pediatr Diabetes. 2009;10(suppl 12):1–2. doi: 10.1111/j.1399-5448.2009.00577.x. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein J, Klingensmith G, Copeland K, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 4.Kordonouri O, Hartmann R, Deiss D, et al. Natural course of autoimmune thyroiditis in type 1 diabetes: association with gender, age, diabetes duration, and puberty. Arch Dis Child. 2005;90:411–414. doi: 10.1136/adc.2004.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2002;25((suppl 1)):s5–s20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 6.Ghawil M, Tonutti E, Abusrewil S, et al . Autoimmune thyroid disease in Libyan children and young adults with type 1 diabetes mellitus. Eur J Pediatr. 2011;170:983–987. doi: 10.1007/s00431-010-1386-1. [DOI] [PubMed] [Google Scholar]
- 7.Kordonouri O, Deiss D, Danne T, et al. Predictivity of thyroid autoantibodies for the development of thyroid disorders in children and adolescents with type 1 diabetes. Diabet Med J Br Diabet Assoc. 2002;19:518–521. doi: 10.1046/j.1464-5491.2002.00699.x. [DOI] [PubMed] [Google Scholar]
- 8.Menon PS, Vaidyanathan B, Kaur M. Autoimmune thyroid disease in Indian children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2001;14:279–286. doi: 10.1515/jpem.2001.14.3.279. [DOI] [PubMed] [Google Scholar]
- 9.Radaideh A, El-Khateeb M, Batieha AM, et al. Thyroid function and thyroid autoimmunity in patients with type 1 diabetes mellitus. Saudi Med J. 2003;24:352–355. [PubMed] [Google Scholar]
- 10.Shaltout AA, Qabazard MA, Abdella NA, et al. High incidence of childhood-onset IDDM in Kuwait. Kuwait Study Group of Diabetes in Childhood. Diabetes Care. 1995;18:923–927. doi: 10.2337/diacare.18.7.923. [DOI] [PubMed] [Google Scholar]
- 11.Shaltout AA, Moussa MA, Qabazard M, et al. Further evidence for the rising incidence of childhood type 1 diabetes in Kuwait. Diabet Med J Br Diabet Assoc. 2002;19:522–525. doi: 10.1046/j.1464-5491.2002.00703.x. [DOI] [PubMed] [Google Scholar]
- 12.Karvonen M, Viik-Kajander M, Moltchanova E, et al. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23:1516–1526. doi: 10.2337/diacare.23.10.1516. [DOI] [PubMed] [Google Scholar]
- 13.Abdullah MA, Salman H, Bahakim H, et al. Antithyroid and other organ-specific antibodies in Saudi Arab diabetic and normal children. Diabet Med J Br Diabet Assoc. 1990;7:50–52. doi: 10.1111/j.1464-5491.1990.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 14.Magzoub MM, Abdel-Hameed AA, Bottazzo GF. Prevalence of islet cell and thyrogastric autoantibodies in Sudanese patients with type 1 diabetes. Diabet Med J Br Diabet Assoc. 1994;11:188–192. doi: 10.1111/j.1464-5491.1994.tb02018.x. [DOI] [PubMed] [Google Scholar]
- 15.Berhman R, Kliegman R, Arvin A, editors. Nelson Textbook of Paediatrics. ed 15. Philadelphia: Saunders; 1996. [Google Scholar]
- 16.Sharifi F, Ghasemi L, Mousavinasab N. Thyroid function and anti-thyroid antibodies in Iranian patients with type 1 diabetes mellitus: influences of age and sex. Iran J Allergy Asthma Immunol. 2008;7:31–36. [PubMed] [Google Scholar]
- 17.Warren RE, Frier BM. Thyroid autoimmunity at onset of type 1 diabetes as a predictor of thyroid dysfunction: response to Gonzalez et al. Diabetes Care. 2007;30:e120. doi: 10.2337/dc07-1292. [DOI] [PubMed] [Google Scholar]
- 18.Haider MZ, Shaltout A, Alsaeid K, et al. Prevalence of human leukocyte antigen DQA1 and DQB1 alleles in Kuwaiti Arab children with type 1 diabetes mellitus. Clin Genet. 1999;56:450–456. doi: 10.1034/j.1399-0004.1999.560608.x. [DOI] [PubMed] [Google Scholar]
- 19.Al-Awadhi AM, Olusi S, Hasan EA, et al. Frequency of abnormal thyroid function tests in Kuwaiti Arabs with autoimmune diseases. Med Princ Pract. 2008;17:61–65. doi: 10.1159/000109592. [DOI] [PubMed] [Google Scholar]
- 20.Ali FE, Bayoumy HA, Mohammad ASR, et al. Thyroid function in Kuwaiti subjects with Down's syndrome. Med Princ Pract. 2002;11:206–209. doi: 10.1159/000065807. [DOI] [PubMed] [Google Scholar]
- 21.Severinski S, Banac S, Severinski NS, et al. Epidemiology and clinical characteristics of thyroid dysfunction in children and adolescents with type 1 diabetes. Coll Antropol. 2009;33:273–279. [PubMed] [Google Scholar]
- 22.Kakleas K, Paschali E, Kefalas N, et al. Factors for thyroid autoimmunity in children and adolescents with type 1 diabetes mellitus. Ups J Med Sci. 2009;114:214–220. doi: 10.3109/03009730903276381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umpierrez GE, Latif KA, Murphy MB, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care. 2003;26:1181–1185. doi: 10.2337/diacare.26.4.1181. [DOI] [PubMed] [Google Scholar]
- 24.Shun CB, Donaghue KC, Phelan H, et al. Thyroid autoimmunity in type 1 diabetes: systematic review and meta-analysis. Diabet Med J Br Diabet Assoc. 2014;31:126–135. doi: 10.1111/dme.12318. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani RM, Mantovani LM, Dias VMA. Thyroid autoimmunity in children and adolescents with type 1 diabetes mellitus: prevalence and risk factors. J Pediatr Endocrinol Metab. 2007;20:669–675. doi: 10.1515/jpem.2007.20.6.669. [DOI] [PubMed] [Google Scholar]
- 26.O'Grady MJ, Cody D. Subclinical hypothyroidism in childhood. Arch Dis Child. 2011;96:280–284. doi: 10.1136/adc.2009.181800. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin G, Volkening LK, Laffel LMB. Younger age at onset of type 1 diabetes in concordant sibling pairs is associated with increased risk for autoimmune thyroid disease. Diabetes Care. 2006;29:1397–1398. doi: 10.2337/dc06-0098. [DOI] [PubMed] [Google Scholar]
- 28.Barker JM. Clinical review: type 1 diabetes-associated autoimmunity: natural history, genetic associations, and screening. J Clin Endocrinol Metab. 2006;91:1210–1217. doi: 10.1210/jc.2005-1679. [DOI] [PubMed] [Google Scholar]
- 29.McDermott MT, Ridgway EC. Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab. 2001;86:4585–4590. doi: 10.1210/jcem.86.10.7959. [DOI] [PubMed] [Google Scholar]
- 30.Chase HP, Garg SK, Cockerham RS, et al. Thyroid hormone replacement and growth of children with subclinical hypothyroidism and diabetes. Diabet Med J Br Diabet Assoc. 1990;7:299–303. doi: 10.1111/j.1464-5491.1990.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 31.Kaplowitz PB. Subclinical hypothyroidism in children: normal variation or sign of a failing thyroid gland? Int J Pediatr Endocrinol. 2010;2010:281453. doi: 10.1155/2010/281453. [DOI] [PMC free article] [PubMed] [Google Scholar]