Abstract
Objective
We aimed to evaluate the contributions of acute one-stop cardiovascular magnetic resonance (CMR) imaging to the differential diagnosis of acute coronary syndrome (ACS) and unobstructed coronary arteries.
Subjects and Methods
In this study, 32 consecutive patients who presented with ACS and unobstructed coronary arteries on angiography were enrolled between January 2010 and December 2012. Acute one-stop CMR, including cine, angiography, black-blood, first-pass perfusion and late gadolinium enhancement (LGE) imaging, was performed with a pre-specified algorithm which was decided on by the doctors for all patients. The intimal flap in the aorta and the filling defect in the pulmonary artery were detected on MR angiography imaging. Left ventricular wall motion and ventricular thickness were analyzed in cine-mode sequences. The LGE images were reviewed for the presence, anatomical distribution and extent of contrast enhancement.
Results
The acute one-stop CMR study was completed in all the 32 patients without adverse events. The overall time duration was between 15 and 60 min. Of the 32 patients, a CMR diagnosis was made in 30 (93.8%). Aortic dissection was detected in 3 patients, pulmonary embolism in 2, hypertrophic cardiomyopathy in 2, acute myocardial infarction in 5, acute myocarditis in 16 and stress cardiomyopathy in 2. No confirmed diagnosis was established in the remaining 2 patients with normal CMR.
Conclusion
Acute one-stop CMR allowed for the identification of an aetiology in most of the patients in this study. It may prove to be of immense help in establishing a differential diagnosis in patients presenting with acute chest pain, elevated troponin I and normal coronary arteries.
Key Words: Acute coronary syndrome, Chest pain, Magnetic resonance imaging, Coronary artery disease
Introduction
Patients who arrive at hospital with prolonged chest pain, elevated levels of the biomarkers of myocardial damage and abnormal electrocardiography (ECG) are admitted with an initial diagnosis of acute coronary syndrome (ACS) [1]. It is necessary for high-risk patients to undergo primary percutaneous coronary angioplasty. Approximately 10% of these patients do not have significant coronary artery stenosis [1,2]; this represents a serious diagnostic challenge. Although the situation is sometimes one of acute myocardial infarction (AMI) without the angiographic features of coronary lesions, other non-ischaemic cardiovascular diseases such as pulmonary embolism (PE), aortic dissection (AD) or acute myocarditis can present a clinical picture similar to ACS [3,4]. Establishing a correct diagnosis has significant prognostic and therapeutic implications for such patients.
Cardiovascular magnetic resonance (CMR) is a non-invasive, comprehensive imaging technique that allows for the simultaneous assessment of cardiovascular anatomy, tissue characterization and functional analysis [2]. Gadolinium can be utilized for high-resolution pulmonary and aortic magnetic resonance angiography (MRA) to exclude PE or AD [5,6]. Cardiac dimensions, hypertrophy patterns and motion abnormalities can be easily visualized in cine-mode sequences [7,8]. In addition, inflammation, myocardial hypoperfusion and infarct-related necrosis are distinctly detectable using T2-oedema or late gadolinium enhancement (LGE) imaging techniques [9,10,11]. Finally, CMR is playing an increasingly important role in the initial diagnosis and management of various cardiovascular diseases [12].
In this study, we sought to implement acute one-stop CMR as a non-invasive imaging method for differential diagnosis in patients presenting with ACS and unobstructed coronary arteries.
Subjects and Methods
Of the 1,215 patients who were admitted to the Department of Cardiology with an initial diagnosis of ACS and who underwent primary coronary angiography (CAG) between January 2010 and December 2012, 118 presented with unobstructed coronary angiograms. Of these 118 eligible patients, 75 were admitted outside the CMR operational hours, 8 were contraindicative of CMR and 3 had a history of myocardial infarction or hypertrophic cardiomyopathy. Hence, 32 patients (age range 24-78 years) were enrolled in the study. They presented with an acute onset of chest pain (>30 min), elevated levels of cardiac troponin I (>0.1 ng/ml), ECG changes indicative of new ischaemia (new ST-T changes, the development of pathological Q waves or new left bundle branch block) and unobstructed coronary arteries (stenosis <50% of the diameter of the vessel) on angiography. Patients were excluded when they were admitted outside the CMR working hours (between 6 p.m. and 8 a.m. or on a Sunday) and when they had acute heart failure, critical arrhythmia, an unstable haemodynamic condition, a history of myocardial infarction or hypertrophic cardiomyopathy, claustrophobia and implanted pacemakers or other metal devices. All the enrolled patients underwent serial ECG, serial measurement of myocardial damage marker levels, chest X-ray, CAG and one-stop CMR. Primary CAG was performed no later than 2 h after admission. Acute CMR was conducted 2-4 h immediately after negative cardiac catheterization. The study was approved by the Institutional Ethics Committee and Review Board of the hospital.
Acute One-Stop CMR Study
All one-stop CMR procedures were performed using a Signa EXCITE 1.5-tesla clinical imaging system (GE Medical Systems, Milwaukee, Wis., USA) that provides the possibility of monitoring blood pressure, heart rate and blood oxygen saturation simultaneously. The pre-specified algorithm for stepwise evaluation was decided by the authors. The algorithm could be followed step-by-step or interrupted at each point to minimize the examination time for the patient.
First, due to the severe life-threatening risk of AD and PE, these were ruled out before the cardiac MR was conducted. Which examination was performed first depended on the symptoms of the patient. After scout and reference scans, 6 steady-state free-precession (SSFP) cine images with parallel imaging (slice thickness: 8 mm and sense factor: 2) were acquired through the thoracic aorta with subsequent MRA if AD was suspected. The non-breath-hold SSFP sequence scans, contrast-enhanced 3-dimensional (3D)-MR pulmonary angiography (half-scan data acquisition; rec voxel: 0.7/0.7/2 mm and sense factor: 2) and 3D gradient-echo (GRE) imaging were performed using double-dose gadolinium-DPTA if PE was suspected.
The one-stop CMR study ended when the diagnosis of AD or PE was confirmed; otherwise, cardiac MR was performed. After scout views to determine cardiac axis locations, the typical protocol involved 4 stages.
Cine Mode
The SSFP sequences were acquired in 3 cardiac conventional planes [left ventricular (LV) long-axis, short-axis and 4-chamber view] with these typical settings: breath-hold acquisition/each slice, a slice thickness of 7-10 mm with no gap, the shortest TR/TE typically 3.7/1.6 ms, a flip angle of 45°, a field of view (FOV) of 320-380 mm and a matrix of 224 × 192. In the short-axis view, 10-12 slices were acquired with complete coverage of the left ventricle from base to apex.
Double/Triple Inversion Recovery
Double/triple inversion recovery (IR) preparation was done in the dual-contrast fast-spin echo sequence. The double IR preparation consisted of a non-selective adiabatic inversion pulse followed immediately by a selective inversion pulse to suppress the blood signal. This is similar to T1-weighted imaging. The triple IR preparation consisted of the double IR pulse and an extra radiofrequency pulse to suppress the fat signal. This is similar to T2-weighted imaging. The dual contrast was performed through a split-echo train. The following imaging parameters were applied: a TE1 of 42 ms, a TE2 of 102 ms, echo spacing of 4.2 ms, a TI of 575 ms, a TR of 2 × R-R intervals, an FOV of 320-400 mm, a matrix of 256 × 256 and a slice thickness of 8-10 mm without a gap. Eight contiguous slices in a short-axis view were placed to cover the LV basal to apical planes by breath holding during each section.
First-Pass Perfusion Imaging
Short-axis, first-pass perfusion CMR was performed using a T1-weighted fast GRE sequence with saturation-recovery magnetization preparation (FGRET) with these typical settings: 8 contiguous slices in the short-axis view placed to cover the LV basal to apical planes and recorded continuously for each cardiac cycle, a slice thickness of 8-10 mm without an intersection gap, the shortest TR/TE typically 7.6/2.4 ms, a matrix of 128 × 128, an FOV of 270-360 mm. The total duration was approximately 1 min with partial breath holding during the initial myocardial enhancement. The acquisition was synchronized with the intravenous injection of 0.1 mmol/kg of gadolinium-DPTA at a rate of 3.5-4 ml/s, followed by a flush infusion at the same rate.
LGE Imaging
After perfusion imaging, an additional dose of 0.1 mmol/kg of gadolinium-DPTA was administered at a rate of 0.5 ml/s. With a 12-min delay, LGE MRI images were acquired with breath-holding for each stack, utilizing an IR-prepared, segmented GRE sequence. These settings were typically: a stack of 8 contiguous slices in the short-axis view with the orientations identical to perfusion imaging, a slice thickness of 8-10 mm without an intersection gap, a TR/TE/inversion time of 4.0/1.8/200-360 ms, an FOV of 320-360 mm, a matrix of 192 × 160 and a flip angle of 20°.
The cardiac MR images were assessed by 2 experienced radiologists (H.Z. and M.Z.). Ventricular volumes, LV ejection fraction (LVEF) and ventricular thickness were calculated. LV wall motion was measured according to the classification of the American Heart Association [13]. T2-weighted images were used to detect potential areas of high signal intensity consistent with myocardial oedema, which were measured by visual estimation without any quantification method. The presence of microvascular obstruction was visually ascertained on first-pass perfusion images. Finally, the LGE images were also visually reviewed for the presence, anatomical distribution and extent of subendocardial, subepicardial, mid-wall and transmural contrast enhancement. The final diagnosis was based on the delayed contrast-enhancement images. A diagnosis of AMI was made from images that showed areas of subendocardial and transmural contrast enhancement, while diagnoses of other myocardial diseases were made with subepicardial and intramyocardial contrast enhancement.
Results
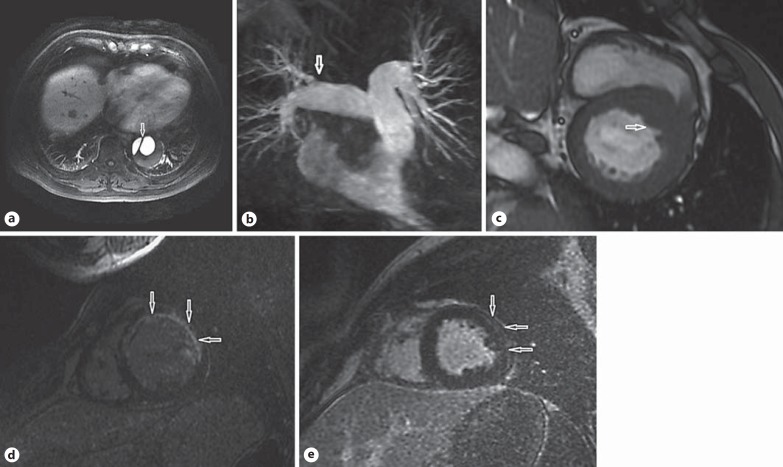
The baseline characteristics of the 32 patients who ultimately underwent acute CMR are presented in table 1. All of the patients completed the CMR imaging without adverse events. The mean duration time of one-stop CMR was 42.5 ± 18.2 min (range 15-60 min) and the findings are presented in table 2. Of the 32 patients, a CMR diagnosis was made in 30 (93.8%). The details of the differential diagnosis are summarized in table 3. Three patients were diagnosed with AD. The intimal flap that leads to a widened false lumen of the thoracic aorta and the broad entry as a communication between the two lumens was detected on ECG-triggered cine SSFP images taken in anatomical transversal orientation during the diastolic and systolic periods (fig. 1a). Normal flow in the narrow true lumen and extremely slow flow within the false lumen were observed on dynamic contrast-enhanced 3D-MRA of the aorta taken in coronal orientation. The filling defect or vascular truncation signs in the left and right pulmonary arterial branch were detected on SSFP imaging, gadolinium chelate-based contrast-enhanced breath-hold MR pulmonary angiography or recirculation-phase breath-hold 3D GRE imaging in 2 patients who had a diagnosis of PE (fig. 1b).
Table 1.
Baseline characteristics of the study population (n = 32)
| Age, years | 47.5 ± 18.2 |
| Men | 18 (56.3) |
| Cardiovascular risk factors | |
| Smoking | 20 (62.5) |
| Diabetes | 13 (40.6) |
| Hyperlipidaemia | 10 (31.3) |
| Obesity | 16 (50.0) |
| Hypertension | 15 (46.9) |
| Family history | 14 (43.8) |
| Serum parameters | |
| Cardiac troponin I admission, ng/ml | 8.3 ± 6.2 |
| C-reactive protein, mg/l | 42.5 ± 21.5 |
| D-dimer, mg/l | 1.8 ± 5.3 |
| ECG | |
| ST-segment elevation | 13 (40.6) |
| ST-segment depression | 7 (21.9) |
| T-wave abnormalities | 5 (15.6) |
| Patdological Q waves | 3 (9.4) |
| New left bundle branch block | 1 (3.1) |
| Normal | 3 (9.4) |
| Risk stratification | |
| GRACE score | 135.3 ± 38.6 |
Values are n (%) or mean ± SD.
Table 2.
Findings on cardiac MR (n = 27)
| Cine mode | |
| LVEF | 52.4 ± 9.2 |
| Septal or apical segment hypertrophy | 2 (7.4) |
| Wall motion abnormalities | 17 (63.0) |
| T2-weighted spin-echo sequences | |
| Oedema | 14 (51.9) |
| First-pass perfusion imaging | |
| Microvascular obstruction | 1 (3.7) |
| Presence of LGE | |
| Subendocardial | 4 (14.8) |
| Transmural | 2 (7.4) |
| Subepicardial | 12 (44.4) |
| Intramyocardial | 5 (18.5) |
| Normal cardiac MR | 2 (7.4) |
Values are n (%) or mean ± SD.
Table 3.
Final diagnosis from the CMR study (n = 32)
| AD | 3 (9.3) |
| PE | 2 (6.3) |
| Hypertrophic cardiomyopatdy | 2 (6.3) |
| AMI | 5 (15.5) |
| Acute myocarditis | 16 (50.0) |
| Stress cardiomyopathy | 2 (6.3) |
| No diagnosis | 2 (6.3) |
Values are n (%).
Fig. 1.
CMR images of the patients suspected of having ACS. a Contrast-enhanced T1-weighted images taken in an anatomical transversal orientation showing an intimal flap (arrow) in the thoracic aorta. b 3D-MR pulmonary angiography showing filling-defect signs (arrow) in the right upper lobe pulmonary arterial branch. c SSFP images in the short-axis view showing asymmetric increased thickness (arrow) at the base of the interventricular septum. d IR images in the short-axis view of the left ventricle showing transmural delayed contrast enhancement (arrows) in the extensive anterior wall. e IR images in the short-axis view of the left ventricle showing subepicardial delayed contrast enhancement (arrows) in the lateral wall.
Two patients were diagnosed with hypertrophic cardiomyopathy, presenting as asymmetric increased thickness (15-21 mm) at the base of the interventricular septum or apical segment, and a significant reduction in the LV cavity on the SSFP images (fig. 1c). The punctate or patchy LGE was seen in the hypertrophic hypokinetic segments, which helped to distinguish it from AMI.
A hyperintense signal compatible with oedema was detected on T2-weighted images in 14 patients. It was located in the septal or anterior segments in 4 patients and in the lateral and lower segments in the remaining 10. Areas of subendocardial contrast enhancement were detected on the delayed contrast-enhancement images in 3 patients, and areas of transmural contrast enhancement were detected in 2 patients, one with an anterior location and the other with an inferior location (fig. 1d). These 5 patients who presented subendocardial or transmural contrast enhancement were diagnosed with AMI; 1 of them displayed typical microvascular obstruction on the first-pass perfusion images, and 2 displayed a hyperintense signal compatible with myocardial oedema on the T2-weighted images.
Areas of subepicardial or intramyocardial contrast enhancement were mainly located in the LV lateral segments in 16 patients (fig. 1e). The contrast-enhancement pattern was multifocal and patchy in 12 patients, and 11 patients presented a hyperintense signal on T2-weighted images. These 16 patients were diagnosed as having acute myocarditis. Another CMR checkup 1 month later showed that the myocardial contrast signal had basically returned to normal in 6 patients.
There was no positive finding when using the contrast enhancement on the IR images in 4 patients. Two were post-menopausal women who had an exaggerated sympathetic stimulation with an antecedent stress event. They displayed severe contractile abnormalities in the apical segments with a low LVEF (30-35%) on the cine-mode images and an absence of contrast enhancement, ruling out AMI or acute myocarditis. One of them had myocardial oedema on T2-weighted imaging. Thus, the diagnosis of stress cardiomyopathy was clear. CMR re-examination showed that their LV dysfunction was completely recovered and the LVEF was 56-60% at 14 days, which further confirmed the initial diagnosis made on CMR. No confirmed diagnosis was established in the remaining 2 patients on normal CMR during the early stage of hospitalization.
Discussion
There is a broad consensus that CMR imaging, especially with LGE has the ability to differentiate conditions that closely mimic ACS [12]. In this study, acute one-stop CMR enabled the aetiological origin of the clinical picture to be identified in most (93.8%) of the patients versus 90-95% in patients with ACS and normal coronary arteries as reported in 2 previous studies by Laraudogoitia Zaldumbide et al. [2] and Leurent et al. [14], respectively. However, it must be noted that there are some differences in the research scheme between our study and others.
Compared with some other studies [2,14,15,16] in which the one-stop CMR was conducted several days after the primary CAG, in our study it was performed just 2-4 h afterwards. Establishing an accurate diagnosis as quickly as possible has paramount importance for both the prognosis and therapeutic outcome in patients suffering from a potentially life-threatening condition. Treatments for different diseases differ immensely. Anti-thrombotic treatment was withdrawn after acute CMR in 78.1% of the patients in our series who did not have AMI or PE, 6.2% of the PE patients received thrombolytic therapy and 9.7% of the AD patients were transferred to the Department of Vascular Surgery for an operation. The other explanation could be that the extension of the interval between the onset of symptoms and CMR imaging meant that several transient abnormalities detectable on CMR (e.g. dyskinetic wall motion, pericardial effusion and myocardial oedema) might have resolved. This would reduce the diagnostic value of CMR. Definite diagnoses were made on the basis of acute CMR imaging in nearly 95% of our patients whereas Assomull et al. [15] reported that the overall diagnostic contribution of CMR in their patients was only 65%, probably due to the median delay of 14.5 days between the onset of chest pain and CMR.
Due to the high mortality and morbidity associated with AMI, PE and AD, these 3 diseases should be ruled out first in patients presenting with acute chest pain and elevated troponin I. Therefore, after negative CAG, thoracic aorta MR and pulmonary MR were conducted prior to cardiac MR in our series. This procedure was rarely mentioned in previous studies [2,14], although echocardiography was conventionally performed before CMR. Echocardiography is able to detect thrombi in the pulmonary and intimal flap in the thoracic aorta. Sensitivity and specificity are very low in detecting AD and PE, but contrast-enhanced 3D-MRA of the aorta, pulmonary MRA and 3D GRE imaging are much more sensitive and specific [5,6,17,18]. Three cases of AD and 2 cases of PE were diagnosed clearly by the use of the above MR technique in this study. Furthermore, it should be noted that the experienced cardiologist decided on the examination procedures according to the clinical manifestations. For instance, pulmonary MR was performed primarily on the patients with hypoxaemia and elevated levels of D-dimer. This minimized the testing time and made early diagnosis possible for our patients.
In contrast to this study, Steen et al. [19] performed acute CMR before cardiac catheterization in the chest pain unit in 29 patients with elevated troponin I and a low-to-intermediate probability of ACS; they detected PE, AMI, myocarditis and cardiomyopathy step by step, with 37.9% (11/29) of their patients being given a diagnosis of AMI. The point is that approximately 30% of the patients with ACS did not have typical clinical manifestations, conclusive ECG changes or cardiovascular risk factors [20]. Some patients do not have to undergo further invasive procedures after acute CMR, but others may miss the optimal opportunity to undergo primary percutaneous coronary intervention. However, the patients in our study had undergone CAG before the CMR examination. Most of the patients with AMI were ruled out, and the remaining patients had normal coronary flow, which reduced the risk of sudden cardiovascular events during the CMR study. It was indubitably safer and better tolerated. In addition, in view of non-coronary cardiac disease presenting with acute chest pain and the differential diagnosis of the underlying pathology, MRI has unique diagnostic features [21]. Therefore, in our series, acute CMR was conducted after CAG in patients suspected of having ACS.
In this study, no significant abnormality was seen on the CMR images of 2 patients with a modest increase in troponin I (1.2-2.4 ng/ml) above the normal range, which is a sign of minimal myocardial necrosis. One of them had a final diagnosis of AMI, demonstrated by the typically dynamic changes on ECG and the biomarkers of myocardial damage in 1 week. The explanation may be that the absence of contrast enhancement on the CMR was due to the fact that the technique is limited by inadequate spatial resolution when detecting very small necrotic areas [22]. Three days after the onset of chest pain, the symptoms were completely alleviated and a moderate amount of pericardial effusion was detected on echocardiography at the same time in the other patient, who was suspected of having acute perimyocarditis. This could have been due to the above-mentioned reason, or it may have been that there was no wall motion abnormality or pericardial effusion at an early stage, which is nicely depicted in the black-blood-prepared T2-weighted images as well as in the SSFP cine loops [23].
There are some limitations to our study. First, since MRI services in our hospital are not round-the-clock, we only enrolled 32 patients with ACS and unobstructed coronary arteries. So the sample size in this single center was relatively small. Second, no long-term follow-up was implemented in any patients, and only a few patients underwent repeated CMR (this can yield prognostic information). Third, the criteria for deciding who would undergo the acute CMR study were not standardized, and the decision was left to the discretion of the cardiologist.
Conclusion
Acute one-stop CMR safely allowed for the identification of an aetiology in more than 90% of the patients in this study. It may prove to be of immense help in establishing a differential diagnosis in patients presenting with acute chest pain, elevated troponin I and normal coronary arteries.
Guang Chu and Hao Zhang contributed equally to this work.
References
- 1.Arai AE. False positive or true positive troponin in patients presenting with chest pain but ‘normal’ coronary arteries: lessons from cardiac MRI. Eur Heart J. 2007;28:1175–1177. doi: 10.1093/eurheartj/ehl567. [DOI] [PubMed] [Google Scholar]
- 2.Laraudogoitia Zaldumbide E, Perez-David E, Larena JA, et al. The value of cardiac magnetic resonance in patients with acute coronary syndrome and normal coronary arteries. Rev Esp Cardiol. 2009;62:976–983. doi: 10.1016/s1885-5857(09)73263-3. [DOI] [PubMed] [Google Scholar]
- 3.Larson DM, Menssen KM, Sharkey SW, et al. ‘False-positive’ cardiac catheterization laboratory activation among patients with suspected ST segment elevation myocardial infarction. JAMA. 2007;298:2754–2760. doi: 10.1001/jama.298.23.2754. [DOI] [PubMed] [Google Scholar]
- 4.Mahajan N, Mehta Y, Rose M, et al. Elevated troponin level is not synonymous with myocardial infarction. Int J Cardiol. 2006;111:442–449. doi: 10.1016/j.ijcard.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Kalb B, Sharma P, Tigges S, et al. MR imaging of pulmonary embolism: diagnostic accuracy of contrast-enhanced 3D MR pulmonary angiography, contrast-enhanced low-flip angle 3D GRE, and non-enhanced free-induction FISP sequences. Radiology. 2012;63:271–278. doi: 10.1148/radiol.12110224. [DOI] [PubMed] [Google Scholar]
- 6.Krishnam MS, Tomasian A, Malik S, et al. Image quality and diagnostic accuracy of unenhanced SSFP MR angiography compared with conventional contrast-enhanced MR angiography for the assessment of thoracic aortic diseases. Eur Radiol. 2010;20:1311–1320. doi: 10.1007/s00330-009-1672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proctor RD, Shambrook JS, McParland P, et al. Imaging hypertrophic heart diseases with cardiovascular MR. Clin Radiol. 2011;66:176–186. doi: 10.1016/j.crad.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Leurent G, Larralde A, Boulmier D, et al. Cardiac MRI studies of transient left ventricular apical ballooning syndrome (Takotsubo cardiomyopathy): a systematic review. Int J Cardiol. 2009;135:146–149. doi: 10.1016/j.ijcard.2009.03.067. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. JACC. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yalcinkaya E, Bugan B, Celik M, et al. Cardiomyopathies: the value of cardiac magnetic resonance imaging. Med Princ Pract. 2014;23:191. doi: 10.1159/000356379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich MG. Tissue characterization of acute myocardial infarction and myocarditis by cardiac magnetic resonance. JACC Cardiovasc Imaging. 2008;1:652–662. doi: 10.1016/j.jcmg.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Bruder O, Schneider S, Nothnagel D, et al. Euro CMR (European Cardiovascular Magnetic Resonance) Registry: results of the German pilot phase. J Am Coll Cardiol. 2009;54:1457–1466. doi: 10.1016/j.jacc.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 14.Leurent G, Langella B, Fougerou C, et al. Diagnostic contributions of cardiac magnetic resonance imaging in patients presenting with elevated troponin, acute chest pain syndrome and unobstructed coronary arteries. Arch Cardiovasc Dis. 2011;104:161–170. doi: 10.1016/j.acvd.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Assomull RG, Lyne JC, Keenan N, et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin and unobstructed coronary arteries. Eur Heart J. 2007;28:1242–1251. doi: 10.1093/eurheartj/ehm113. [DOI] [PubMed] [Google Scholar]
- 16.Chopard R, Jehl J, Dutheil J, et al. Evolution of acute coronary syndrome with normal coronary arteries and normal cardiac magnetic resonance imaging. Arch Cardiovasc Dis. 2011;104:509–517. doi: 10.1016/j.acvd.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Ohno Y, Koyama H, Matsumoto K, et al. Dynamic MR perfusion imaging: capability for quantitative assessment of disease extent and prediction of outcome for patients with acute pulmonary thromboembolism. J Magn Reson Imaging. 2010;31:1081–1090. doi: 10.1002/jmri.22146. [DOI] [PubMed] [Google Scholar]
- 18.Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14:15–23. doi: 10.1093/ehjci/jes215. [DOI] [PubMed] [Google Scholar]
- 19.Steen H, Madadi-Schroeder M, Lehrke S, et al. Staged cardiovascular magnetic resonance for differential diagnosis of troponin T positive patients with low likelihood for acute coronary syndrome. J Cardiovasc Magn Reson. 2010;12:51. doi: 10.1186/1532-429X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassand JP, Hamm CW, Ardissino D, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 21.Hunold P, Bischoff P, Barkhausen J, et al. Acute chest pain: the role of MR imaging and MR angiography. Eur J Radiol. 2012;81:3680–3690. doi: 10.1016/j.ejrad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 22.Vohringer M, Mahrholdt H, Yilmaz A, et al. Significance of late gadolinium enhancement in cardiovascular magnetic resonance imaging (CMR) Herz. 2007;32:129–137. doi: 10.1007/s00059-007-2972-5. [DOI] [PubMed] [Google Scholar]
- 23.Sa MI, Kiesewetter CH, Jagathesan R, et al. Images in cardiovascular medicine. Acute pericarditis assessed with magnetic resonance imaging: a new approach. Circulation. 2009;119:e183–e186. doi: 10.1161/CIRCULATIONAHA.108.771006. [DOI] [PubMed] [Google Scholar]